Abstract
Recent neuroimaging studies suggest that the superior parietal lobule (SPL) of the human cortex mediates goal-directed attentional orienting, while the temporo-parietal junction (TPJ) mediates stimulus-driven attentional orienting. Here, we investigated these brain-behavior correspondences by examining the performance of patients with an attentional deficit following a right hemisphere lesion. Patients completed two tasks, one sensitive to stimulus-driven attentional orienting and the other to goal-directed attentional orienting. Based on the behavioral profiles obtained on each task, patients were assigned to different groups and their lesion overlap explored. Patients who exhibited difficulties with goal-directed attentional orienting and showed concurrent “hyper-capture” presented with lesion overlap centered over superior portions of the parietal lobule with spared inferior portions of the parietal lobule. Patients who performed normally on the goal-directed orienting task, while remaining abnormally immune to attentional capture, presented with lesion overlap centered over the inferior portions of the parietal lobule but spared superior parietal lobule. The findings from this study clearly suggest that (a) SPL and TPJ are anatomical regions that are recruited for the purposes of top-down and bottom-up orienting, respectively, and that damage to SPL and TPJ leads to disorders of top-down and bottom-up orienting, and (b) albeit dissociable, top-down and bottom-up orienting (and, by extension, SPL and TPJ) are not entirely independent mechanisms.
Keywords: Neglect, Parietal cortex, Attentional control, Superior parietal lobule (SPL), Temporo-parietal junction (TPJ), Top-down attention, Bottom-up attention
Introduction
Over the past several decades, researchers have relied critically on neuropsychological studies of patients with hemispatial neglect to address key questions about attentional dysfunction and its underlying neural substrate. Indeed, much progress has been made in elucidating issues such as the nature and extent of implicit processing on the contralesional side of space (for example, Shomstein et al. 2010; Van Vleet and Robertson 2006), the difference in visual extinction after right versus left hemisphere stroke (for example, Becker and Karnath 2007), and the nature of the hemispatial neglect as defined with respect to different reference frames (for example, Medina et al. 2009). One issue that has received extensive consideration but still remains hotly debated concerns the neuroanatomy of hemispatial neglect and the possible differences in the behavioral profile following lesions to different cortical regions. Whereas the view that dominated the field for a long time considered parietal cortex as a monolithic entity and a unitary attentional deficit resulting from parietal damage as the predominant impairment, more recently, there has been a fragmentation of parietal cortex into subcomponents, on the one hand, and of the attentional disorder into differing subprofiles, on the other. Here, we examine these brain-behavior correspondences in attentional processing in greater detail using a set of fine-grained psychophysical measures to cleave apart the different behavioral patterns and their underlying neural correlates.
In the classical neuropsychological literature, parietal cortex, as an entity, was generally considered the primary lesion site for hemispatial neglect. This view, elaborated in detail by early researchers (Critchley 1953; McFire and Zangwill 1960; Piercy 1964), clearly recognized the association between the parietal lesion and the ensuing ‘visualspatial agnosia’ or ‘neglect’. This perspective was largely held through the 1980s at which point more sophisticated psychological and neurological examinations began to be undertaken (Bisiach and Vallar 1988; Mort et al. 2003; Posner et al. 1984). Indeed, Posner et al. (1984) were among the first to administer a well-developed chronometric tool, the covert visuospatial cueing paradigm, to individuals with parietal injury and, based on the findings from these studies, conjectured that damage to the parietal lobe produces a deficit in the ‘disengage’ operation i.e., a disproportionate impairment in retracting attention from one location and shifting it to another when the target appears on the side of space contralateral to the lesion.
It is the case, however, that attention can be directed to a stimulus in at least two ways, via top-down (goal-directed) selection or via bottom-up (stimulus-driven) attention (perhaps decomposing ‘disengage’ even further). The goal-directed form of attention is one in which attentional orienting results from the explicit will of an organism; this includes, for example, the willful orienting to the upcoming traffic light or, in experimental terms, directing one’s attention to the location indicated by an endogenous cue, such as an arrow pointing to a particular region of the computer screen. The stimulus-driven form of attention occurs via bottom-up attentional selection that is engaged when an intrinsic property of the stimulus is sufficiently salient to capture attention away from the task at hand. For example, a flash of red in a pile of tomatoes will (hopefully) attract attention to the ripe fruit or again, in experimental terms, a change in luminance, would draw the observer’s attention to the salient input. Critically, if an item is selected (either in a top-down or a bottom-up fashion), then its representation is enhanced, resulting in an attentional facilitation in which the speed and accuracy of detecting a target at the attended location is improved relative to target detection in unattended locations (Yantis 2000).
Recent neuroimaging studies with normal subjects suggest that each form of attentional orienting may rely on a somewhat differing neural substrate. Voluntary goal-directed deployments of attention are associated with neural activity in regions of dorsal parietal cortex (intraparietal sulcus; IPS) and superior parietal lobule (SPL) as well as in the frontal eye fields (FEF), whereas stimulus-driven attentional capture is associated with the temporo-parietal junction (TPJ) and ventral frontal cortex (Corbetta et al. 2000; Corbetta and Shulman 2002; Serences et al. 2005; Behrmann et al. 2004). Thus, separate loci within the parietal lobe have been identified as the neural source for goal-directed (superior portions) and stimulus-driven (inferior portions) attentional orienting.
The association of one or the other form of orienting with different lesion sites is more complicated, however. For example, clinical symptoms of hemispatial neglect are strongly associated with damage to the inferior portions of the parietal lobe, which includes TPJ, rather than to superior portions like SPL (Friedrich et al. 1998; Vallar and Perani 1986). Also, previous attempts to examine these two forms of orienting in patients with neglect have not clearly revealed differences in brain-behavior relations with the different forms of attentional orienting. Indeed, in his original work with patients with brain damage, Posner et al. (1984) tested the patients on two different versions of the spatial cueing paradigm, one in which the cues were central arrows, thereby tapping into the endogenous or volitional form of orienting and one in which the cues consisted of the brightening of the peripheral box, thereby tapping into exogenous or bottom-up form of attentional orienting. The patients were impaired on both tasks. However, there was no clear neural distinction between the two forms of orienting among the patients, and the extent of the disengage deficit was simply correlated with extent of parietal lesion. No further subdivisions of the lesion site were considered. Friedrich et al. (1998), in a follow-up study, compared the performance of two groups of patients on the Posner cuing task. One group consisted of patients whose lesion could be localized to the TPJ, and the other group whose lesions were localized to the parietal cortex, while sparing SPL. The authors observed that both groups of patients exhibited a strong validity effect (i.e., faster RTs for detecting targets in the validly cued location), while only the TPJ group exhibited a RT pattern consistent with the disengagement pattern (i.e., difficulty in detecting contralesional targets after the cue engaged attention in the ipsilesional field). The authors argued then that TPJ, rather than SPL, subserves spatial orienting. Note, however, that because the authors had used only the exogenous form of attention cueing in their paradigm (and did not compare it with the endogenous version of the paradigm), they might have failed to observe the deficit associated with goal-directed orienting in the SPL patients. Finally, in a recent study with patients with lesions centered primarily over TPJ and STG but preserved SPL, Corbetta et al. (2005) showed that spatial neglect, as well as its recovery, was associated with restoration of the BOLD signal in both the ventral temporo-parietal and dorsal parietal regions.
As evident from this brief review, there does not seem to be a clear association between lesion site and behavioral profile in the neuropsychological literature. Given the fractionation of attentional selection in the imaging studies and the debate concerning the neural bases of attentional neglect, here, we examine the brain-behavior correspondences of a small group of individuals with documented lesions affecting largely either the superior portions of the parietal lobule (subsuming SPL) or the inferior portions of the parietal lobule (subsuming TPJ) and explore whether a specific behavioral profile is predictive of a corresponding anatomical lesion.
Differentiating between SPL and TPJ function
To distinguish between goal-driven attentional control and salient attentional capture and to examine the mapping of behavior onto the SPL and TPJ, respectively, we adopted two behavioral paradigms, each targeted to one of these forms of attentional selection. To examine the integrity of top-down attentional orienting in the patients, we employed a variant of the Sperling and Reeves (1980) task, which has been successfully used to demonstrate SPL activation in fMRI studies (Yantis et al. 2002). Essentially, in this experiment, participants are required to shift spatial attention from one side of space to another following a top-down attentional cue. Similarly, in order to examine the bottom-up attentional orienting abilities of the patients, a variant of Folk et al.’s (2002) contingent paradigm was employed in which participants detect targets that appear at fixation, while task-irrelevant color singletons are flashed in the periphery. The extent to which task-irrelevant distractors interfere with the central detection task is then used as a measure of bottom-up attentional capture (Bacon and Egeth 1994).
While remaining agnostic as to the lesion-to-impairment association, the predictions are as follows: patients impaired in the Sperling and Reeves (1980) top-down attentional orienting task (with preserved performance on the Folk et al.’s task) will have lesions affecting superior portions of the parietal lobe (including SPL), while patients impaired on the Folk et al. (2002) task (with spared performance on Sperling and Reeves task) will have lesions affecting the inferior portions of the parietal lobe (affecting TPJ). A double dissociation of this form will not only attest to the independent psychological components of attention but will also indicate that these attentional components are mediated by independent neural mechanisms.
Method
Participants
Nine patients with chronic, right-lateralized focal cortical lesions and 9 healthy control participants (matched to the patients on age and education level) consented to participate in the experiments, in accordance with the protocol approved by the Institutional Review Boards of Carnegie Mellon University and the University of Pittsburgh. All patients (4 women and 5 men) had normal or corrected-to-normal vision, were tested at least 8 months following the onset of the cerebrovascular incident (right middle cerebral artery infarcts), and ranged in age between 42 and 78 years (mean of 66.7). All patients scored below 100 (cutoff 132/146 for neglect) on the Behavioral Inattention Test bedside battery (BIT; Wilson et al. 1987), meeting the criterion for hemispatial neglect. The BIT includes line cancellation, letter cancellation, star cancellation, figure and shape copying, line bisection, and representational drawing tasks, thereby sampling neglect across a wide array of visuoperceptual tasks (Table 1).
Table 1.
Patients along with sex, age at the time of testing, and the score on the Behavioral Inattention Test (BIT; Wilson et al. 1987), which included line bisection, line cancellation, letter cancellation, and figure-drawing tasks
| Patient | Sex | Age | BIT score |
|---|---|---|---|
| OL | M | 62 | 129 |
| BB | F | 70 | 86 |
| FD | M | 77 | 82 |
| RK | F | 44 | 114 |
| SW | F | 56 | 86 |
| JM | M | 61 | 128 |
| MF | M | 45 | 114 |
| LB | F | 67 | 84 |
| LD | M | 72 | 86 |
The cutoff score for the BIT is 129 out of 146 possible points and any score of 132 or below is considered as diagnostic of neglect
Top-down attentional shifting paradigm
Apparatus and stimuli
The experiment was conducted on a portable computer with a 14-in color monitor placed roughly 62 cm from the observer. Displays consisted of a central fixation point and two RSVP alphanumeric streams positioned approximately 1.6° to the right and left of fixation. Each RSVP stream consisted of white letters and digits presented on a gray background, drawn pseudorandomly from a pre-defined set (“2”, “4”, “A”, “C”, “F”, “G”, “H”, “J”, “K”, “M”, “N”, “P”, “R”, “T”, “U”, “V”, “X”, and “Y).” Each character in the RSVP stream subtended approximately 0.7° of visual angle horizontally and 0.8° vertically.
Procedure
At the start of each run, a cue appeared (a small line to the left or to the right of the fixation), instructing the subject to attend to the left or right stream (Fig. 1). After the cue disappeared, two RSVP streams were presented, consisting of items that changed their identity rapidly and synchronously every 500 ms. Characters in each stream were chosen randomly with the only restriction being that no letter was repeated consecutively and that the letters presented within the left and the right stream could never be the same. The subjects’ task was to detect target digits (“2” and “4”) embedded among the letters (which are essentially distractors) within the attended stream and to press a left or right mouse button to indicate successful detection of the 2 or 4. For half the subjects, the digit “2” instructed them to shift attention from the currently attended stream to the unattended stream (e.g., left to right), while the digit “4” instructed them to maintain their attention on the currently attended stream; this mapping was reversed for the remaining subjects. The subjects were instructed to hold attention on the currently attended stream if they thought they had missed a target. Only detected target events were included in our analysis.
Fig. 1.
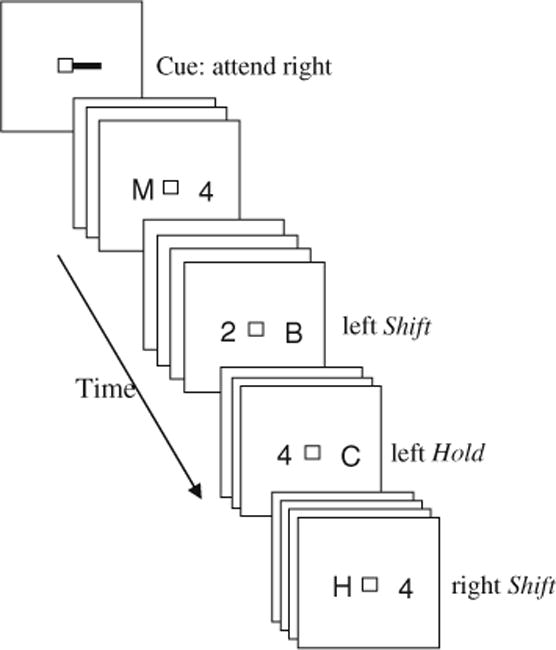
Example of a typical run in the attentional control RSVP paradigm. At the beginning of the run, a cue is presented directing the subject to start paying attention to one of the streams. Subjects are instructed to detect digit targets embedded among letter distractors. If digit ‘4’ is detected, subjects shift their attention to the previously ignored stream. If digit ‘2’ is detected, subjects hold their attention within the attended stream. Targets are then sorted based on the preceding trial such that if the target was presented right after participant shifted attention into the previously ignored stream, it is labeled a shift target. If, on the other hand, the target was presented within the stream that already contained a target digit, then this target is labeled a hold target
Each subject performed 2 practice runs and 10 experimental runs; each run was about 5 min in duration and included 8 occurrences of each of the four target types: attend left, attend right, switch attention from right to left, and switch attention from left to right (a total of 32 target events per run). Participants were encouraged to take breaks between runs.
This design allowed for two trial types: (1) targets that were presented to the attended stream immediately after attention was shifted into it, labeled the shift trial; and (2) targets that appeared within a stream in which another target had already been presented, labeled the hold trial. The two types of targets occurred equally often on the right and the left side of space. If attentional shifting capabilities are perturbed, then exhibiting overall slower RTs for the target on the left side (hold or shift), the detection time for targets appearing on either the right or the left side following a shift should be slower than for hold targets (i.e., reflecting sluggish shifting independent of whether attention is shifted into the intact ipsilesional side or into the neglected side). This sluggishness of executing an attentional shift is expected for both sides of space since the neuroimaging studies point to right SPL as being recruited unilaterally independently of which side of space attention is being shifted to (Shomstein and Behrmann 2006; Shomstein and Yantis 2006; Yantis et al. 2002).
Contingent capture paradigm
Apparatus and stimuli
The experiment consisted of a single RSVP stream of single letters, presented in the center of the display. Each letter measured 1.3° × 1.2°, with a stroke width of 0.3°. One letter in the sequence was red or green, depending on whether the participants were in the ‘red searching’ group or ‘green searching’ group. The color of the remaining letters in the sequence was chosen randomly from a set of four possible colors. For subjects searching for green targets, the non-target colors were dark gray, blue, purple, and red (for subjects searching for red targets, red was replaced with green). One of the letters in the sequence was accompanied by four surrounding #s whose inner edges appeared 5.2° above, below, right, and left of the center of the letter (Fig. 2). Depending on the distractor condition, either all the #s were gray or three were gray and one was red or green (depending on the red or green searching condition).
Fig. 2.
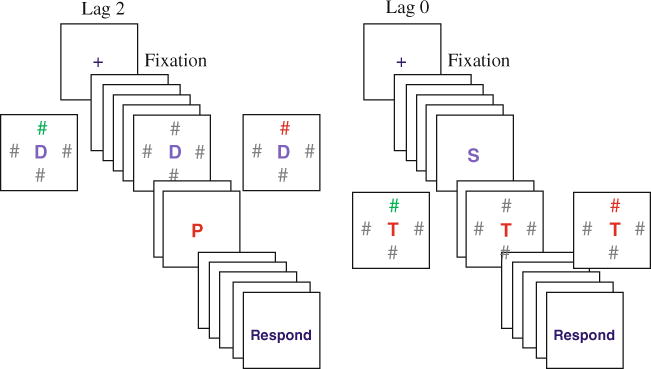
Example of lag 2 and lag 0 trial types crossed with three different types of distractors. Subjects are asked to report the identity of the red central letter (P in the left panel and T in the right panel). Left panel: examples of lag 2 trials in which distractors are all-gray (center) or different-color (left) or target-color (right). Right panel: examples of lag 0 trials for the three types of distractors
Procedure
Each trial began with a 500-ms fixation cross appearing in the middle of the screen, followed, after 200 ms delay, by the sequential presentation of 15 letters. Each letter in the sequence was presented for 60 ms, followed by a 60-ms blank interval. The letters on each trial were selected randomly from the English alphabet (with the exception of I, O, W, and Z that were excluded to match the design of Folk et al. (2002)). Each participant was assigned a target color (red or green). The target could appear in serial positions 8 through 12 determined randomly.
Three different distractor conditions were randomly mixed within a block. In the ‘four-gray’ distractor condition, on the frame that contained four eccentric # distractors, all of #s were gray. In the ‘target-color’ distractor condition, three of the # distractors were gray and one # matched the color of the target letter (e.g., if subjects searched for red, a single distractor # was also red and the other three were gray). In the ‘irrelevant-color’ distractor condition, three of the # distractors were gray and one # was a non-target color (e.g., if a subject was searching for a red target, the distractors would consist of three gray and one green #s). The distractors were presented at two temporal lags in relation to the target, either presented concurrently with the target (lag 0, see Fig. 2 right panel) or presented two frames prior to the target onset (lag 2, see Fig. 2 left panel). The color distractor appeared either above or below the central letter (never to the left or to the right). Across trials, each distractor-type appeared equally often at each lag. The experiment consisted of 10 runs of 30 experimental trials each, for a total of 300 trials. Each run was followed by a break.
This design allowed us to investigate the extent to which a target-colored distractor captures attention in patients, by examining target accuracy when distractor appears simultaneously with the target (lag 0) or two frames before the target onset (lag 2). It has been observed that normal controls exhibit contingent attentional capture only at lag 2 (Folk et al. 2002). If a patient’s ability to be captured is perturbed, then two patterns of performance are possible: (1) the target-colored distractor fails to capture attention, thus yielding accurate performance at lag 0 and lag 2 irrespective of which distractor color is presented in the periphery; or (2) the distractors are so salient that they interfere at both lags (hyper-capture). In contrast, if patients exhibit normal attentional capture, then only the target-colored distractor should interfere with target processing, and it should do so only at lag 2.
Lesion analysis
Raw T2 fluid-attenuated inversion recovery (FLAIR) images (0.86 × 0.86 × 12 mm) were obtained for 6 patients, and non-digital picture images of a lesion (T2 FLAIR, T1 weighted, and CT scan) for the remaining 3 patients. Since there is no FLAIR template for normalization, and T1 and T2 image-averaging procedures led to sub-optimal normalization template, the axial 28 slices T2 FLAIR images were reoriented and re-sliced into 91 slices using SPM5 to match the dimension of a T1 single subject SPM5 template with 2-mm3 resolution. For each of the 6 patients, the lesion was then mapped onto the standard space template. For the remaining patients, the lesion, identified on the non-digital picture image, was traced onto the normalized template. Four trained raters traced the lesion slice-by-slice onto the corresponding slice on the template image using MRIcro’s region of interest (ROI) function. The region overlapped by at least three raters was defined as the final lesion ROI of each patient. After patients were grouped according to their behavioral profiles (see below), lesion ROIs were overlapped for each group to visualize commonly affected lesion areas.
Results
Top-down attentional shifting
The mean reaction times (RT) for targets (digits ‘2’ and ‘4’) were sorted according to the following rule: if the target appeared right after a shift, it was labeled as a shift target (e.g., a digit ‘4’ or a digit ‘2’ that appeared after a cue to shift attention to the other stream); if the target appeared after another target was already detected in that stream, it was labeled as a hold target (e.g., a digit ‘4’ or a digit ‘2’ appearing in the stream in which participant has already detected another target)1. RTs for each patient and the mean of the control group in the four conditions are shown in Fig. 3a.
Fig. 3.
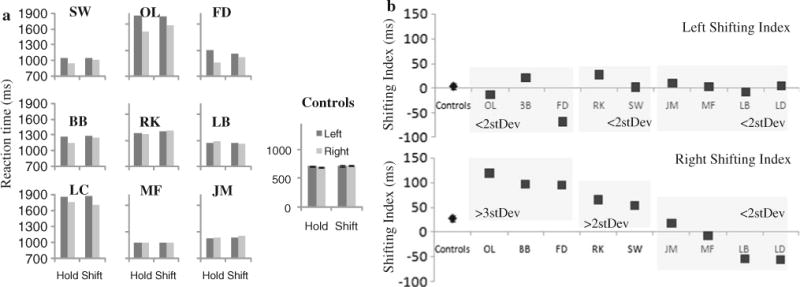
a Reaction times as a function of target type (shift or hold) and side of occurrence for each of the 9 patients and the control group. b Left (top panel) and right (bottom panel) side shifting indices were computed for the control group (as an average) and for each patient individually as a difference between shift and hold target types, reflecting efficiency of an attentional shift into the right (unaffected) or left (neglected) side of space
To capture and quantify the potential difficulty in the ability to shift attention in a goal-directed manner, we computed the left and right shifting index by subtracting shift targets from hold targets for each side, respectively, to reflect the difficulty with top-down attentional shifting (i.e., slowing in executing an attentional shift). While we calculate the shifting index for both the left and the right side, we particularly focus only on the right shift targets for two reasons: (1) based on numerous neuroimaging studies described previously, goal-directed spatial attentional shifting selectively recruits right SPL independent of where in space attention is shifted to (i.e., both left and right), thus patients should be affected while executing attentional shifts either to the left or to the right; and (2) shifts into the unaffected right side of space provide an uncontaminated (free of possible floor effects on the neglected side) measure of shifting efficiency. The shifting index is presented in Fig. 3b for both the left (top panel) and the right side (bottom panel). While the left shifting index shows no difference among patients and controls (all patient’s values are within the 2 standard deviations of the controls), the right shifting index clearly reflects that some patients are less efficient when executing attentional shifts, i.e., yielding a larger shift index, than other patients and than controls. To explore the individual differences, patients whose right shifting index exceeded that of three standard deviations (1SD = 31 ms) of the control group were assigned to one group (patients OL, BB, and FD), patients whose index was greater than two standard deviations of the controls were assigned to a second group (patients SW and RK), and those within the variance of controls were assigned to a third group (patients JM, MF, LB, and LD).
The lesion overlap for individuals in the three groups was then examined (see Fig. 4a). The common region affected in the most deviant group (>3SDs of the control group) was located within the superior portion of parietal cortex, and each patient’s lesion subsumed SPL. We thus call this group “the SPL group.” At the opposite end, the group whose shifting index was well within the normal bounds had a lesion overlap in the inferior portions of the parietal cortex (subsuming TPJ), we thus label this group as a “TPJ group.” The third group, whose shifting index exceeded two standard deviations of the control group, only included two patients, one with a diffuse and one with a circumscribed lesion (Fig. 4c), thus we only focus on the SPL and the TPJ groups.
Fig. 4.
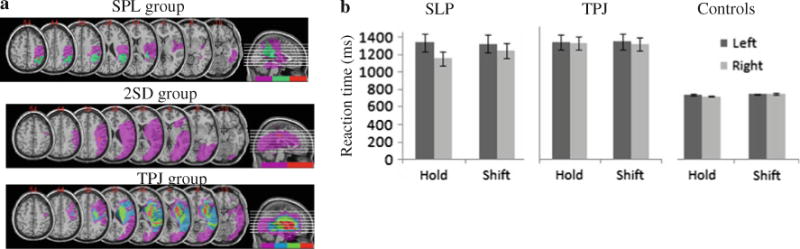
a Lesion overlaps. Top panel Patients whose performance differs from control—patients OL, BB, and FD—exhibit lesion overlap over the superior parts of the parietal cortex, labeled here as the “SPL group.” Middle panel Patients whose shifting index fell within two standard deviations of the mean of controls (patients RK and SW), labeled as the “2SD group.” Bottom panel Patients whose performance did not differ from that of controls—patients JM, MF, LB, and LD—exhibit lesion overlap over the inferior portions of the parietal cortex, labeled here as the “TPJ group.” b Reaction times as a function of target type (shift or hold) and side of occurrence for the SPL, TPJ, and control groups
RTs for correct responses for the SPL and the TPJ group were subjected to an omnibus ANOVA with the target type (hold or shift), side of occurrence (left or right) as within-subject factors, and group (TPJ, SPL, and controls) as a between-subject factor (see Fig. 4b). The analysis revealed a significant main effect of side [F(1, 13) = 20.11, P < 0.001] with targets appearing on the left yielding slower responses than those on the right; of type [F(1, 13) = 4.53, P < 0.05] with shift targets detected slower than the hold targets; and of group [F(2, 13) = 13.76, P < 0.01]. Importantly, we observed a significant interaction of side × group [F(2, 13) = 10.41, P < 0.01], of side × type [F(1, 13) = 9.53, P < 0.01], as well as a three-way interaction of side × type × group [F(2, 13) = 9.30, P < 0.01] (see Fig. 4b).
Planned comparison ANOVAs comparing the performance of the groups with one another demonstrated that, in line with our predictions, the SPL group differed significantly from the TPJ group [side × type × group interaction: F(1, 5) = 28.15, P < 0.01] as well as from the controls [side × type × group interaction: F(1, 10) = 9.03, P < 0.02], while the TPJ group did not differ from controls [F = 3.3, NS]. The important difference between the SPL group and the other two groups is that, in addition to showing neglect on the left side, patients exhibit impaired slowed identification of shift targets appeared in the unaffected (right) hemispace—note the difference between hold right and shift right targets for the SPL group in Fig. 4b. This slowing in shifting even into the right field is a clear illustration of the difficulty in goal-directed attentional shifting.
Contingent capture
The pattern of performance elicited by the control group was examined first to ensure that we were able to replicate the lag 2 capture of Folk et al. (2002). The mean percentage of correct target identification as a function of distractor condition and distractor-to-target lag is presented in Fig. 5a (left panel). These data were subjected to an ANOVA with lag (0 and 2) and distractor-type (gray, target color, different color) as within-subject factors. ANOVA yielded a main effect of lag [F(1,8) = 58.59, P < 0.01] such that accuracy was greater at the lag 0 condition when compared to lag 2; a main effect of distractor-type [F(2,16) = 21.63, P < 0.01] such that accuracy was lower for the target color distractor, as well as a significant distractor-type by lag interaction [F(2,16) = 13.30, P < 0.01], reflecting the fact that target-colored distractor led to decreased target accuracy at lag 2 only. These findings confirm the expected capture profile in our control group.
Fig. 5.
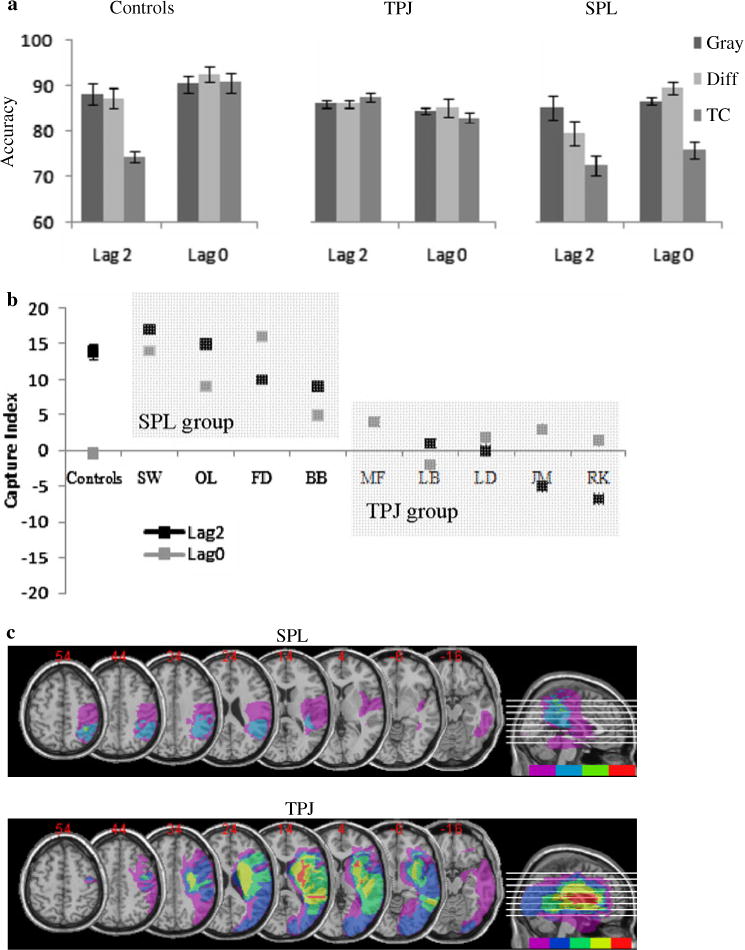
a Mean accuracy for lag 2 and lag 0 conditions as a function of distractor-type (all-gray, target-colored, different-colored) for controls and the two patient groups. b The capture index was computed for each individual patient by subtracting the target-colored distractor trials accuracy from the all-gray distractor accuracy, reflecting the extent of attentional capture by a task-irrelevant distractor. c Lesion overlaps. Top panel for the SPL group that included patients SW, OL, FD, and BB. Bottom panel for the TPJ group that included patients MF, LB, LD, JM, and RK
To explore the patient’s performance, we calculated a capture index (see Fig. 5b, black lines) for each individual: this index summarizes the extent to which target-colored distractors interfere with target processing at both lags (i.e., the extent of attentional capture) by subtracting the accuracy for target detection when one of the distractors is the same color as the target (capture) from the accuracy for target detection when all of the distractors are gray (least distraction). The derived capture index (see Fig. 5b) demonstrates that controls have a high capture index for target-colored distractors at lag 2 only.
The patients on the other hand showed two different patterns: ‘normal’ capture for target-colored distractor at lag 2 that was accompanied by practically as much capture at lag 0; or complete immunity to capture such that target-colored distractors did not interfere with target processing at either lag. In order to distil these differences among the individuals, the extent of target-colored capture at lag 2 was calculated for each patient. If a patient’s lag 2 capture index exceeded 3 standard deviations from the mean of the control, they were placed in one group (MF, LB, LD, JM, and RK), and those patients who performed within the variance of the control groups were placed into another group (SW, OL, FD, and BB).
Lesion overlap was assessed for the two groups (Fig. 5c). The group whose performance was within the normal range shows an area of lesion overlap in the SPL region and thus is labeled the “SPL group”. On the other hand, the lesion overlap for the group with a capture index that exceeds that of the controls was located around TPJ and the insula and we label this group the “TPJ group.” The accuracy data from three groups (Fig. 5a) were then subjected to an ANOVA with lag (0 or 2) and distractor-type (gray, target color, different color) as within-subject factors, and group (SPL, TPJ, controls) as a between-subject factor. ANOVA yielded main effects of lag [F(1,15) = 13.89, P < 0.01] with greater accuracies at lag 0 when compared to lag 2, and of type [F(2,130) = 27.79, P < 0.001] with lower accuracy for target-colored dis-tractors. In addition, a lag × group [F(2,15) = 11.86, P < 0.01], type × group [F(4,30) = 6.63, P < 0.01], and lag × type [F(2,30) = 3.31, P = 0.05] interaction were significant. More importantly, a three-way lag × type × group interaction was also significant [F(4,30) = 7.16, P < 0.001] (see Fig. 5a).
Planned comparison ANOVAs comparing the performance of each group with one another demonstrated that, in line with our predictions, the SPL group differed significantly from the TPJ group as reflected by a significant lag × group [F(1,7) = 5.98, P < 0.05] and a type × group [F(2,14) = 11.65, P < 0.01] interaction. Additionally, each patient group differed significantly from the control group as reflected by significant three-way interactions: patients with superior lesions from controls [F(2,22) = 7.67, P < 0.01], and patients with inferior lesions from controls [F(2,24) = 8.52, P < 0.01]. Note that while the TPJ group bears out the prediction of an alteration in attentional capture, the SPL group violates the prediction of preserved performance and also shows a pattern that differs from controls, albeit a different form than the TPJ group.
Stability of group assignment and subtraction analysis
It is important to note that each patient retained their status as belonging to the SPL or the TPJ group, independent of the task employed. Patients JM, MF, LB, and LD were all placed into the TPJ group based on their performance profile in the goal-directed task and also based on their performance in the attentional capture task, attesting to the robustness of their assignment to the same group. The same was true of the patients BB, FD, and OL, all of whom were placed into the SPL group based on the fact that their attentional shifts were slower than that of controls. Interestingly, all three patients exhibited hyper-capture, which is not consistent with the predicted double dissociation as discussed below. Of relevance here is the stability of the group assignment in the majority of cases (the two remaining cases both showed variable performance but also variable lesion locations), attesting to the robustness and sensitivity of the behavioral tasks employed here to differentiate deficits in either top-down or bottom-up attentional orienting.
While overlap analyses are robust and informative, they can sometimes be misleading since they do not take into account areas of the brain commonly damaged regardless of a behavioral deficit. In order to verify further the key brain-behavior correspondences, we also performed a subtraction analysis. Two groups of patients were created, those who performed well on the top-down task while performing poorly on the bottom-up task, consisting of patients JM, MF, LB, and LD, were assigned to group 1; and those who performed well on the bottom-up task while performing poorly on the top-down task, consisting of patients BB, FD, and OL, were assigned to group 2. Subtraction analysis was then performed (see Fig. 6) uncovering areas of the brain more affected in group 1 than in group 2 and vice versa. The results of the subtraction analysis are remarkably similar to those observed with the overlap analyses (due to the fact that group1 (BB, FD, OL) intersection and group 2 (JM, MF, LB, LD) intersection had no overlapping area; namely patients who performed poorly on the top-down task mostly sustained damage to the superior portions of the parietal lobe, while patients who performed poorly on the bottom-up task sustained damage to areas in the inferior parts of the parietal lobule.
Fig. 6.
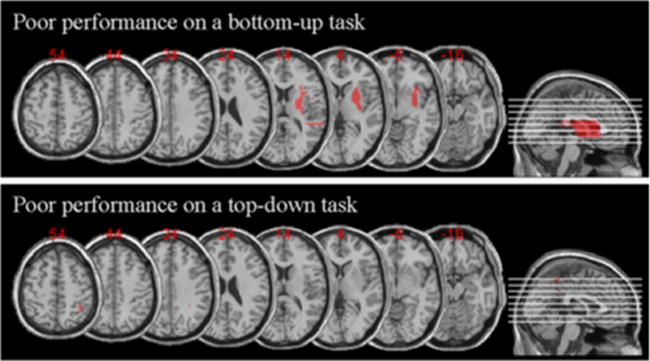
Results of the subtraction analysis for group 1—patients JM, MF, LB, and LD who performed well on the top-down attentional task and performed poorly on the bottom-up attentional task; and group 2—patients OL, BB, and FD who performed poorly on the top-down attentional task while performing well on the bottom-up attentional task. Note that there was no need to adopt a cutoff frequency as there was no overlap between the two groups
Discussion
Recent neuroimaging evidence suggests that attentional selection is subserved by a network that includes parietal cortex as a key component (Corbetta and Shulman 2002); it is the case, however, that two distinct anatomical sites within the parietal cortex appear to be critical, one to mediate top-down attentional orienting (superior parietal lobe; SPL) and the other to mediate bottom-up capture of attention (temporo-parietal junction; TPJ). Much of the evidence for this separable brain-behavior relationship comes from studies using neuroimaging. Here, in an investigation into neuropsychological patients with a lesion to one or the other of these distinct anatomical sites, we examine the relative contribution of SPL and TPJ for attentional orienting and explore the extent to which these regions are independent of each other and dissociable in function.
Goal-directed and stimulus-driven orienting in hemispatial neglect
In this study, each patient’s behavioral profile, delineated based on their performance on two behavioral tasks, each taxing either top-down or bottom-up attentional orienting, was used as a marker of whether the ability to orient in a top-down or a bottom-up fashion was perturbed. Following the characterization of the behavioral profile, each patient was then assigned to a group whose ability to orient deviated from that of a normal control group or to a group whose ability was similar to that of the control group. The lesion overlap analysis was then performed to reveal the overlapping/shared region of cortex that was damaged in the individuals in each group.
The findings from this study clearly suggest that (a) SPL and TPJ are anatomical regions that are necessarily recruited for the purposes of top-down and bottom-up orienting and that damage to SPL and TPJ leads to disorders of top-down and bottom-up orienting,2 and (b) top-down and bottom-up orienting (and subsequently SPL and TPJ) are not entirely independent neural mechanisms. Each of these conclusions has theoretical implications and we consider each in turn.
SPL and goal-directed orienting
Evidence for the conclusion that the SPL is necessary for top-down attentional orienting is supported by the finding that patients who performed poorly on the task requiring top-down shifts of attention (with preserved capabilities to shift attention in a bottom-up fashion) shared a lesion location positioned over the superior portions of the parietal cortex that subsumed the SPL. It is important to note that the SPL group exhibited difficulty in shifting of attention not only into the neglected (i.e., left side) but also into the preserved right side of space. The TPJ lesioned group (with preserved SPL) performed normally on this task. Therefore, our data suggest that patients with lesions to the SPL region of the parietal cortex exhibit a specific deficit in shifting spatial attention (regardless of where in space those shifts are destined for) rather than some unspecified general attentional deficit as patients with neglect have generally been labeled.
Recent neuroimaging studies suggest that selective activation of SPL is not restricted to spatial shifts alone, and this region is activated when subjects shift their attention between any two dimensions of the input; for example, shifts between superimposed houses and faces (Yantis and Serences 2003), between features of an object (Liu et al. 2003) or between and within sensory modalities (Shomstein and Yantis 2004, 2006). It therefore remains unexplored whether patients with SPL damage will also be impaired in executing non-spatial shifts of attention (e.g., from one feature of the same object to another, or from one sensory modality to another) (Driver and Vuilleumier 2001; Frassinetti et al. 2005; Van Vleet and Robertson 2006).
TPJ and stimulus-driven orienting
Evidence for the conclusion that TPJ is necessary for bottom-up attentional orienting is supported by the finding that patients who performed poorly on the attentional capture task evinced a lesion overlap region in or around the TPJ. It is important to note that while controls show a typical contingent capture pattern of performance evidenced by lower accuracies for distractors that precede the target by two frames (reflecting the fact that spatial attention was captured by the location of the distractor # thus not leaving enough time to get back to the central target), patients with TPJ damage do not exhibit such decrement in performance. As a result, patients with TPJ damage perform better than controls (i.e., their accuracy is higher) by exhibiting immunity to capture. This, however, should not be interpreted as advantageous since being distracted reflects an important ability of the organism to be distracted from the task at hand in lieu of a more important (i.e., more salient) stimulus (Yantis 2000). On the other hand, patients with preserved TPJ and lesioned SPL (i.e., SPL-lesioned patients) show hyper-distractibility as reflected by the fact that the target-colored distractor is not only distracting at lag 2 (as in controls) but also at lag 0 (see discussion of the possible mechanism in the next section).
Several fMRI studies have documented that bottom-up attentional capture, mediated by stimulus salience and/or relevance, is subserved by the temporo-parietal junction (TPJ). For example, when subjects attend to and monitor a change in either a visual or auditory stimulus presented simultaneously, BOLD activation of the TPJ region of the right parietal lobe is enhanced; this is only the case, however, when the stimulus change occurs in the modality that is relevant to the current behavior (Downar et al. 2001). While our results clearly demonstrate that patients with a lesion to TPJ fail to be captured by the distracting items presented within the same modality (vision in this case), it remains unexplored whether items presented in another modality (e.g., audition) would also fail to capture attention. Our prediction is that patients whose neglect is a result of a TPJ lesion will show a cross-modal immunity to capture. It will be of further interest to explore whether patients with TPJ damage show an overall immunity to distractions independent of whether such distraction is task relevant or irrelevant (Downar et al. 2002). This type of investigation would further explore whether TPJ is a general mechanism that serves as a circuit breaker of ongoing cognitive activity, or whether it “breaks the circuit” only when behaviorally relevant stimuli are detected (Arrington et al. 2000; Corbetta et al. 2000; Corbetta and Shulman 2002).
Dissociability of goal-directed and stimulus-driven forms of orienting?
Although there is apparently a strong association between goal-directed orienting and SPL and stimulus-driven orienting and TPJ, our data suggest that these two systems are not entirely independent. This conclusion is supported by the finding that patients with SPL damage showed a pattern of performance that we label “hyper-capture” rather than showing the normal capture profile which is expected if SPL played no role in attentional capture. Unlike controls, for whom only target-colored distractor captured attention (leading to lower target accuracy), irrelevant-colored distractors also proved to be distracting for patients with SPL lesions (see Fig. 5a, right panel). In addition, whereas for controls, attention was captured by distractors only when they preceded the onset of the target (i.e., lag 2), for patients with SPL lesions, attention was even captured by distracters presented simultaneously with the target (i.e., lag 0). This pattern of performance can be explained by the following framework: SPL is responsible for top-down guidance of attention that includes determining the aspects of the stimuli that are task relevant (e.g., search for red target) (Corbetta and Shulman 2002; Serences et al. 2005). This attentional set then constrains TPJ, such that the capture of attention mechanism that is mediated by TPJ is only triggered by the task-relevant information (e.g., red distractors capturing attention, and gray distractors not capturing attention). The absence of SPL prevents the establishment of a task-relevant attentional set and thus any stimulus, task relevant or not, is deemed important therefore capturing attention (e.g., task irrelevant distractor capturing attention for the SPL group) indiscriminately.
It has been suggested that SPL and TPJ could interact in at least one of two possible ways. The first possibility is that TPJ serves as an alerting system that detects behaviorally relevant stimuli but lacks high spatial resolution; thus, when a behaviorally relevant stimulus is detected, its precise location is supplied by the SPL that stores fine-grained spatial maps along with information about salient locations (Bisley and Goldberg 2003; Kastner et al. 1999; Silver et al. 2005; Wojciulik and Kanwisher 1999). A related hypothetical possibility is that the capture mechanism (that includes TPJ) acts as a circuit breaker of ongoing cognitive activity when a behaviorally relevant stimulus is presented (Corbetta and Shulman 2002). The “hyper-capture” pattern of activity observed in patients with preserved TPJ but lesioned SPL provides further evidence for the hypothesis that TPJ issues a control signal that terminates the task at hand, thus serving as a circuit breaker (Corbetta and Shulman 2002; Serences et al. 2005).
In summary, the findings from the present study reveal that visuo-spatial neglect is not a monolithic disorder, but, rather, that it can arise due to dysfunction of either top-down or bottom-up attentional orienting. This behavioral distinction can be predicted by the locus of the lesion, such that a lesion to SPL is likely to lead to difficulties with top-down orienting, while a lesion to TPJ is likely to lead to difficulties with bottom-up orienting. This dissociation provides strong evidence that SPL and TPJ are necessary for top-down and bottom-up attentional orienting. Furthermore, our results indicate that goal-directed attentional control, subserved by SPL, carries information regarding what is important for the task at hand, thereby constraining bottom-up attentional capture (subserved by TPJ).
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS07391-09 (to S.S.) and National Institute of Mental Health Grant MH54246 (to M.B.). We thank the patients and their families, as well as the participants of the Osher Lifelong Learning Program at Carnegie Mellon University for their participation in this research.
Footnotes
Accuracy performance was also subjected to an ANOVA with effects mirroring those observed in RT. We therefore only focus on the RT analysis.
It should be mentioned that the results from the lesion analysis might not generalize to neglect patient population with different lesion location (e.g., inferior frontal lesions). It should also be noted that the results from this lesion analysis are drawn from a small sample of patients.
Contributor Information
Sarah Shomstein, Department of Psychology, George Washington University, 2125 G Street, NW, Washington, DC 20052, USA.
Jeongmi Lee, Department of Psychology, George Washington University, 2125 G Street, NW, Washington, DC 20052, USA.
Marlene Behrmann, Department of Psychology, Carnegie Mellon University, Pittsburgh, PA, USA.
References
- Arrington CM, Carr TH, Mayer AR, Rao SM. Neural mechanisms of visual attention: object-based selection of a region in space. J Cogn Neurosci. 2000;12(Suppl 2):106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- Bacon WF, Egeth HE. Overriding stimulus-driven attentional capture. Percept Psychophys. 1994;55(5):485–496. doi: 10.3758/bf03205306. [DOI] [PubMed] [Google Scholar]
- Becker E, Karnath HO. Incidence of visual extinction after left versus right hemisphere stroke. Stroke. 2007;38(12):3172–3174. doi: 10.1161/STROKEAHA.107.489096. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14(2):212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Vallar G. Hemineglect in humans. In: Boller F, Graffman J, editors. Handbook of neuropsychology. Elsevier; Amsterdam: 1988. pp. 195–222. [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299(5603):81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8(11):1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Critchley M. The parietal lobes. Hafner Press; London: 1953. [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. NeuroImage. 2001;14:1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol. 2002;87(1):615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Driver J, Vuilleumier P. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition. 2001;79(1–2):39–88. doi: 10.1016/s0010-0277(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Folk CL, Leber AB, Egeth HE. Made you blink! Contingent attentional capture produces a spatial blink. Percept Psychophys. 2002;64(5):741–753. doi: 10.3758/bf03194741. [DOI] [PubMed] [Google Scholar]
- Frassinetti F, Bolognini N, Bottari D, Bonora A, Ladavas E. Audiovisual integration in patients with visual deficit. J Cogn Neurosci. 2005;17(9):1442–1452. doi: 10.1162/0898929054985446. [DOI] [PubMed] [Google Scholar]
- Friedrich FJ, Egly R, Rafal RD, Beck D. Spatial attention deficits in humans: a comparison of superior parietal and temporal-parietal junction lesions. Neuropsychology. 1998;12(2):193–207. doi: 10.1037//0894-4105.12.2.193. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22(4):751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Liu T, Slotnick SD, Serences JT, Yantis S. Cortical mechanisms of feature-based attentional control. Cereb Cortex. 2003;13(12):1334–1343. doi: 10.1093/cercor/bhg080. [DOI] [PubMed] [Google Scholar]
- McFire J, Zangwill OL. Visuo-constructive disabilities associated with lesions of the left cerebral hemisphere. Brain. 1960;82:243–260. [Google Scholar]
- Medina J, Kannan V, Pawlak MA, Kleinman JT, Newhart M, Davis C, et al. Neural substrates of visuospatial processing in distinct reference frames: evidence from unilateral spatial neglect. J Cogn Neurosci. 2009;21(11):2073–2084. doi: 10.1162/jocn.2008.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, et al. The anatomy of visual neglect. Brain. 2003;126(Pt 9):1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Piercy M. The effects of cerebral lesions on intellectual function: a review of current research trends. Br J Psychiatry. 1964;110:310–352. doi: 10.1192/bjp.110.466.310. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4(7):1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol Sci. 2005;16(2):114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Behrmann M. Cortical systems mediating visual attention to both objects and spatial locations. Proc Natl Acad Sci. 2006;103(30):11387–11392. doi: 10.1073/pnas.0601813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Control of attention shifts between vision and audition in human cortex. J Neurosci. 2004;24(47):10702–10706. doi: 10.1523/JNEUROSCI.2939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Parietal cortex mediates voluntary control of spatial and nonspatial auditory attention. J Neurosci. 2006;26(2):435–439. doi: 10.1523/JNEUROSCI.4408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Kimchi R, Hammer M, Behrmann M. Perceptual grouping operates independently of attentional selection: evidence from hemispatial neglect. Atten Percept Psychophys. 2010;72:607–618. doi: 10.3758/APP.72.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94(2):1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling G, Reeves A, editors. Attention and performance VIII. Erlbaum; Hillsdale: 1980. [Google Scholar]
- Vallar G, Perani D. The anatomy of unilateral neglect after right-hemisphere stroke lesions. A clinical/CT-scan correlation study in man. Neuropsychologia. 1986;24(5):609–622. doi: 10.1016/0028-3932(86)90001-1. [DOI] [PubMed] [Google Scholar]
- Van Vleet TM, Robertson LC. Cross-modal interactions in time and space: auditory influence on visual attention in hemispatial neglect. J Cogn Neurosci. 2006;18(8):1368–1379. doi: 10.1162/jocn.2006.18.8.1368. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Cockburn J, Halligan PW. Behavioral innatention test. Pearson; San Antonio: 1987. [Google Scholar]
- Wojciulik E, Kanwisher N. The generality of parietal involvement in visual attention. Neuron. 1999;23(4):747–764. doi: 10.1016/s0896-6273(01)80033-7. [DOI] [PubMed] [Google Scholar]
- Yantis S. Goal-directed and stimulus-driven determinants of attentional control. In: Monsell S, Driver J, editors. Attention & performance XVIII. MIT Press; Cambridge: 2000. pp. 71–107. [Google Scholar]
- Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Curr Opin Neurobiol. 2003;13(2):187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, et al. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5(10):995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]


