Abstract
A goal of clinical trials is to identify unique baseline characteristics that can inform treatment planning. One such target is emotion dysregulation (ED), which contributes to the maintenance of co-occurring posttraumatic stress disorder (PTSD) and substance use disorder (SUD) and may be a potential moderator of treatment response. We examined the moderating impact of ED severity on treatment outcomes in an urban, socioeconomically disadvantaged, and racially/ethnically diverse sample with complex trauma and severe SUDs. Participants with co-occurring PTSD and SUD (PTSD+SUD) were randomized to Concurrent Treatment with Prolonged Exposure (COPE, n = 39), Relapse Prevention Therapy (RPT, n = 43), or an active monitoring control group (AMCG, n = 28). Baseline ED severity moderated treatment outcomes such that high ED was associated with greater reduction in PTSD severity among those who received COPE relative to RPT and AMCG. In contrast, low ED was associated with greater reduction in substance use among those in RPT relative to COPE and AMCG. Implications for individualizing and optimizing treatment selection for PTSD+SUD are discussed.
Keywords: posttraumatic stress disorder, emotion regulation, substance use disorders, prolonged exposure, treatment moderator
Posttraumatic stress disorder (PTSD) is a highly debilitating disorder affecting 6.8% of American adults age 18 and older in their lifetime (Kessler, 1995). For individuals with substance use disorders (SUD), the likelihood of PTSD is magnified. When compared to those without SUD, those with SUD have greater current (25–42%) and lifetime (36–50%) PTSD prevalence rates. A host of negative consequences accompany comorbid PTSD and SUD (PTSD+SUD). Those with PTSD+SUD suffer from more severe symptomatology, early treatment drop-out, and increased chances of relapse than their non-traumatized counterparts (Norman et al., 2012).
Although empirically-supported treatments exist for PSTD+SUD, (Back et al., 2014; Foa et al., 2013; van Dam, Vedel, Ehring, & Emmelkamp, 2012), this population is associated with significant unmet therapeutic needs. Nearly half continue to meet criteria for PTSD following cognitive behavior therapy (Bradley, Greene, Russ, Dutra, & Westen, 2005) and a majority of patients with SUD (60–75%) misuse substances after treatment (Dutra et al., 2008). While effective treatments have been identified, less is known about what intervention works best for whom (Hien et al., 2015). In order to advance treatment efficacy and provide an empirical basis for targeted PTSD+SUD treatment referrals, research must identify the fundamental moderators of validated therapies. One of the ultimate goals of randomized controlled trials (RCTs) is to recognize who should get the treatment under examination (Kraemer, 2016). Among those with PTSD+SUD, emotional dysregulation (ED) is one key potential treatment moderator. ED varies across patients and can cause significant clinical management issues such as emotional lability and even suicidal gestures (e.g., Cloitre, Miranda, Stovall, & Han, 2005; Lanius et al., 2010).
1.1 The role of ED in PTSD+SUD
Prevailing models of PTSD have defined it as primarily a fear-based disorder. Interventions efforts have focused on ameliorating disturbances found in the fear conditioning and extinction networks. However, a growing body of research suggests that in addition to pathological fear, regulation of other emotional responses (e.g., anger, shame, and guilt) may be central to the development, maintenance, and treatment of trauma-related disorders (Resick & Miller, 2009; Saraiya & Lopez-Castro, 2016). Emotion regulation is broadly defined as the ability to monitor, assess and modulate emotional reactions, particularly in the context of goal-oriented behavior (Gratz & Roemer, 2004). In turn, ED is understood as difficulties across a multidimensional range of processes: (a) limited awareness and understanding of emotions, (b) difficulty accepting negative emotions, (c) inability to control impulsive behaviors in the context of emotionally distressing experiences, (d) limited use of situationally-appropriate emotion regulation strategies, and (e) inability to engage in goal-directed behaviors when distressed (Gratz & Roemer, 2004; Gratz, Tull, Matusiewicz, Breetz, & Lejuez, 2013). Several pathways have been hypothesized for the role of ED in PTSD including ED as a consequence of fear conditioning or ED as a vulnerability that contributes to the development and maintenance of PTSD (Lanius et al., 2010). A positive empirical association between ED and PTSD has been established (Seligowski, Lee, Bardeen, & Orcutt, 2015) and evidenced in a diverse set of populations, settings, and trauma types (Ehring & Quack, 2010; O’Bryan, McLeish, Kraemer, & Fleming, 2015; Tull, Barrett, McMillan, & Roemer, 2007; Weiss, Tull, Viana, Anestis, & Gratz, 2012).
Research has suggested that ED may be the underlying vulnerability linking PTSD and SUD (Wolff et al., 2016). In populations who abuse substances, deficits in emotion regulation correlate with and predict PTSD diagnosis and symptom severity as well as mediate the association between PTSD severity and the use of substances as a coping mechanism (McDermott, Tull, Gratz, Daughters, & Lejuez, 2009; Tull et al., 2007; Weiss et al., 2012). Moreover, ED at the time of trauma exposure has been shown to moderate the relationship between PTSD symptoms and substance use, further suggesting its critical role within PTSD+SUD development and progression (Tull, Bardeen, DiLillo, Messman-Moore, & Gratz, 2015; Weiss, Tull, Anestis, & Gratz, 2013).
For those with PTSD+SUD, ED may fuel compulsive substance use in response to trauma-related cues and associated psychological distress (Hien, Litt, Cohen, Miele, & Campbell, 2009). In the short run, substance use may be reinforced by its reduction of PTSD-related distress and its regulation of trauma-cued emotions. In the long run, a vicious cycle emerges whereby chronic substance use worsens PTSD symptomatology. Given the pre-existing ED vulnerabilities, substance use is then perpetuated to further soothe painful emotions. Beyond a general model of ED’s influence on PTSD+SUD, a number of studies have implicated specific disrupted dimensions of emotion regulation in the relationship between traumatic stress and substance misuse. Due in part to differences in settings and samples of PTSD+SUD research, findings have differed as to which particular facet of ED is most critical to the association between PTSD and SUD. In a sample of 42 individuals with PTSD and cocaine dependence receiving inpatient treatment, the ED dimension of difficulties controlling impulsive behaviors was the mechanism through which PTSD symptoms were associated to trauma-related and cocaine cues (Tull et al., 2016). In turn, Goldstein et al. (2017) examined a sample of 260 women with PTSD and alcohol dependence (AD) and found the dimension of difficulty engaging in goal-directed behavior when emotionally distressed fully mediated the association between PTSD and AD, above and beyond other components of ED.
1.2 Impact of ED on Treatment Response
Efforts have been made to characterize the specific impact of ED on PTSD treatment response and outcome (Boden et al., 2013; Miles, Smith, Maieritsch &Ahearn, 2015; Price, Monson, Callahan, & Rodriguez, 2006). To date, such studies have yielded a complicated picture. In part due to variations in methodologies and divergent conceptual and operational definitions of the construct, emotion regulation as a predictor of PTSD change in treatment has proven difficult to consistently demonstrate. For example, in a sample of Afghanistan and Iraq veterans who received cognitive processing therapy, Miles et al. (2015) examined the link between fear of emotions and posttreatment PTSD severity and encountered a complex, multidimensional relationship. More baseline fear of anxiety was significantly and positively associated with PTSD avoidance symptoms and predicted treatment drop-out; however, greater baseline fear of anger predicted lower PTSD symptoms following treatment. In a veteran sample receiving an intensive day treatment PTSD intervention, improvements in emotion regulation were correlated to, but not predictive of changes in PTSD (Price et al., 2006). Conversely, a prospective study of PTSD residential treatment for veterans found that pre-to posttreatment changes in the maladaptive emotion regulation strategy of expressive suppression predicted lower PTSD symptomatology at posttreatment (Boden et al., 2013).
Resting on the assumption that basic emotional resources must be in place to benefit from traditional PTSD interventions, ED has also been implicated as one of the factors limiting the effectiveness of empirically validated interventions (Zlotnick et al., 1997). Since cognitive behavioral treatments involving prolonged exposure (PE) demand confrontation of intense emotional memories, specific concerns have been raised that these types of treatments may potentially overwhelm individuals who present with compromised emotion regulation (Ford, Steinberg, Hawke, Levine, & Zhang, 2012). In response, phase-based treatments have been developed wherein emotional and interpersonal deficits are addressed prior to exposure work (Cloitre et al., 2009). Findings from several RCTs have supported a phase-based approach with evidence of less treatment drop-out and more PTSD symptom amelioration than standard treatment without preliminary emotion regulation training (Bryant, 2010, 2011; Cloitre et al., 2011). Such findings underscore that for patients struggling with ED, treatments with PE alone may limit successful outcomes. In contrast, a recent study comparing treatment with sertraline to PE found that individuals with and without child abuse (the former presumed to be associated with greater ED than the latter) had significant and equivalent improvements in both PTSD and ED domains (Jerud et al., 2014) regardless of treatment type.
For individuals undergoing treatment for PTSD+SUD, recovery is complicated by complex trauma histories, other comorbid mood or anxiety disorders, and multiple life burdens (Najavits & Hien, 2013). Moreover, ED is likely to be more severe in populations with PTSD+SUD than those diagnosed with either PTSD or SUD alone (Weiss et al., 2013). Despite its salience to the treatment of comorbid addictions and PTSD, few studies have explored the impact of ED on an individual’s capacity to make use of PTSD+SUD interventions. What is known is that ED contributes to the historically low engagement and retention rates of PTSD+SUD treatments (Westphal, Aldao, & Jackson, 2017). For some with PTSD+SUD, the real or feared expectation of facing trauma memories and related emotions may be overwhelming and lead to premature drop-out or termination. In support of ED’s unique influence, Tull et al. (2013) examined the role of emotion regulation in treatment attrition and found that males with current PTSD+SUD completed less residential substance abuse treatment if they had low distress tolerance.
1.3 Study Rationale and Hypotheses
To date, no RCT for PTSD+SUD has examined ED as a moderator of treatment outcomes. In a recently completed RCT for individuals with PTSD+SUD, Ruglass et al. (2017) compared an integrated, exposure-based treatment (Concurrent Treatment with Prolonged Exposure [COPE]; Back et al., 2014) to cognitive behavior therapy for substance dependence alone (Relapse Prevention Therapy [RPT]; Carroll, 1996). Both treatments were associated with significant benefits in PTSD and SUD domains. Although the difference between COPE and RPT was not significant in the whole sample, the subset of participants with full (versus subthreshold) PTSD demonstrated significantly greater reduction of PTSD severity (measured by the Clinician Administered PTSD Scale) in COPE relative to RPT. At end-of-treatment, COPE and RPT demonstrated greater reduction in mean PTSD symptom severity relative to an active monitoring control group (AMCG; COPE-AMCG = −34.06, p<.001; RPT-AMCG = −22.58, p = .002). Both treatments were superior to AMCG in reducing use of primary substance in the past seven days (COPE-AMCG = −0.97, p = .01; RPT-AMCG = −2.07, p<.001). The present study was a secondary analysis of the aforementioned (clinicaltrials.gov #NCT01365247, Ruglass et al., 2017). We specifically examined ED as a moderator of treatment and compared impact on PTSD and SUD outcomes by treatment type. To determine potential indications for treatment matching, we examined whether overall ED at baseline moderated treatment effects on PTSD symptoms and substance use, comparing patients receiving COPE to RPT as well as an active monitoring control group. We hypothesized a priori that deficits in emotion regulation would emerge as a significant treatment moderator of both outcomes. Given the lack of previous treatment research regarding the impact of ED on PTSD+SUD outcomes, we refrained from a directional prediction.
Method
2.1 Recruitment and Procedures
Participants were recruited through advertisements and outpatient referrals from community-based substance abuse treatment programs in New York City between September 2008 and January 2014. Written informed consent was collected prior to baseline assessment. Inclusion criteria were: 1. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR; American Psychiatric Association, 2000) criteria for full PTSD, or subthreshold PTSD (Grubaugh et al., 2005). Subthreshold PTSD is defined as meeting Criteria A (exposure to a traumatic stressor), B (re-experiencing symptoms), either C (symptoms of avoidance and/or numbing) or D (increased arousal symptoms), E (symptom duration of at least 1 month), and F (significant distress or impairment of functioning); and 2. DSM-IV-TR criteria for lifetime alcohol or substance dependence plus substance use in the prior 3 months. Exclusion criteria were: 1. Psychotic, schizoaffective or bipolar disorder; 2. Current severe depression or significant suicide risk (ideation without plan was not an exclusion); 3. Participation in PTSD-specific treatment; 4. Commencement of anxiolytic, antidepressant, or mood stabilizing medications within the 8 weeks prior to study participation; and 5. Cognitive impairment as identified by a score of >21 on the Mini Mental Status Exam (Folstein, Folstein, & McHugh, 1975). The institutional review board of the City College of New York approved all procedures. Detailed descriptions of all study procedures including randomization, the interventions and fidelity, and data collection are described in Ruglass et al. (2017).
2.2 Interventions
The two manualized study psychotherapy treatments consisted of 12 individual weekly sessions lasting 90 minutes.
COPE integrates the empirically supported models of PE for PTSD (Back et al., 2014; Foa, Hembree, & Rothbaum, 2007) and RPT for SUD (Carroll, 1998; Marlatt & Donovan, 2005). COPE begins with psychoeducation about the relationship between PTSD and substance misuse. To address behavioral avoidance and fear associated with trauma memories, in-vivo and imaginal exposures are conducted. For the treatment of SUD, relapse prevention strategies are incorporated into each 90-minute session. In-session imaginal narratives were audio-recorded for daily listening between study appointments. Participants logged their weekly progress of exposure exercises, substance use cravings, and use of coping skills.
RPT (Carroll, 1998; Marlatt & Donovan, 2005) is a cognitive behavioral intervention for SUDs that focuses on the acquisition of coping strategies to manage situations that increase risk of substance use relapse. Coping strategies are acquired through psychoeducation, role-plays and active problem-solving exercises combined with at-home assignments all geared towards increasing participants’ self-efficacy in preventing relapse.
The active monitoring control group (AMCG) participants met weekly with research assistants to complete self-report measures, urine toxicology, alcohol breathalyzer, and confirm general health and safety.
2.3 Measures and Constructs
Age, sex, race/ethnicity, education, employment pattern, and income were collected during the baseline interview. The Structured Clinical Interview for DSM-IV for Axis I Disorders (SCID; First, Spitzer, Gibbon, & Williams, 2002) was used to assess alcohol and substance-related diagnoses and age of onset, as well as the presence of other current or past anxiety, mood, or psychotic disorders. The Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995) was used at baseline to diagnose PTSD.
At each weekly visit including baseline, randomization, and sessions 1 through 12 of treatment, the modified PTSD Symptom Scale Self-Report (MPSS-SR; Falsetti, Resnick, Resick, & Kilpatrick, 1993) was utilized to assess the past 7 days of self-reported PTSD symptom severity. The MPSS-SR yields a total score comprised of the sum of frequency and intensity ratings of each of the 17 DSM-IV-TR PTSD symptoms. Psychometric studies of the MPSS-SR with similar comorbid PTSD+SUD treatment samples demonstrate its high concurrent validity with the CAPS, and suggest it is a reliable tool for monitoring PTSD symptoms (Ruglass, Papini, Trub, & Hien, 2014).
Primary substance diagnosis was based on the number of dependence criteria from the SCID and level of use in the past month. The Substance Use Inventory (SUI; Weiss, Hufford, & Najavits, 1995) is a self-report measure of past 7 day primary substance use (as identified by the SCID) frequency and quantity and was collected weekly during treatment confirmed with urine testing.
In order to maximize power necessary to detect moderation effects, the current study used self-reported measures of PTSD symptoms and days of primary substance use (urine confirmed) assessed weekly throughout the course of treatment. Clinician-assessed symptoms for the main outcomes are reported in Ruglass et al. (2017).
ED was measured with the Difficulties in Emotion Regulation Scale (DERS; Gratz & Roemer, 2004). The DERS is a 36-item self-report measure developed to assess clinically significant difficulties in emotion regulation. DERS items reflect difficulties within various dimensions of emotion regulation: (a) awareness and understanding of emotions; (b) acceptance of emotions; (c) ability to engage in goal-directed behavior and refrain from impulsive behavior when experiencing negative emotions; and (d) access to emotion regulation strategies perceived as effective. Responses range from 1 to 5 with higher scores indicating greater difficulties in emotion regulation. Total DERS has been found to have good test–retest reliability (r = .88) and high internal consistency (α = .93); the subscales also have high internal consistency (i.e., Cronbach’s α > .80 for each). Preliminary findings suggest adequate construct and predictive validity (Gratz & Roemer, 2004; Sloan & Kring, 2007).
2.4 Statistical Analyses
We examined the moderating impact of overall ED at baseline (DERS total score) on within-treatment change in PTSD symptom severity and days of primary substance use. Following the intent-to-treat principle, we used generalized linear mixed models (GLMM, (Stroup, 2012) which can estimate missing data so that all participants can be included in the analysis. Outcome distributions and respective link functions were specified to be consistent with the main outcome analyses of this trial (i.e., normal for MPSS-SR total score, and negative binomial for days of use). Models included fixed effects of group (COPE, RPT, or AMCG), time (coded as a continuous variable that included all measurement time points from baseline to end of treatment), ED (DERS total score), and all interactions between these variables, as well as random intercepts to account for individual differences in baseline values of the outcome measures. Model fixed effects were examined by first looking at the highest order interactions. To aid in the interpretation of interactions that were significant at the p < .05 level, we report test statistics along with estimates and 95% confidence intervals (CI) for subsequent comparisons. When treatment played a role in an interaction, we calculated unstandardized mean differences with 95% CI of the pairwise comparisons between the three treatment groups at different levels of the other variables in the interaction. These unstandardized mean differences provide interpretable estimates of effect sizes in original units of measurement (i.e., difference in number of points in the MPSS-SR for the PTSD outcome, and difference in the number of use days for the SUD outcome). IBM SPSS version 22 was used to conduct all analyses.
Results
3.1 Baseline characteristics
There were no significant differences among groups in key demographics and baseline PTSD, substance use, and ED (see Table 1). Participants were between the ages of 23 and 64 (M = 45), mostly men (64%), and most self-identified as Black/African American (59%). The average number of days of substance use in the week prior to baseline was 3.93. The mean PTSD severity score on the MPSS-SR was 54.50 (SD = 24.16) and mean total score on the DERS was 90.00 (SD = 22.32). Recruitment and study flow are presented in the CONSORT diagram (Figure 1). Examination of baseline correlations between DERS and measures of PTSD severity and days of primary substance use revealed that baseline DERS was positively correlated with PTSD severity (r = .44, p < .001) and negatively correlated with days of primary substance use (r = −.21, p = .03).
Table 1.
Demographic and baseline characteristics (N = 110)
| COPE (n = 39) |
RPT (n = 43) |
AMCG (n=28) |
|
|---|---|---|---|
| Demographic Characteristics | M(SD) or N(%) | M(SD) or N(%) | M(SD) or N(%) |
|
| |||
| Age | 43.08 (10.00) | 44.21 (9.05) | 47.18 (8.21) |
| Female | 11 (28.2%) | 16 (37.2%) | 13 (46.4%) |
| Race/Ethnicity | |||
| Black/African American | 21 (53.8%) | 28 (65.1%) | 16 (57.1%) |
| Hispanic/Latino | 10 (25.6%) | 9 (20.9%) | 3 (10.7%) |
| White | 6 (15.4%) | 6 (14.0%) | 8 (28.6%) |
| Other | 2 (5.1%) | 0 | 1 (3.6%) |
|
| |||
| Clinical Baseline Characteristics | M(SD) | M(SD) | M(SD) |
|
| |||
| Prior week primary substance use days | 3.90 (2.69) | 4.05 (2.35) | 3.79 (2.27) |
| PTSD total severity and subscales | |||
| MPSS-SR total | 54.31 (24.56) | 57.49 (24.33) | 50.21 (23.58) |
| Re-experiencing | 15.33 (8.17) | 15.30 (7.97) | 13.75 (8.03) |
| Avoidance | 7.38 (4.53) | 8.33 (3.83) | 8.00 (4.06) |
| Dysphoria | 14.00 (8.89) | 15.70 (9.95) | 13.75 (8.99) |
| Hyperarousal | 17.59 (8.71) | 18.16 (8.98) | 14.71 (7.70) |
| Emotion dysregulation and subscales | |||
| DERS Total | 88.19 (24.01) | 95.39 (21.16) | 84.25 (20.47) |
| Impulse Control Difficulties | 13.25 (5.39) | 15.01 (5.57) | 12.93 (5.09) |
| Inability to Engage in Goal-Directed Behavior | 15.85 (4.50) | 16.33 (5.26) | 14.97 (4.36) |
| Lack of Emotion Regulation Strategies | 19.32 (6.74) | 20.73 (5.62) | 18.19 (6.19) |
| Lack of Emotional Acceptance | 14.37 (5.81) | 15.11 (5.54) | 13.95 (5.74) |
| Lack of Emotional Clarity | 10.72 (4.30) | 11.68 (3.60) | 10.68 (3.37) |
| Lack of Emotional Awareness | 14.68 (5.63) | 16.52 (4.98) | 13.53 (4.96) |
Note. COPE = Concurrent Treatment with Prolonged Exposure, RPT = Relapse Prevention Therapy, AMCG = Active Monitoring Control Group, PTSD = posttraumatic stress disorder, MPSS-SR = Modified PTSD Symptom Scale Self Report (Range 0–119), DERS = Difficulties in Emotion Regulation Scale (Range 41–205).
Figure 1.
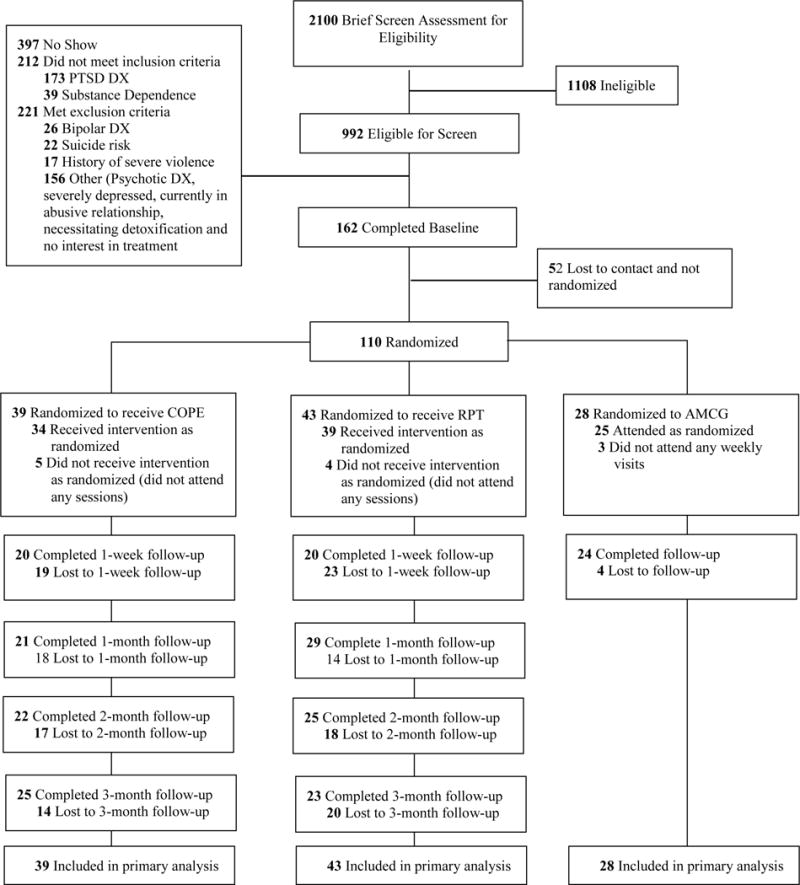
CONSORT Diagram of participant flow through the protocol. PTSD = posttraumatic stress disorder; DX = diagnosis; COPE = Concurrent Treatment of PTSD and SUD using Prolonged Exposure; RPT = Relapse Prevention Therapy; AMCG = Active Monitoring Control Group.
3.2 Baseline ED as a moderator of treatment effect
The three-way interaction between group, time, and DERS total was significant in the model of PTSD outcome, F(2,968) = 8.95, p < .001, and in the days of primary substance use model, F(2,957) = 3.74, p = .02. To aid in the interpretation of these three-way interactions (Aiken, West, & Reno, 1991), each model was refit and plotted with DERS total scores centered either one standard deviation above or below the mean (M = 90.00, SD = 22.33, High DERS= 112.33, Low DERS = 67.68). This approach allows the examination of DERS score as a continuous moderator, and has been recommended over approaches that dichotomize continuous moderators such as median splits or dividing the sample based on a cutoff, which can result in loss of information, reduction of statistical power, and misleading results (MacCallum, Zhang, Preacher, & Rucker, 2002). For each model, the entire sample is retained, and the statistical properties of the overall model’s fixed effects are identical. However, changing the centering of the moderating variable provides estimates that reflect what the treatment effects are when ED is low or high. Similarly, time was re-centered to provide estimates at the beginning and end of treatment. Model estimated means and confidence intervals for all outcome measures at baseline and end of treatment are reported in Table 2. This table provides estimates of models centered at high and low ED, but note these do not represent a “split” in the sample (just as the estimates for baseline and end-of-treatment do not reflect a split, but rather how the outcome varies as a function of time).
Table 2.
Model estimated means and confidence intervals for PTSD and substance use outcomes centered by high and low emotion dysregulation.
| COPE (n = 39) |
RPT (n = 43) |
AMCG (n = 28) |
|
|---|---|---|---|
|
| |||
| Outcome measure and time point | M [95% CI] | M [95% CI] | M [95% CI] |
| High emotion dysregulation | |||
| PTSD severity | |||
| Baseline | 63.92 [55.42, 72.41] | 66.05 [58.99, 73.10] | 63.31 [53.31, 73.31] |
| End of treatment | 12.22 [3.39, 21.05] | 29.48 [16.48, 42.48] | 60.04 [39.89, 80.19] |
| Substance use days | |||
| Baseline | 2.43 [1.44, 4.10] | 2.89 [2.18, 3.85] | 2.56 [1.57, 4.18] |
| End of treatment | 0.23 [0.10, 0.53] | 0.39 [0.17, 0.91] | 1.68 [0.81, 3.49] |
| Low emotion dysregulation | |||
| PTSD severity | |||
| Baseline | 45.48 [34.71, 56,25] | 35.96 [26.36, 45.55] | 37.47 [26.98, 47.97] |
| End of treatment | 28.78 [18.71, 38.86] | 16.29 [4.17, 28.41] | 26.48 [11.75, 41.21] |
| Substance use days | |||
| Baseline | 3.74 [2.70, 5.16] | 3.98 [2.87, 5.51] | 3.39 [2.44, 4.72] |
| End of treatment | 1.38 [0.68, 2.77] | 0.22 [0.07, 0.76] | 2.57 [1.67, 3.96] |
Note. COPE = Concurrent Treatment with Prolonged Exposure, RPT = Relapse Prevention Therapy, AMCG = Active Monitoring Control Group, PTSD = posttraumatic stress disorder. No significant baseline differences in outcome variables across analyses.
3.3 PTSD Outcomes
For participants with low DERS, the PTSD outcome (Figure 2) was not significantly different among COPE and RPT. For those with high DERS, PTSD symptom severity at end of treatment was lower for COPE relative to RPT, M difference = −17.26, 95% CI [−32.98, −1.55], t(968) = 2.16, p = .03, COPE relative to AMCG, M difference = −47.83, 95% CI [−69.83, −25.83], t(968) = 4.27, p < .001, and RPT relative to AMCG, M difference = −30.56, 95% CI [−54.55, −6.58], t(968) = 2.50, p = .01. In addition to the significant differences between each treatment group and the control condition at end-of-treatment, the 17-point difference in mean PTSD symptom severity between COPE and RPT for the high DERS group was also clinically significant (Weathers, Keane, & Davidson, 2001). However, it is important to interpret these mean differences in the context of the wide confidence intervals, which are reflective of the broad range of outcomes in this trial that recruited participants with full and subthreshold PTSD.
Figure 2.
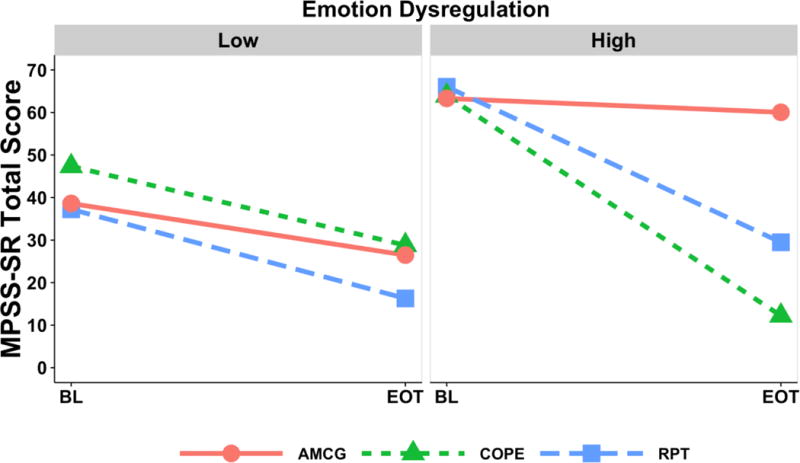
PTSD outcome for each of the three groups at low and high emotion dysregulation. MPSS-SR = Modified PTSD Symptom Scale, BL = baseline measure, EOT = end-of-treatment. COPE = Concurrent Treatment of PTSD and SUD using Prolonged Exposure; RPT = Relapse Prevention Therapy; AMCG = Active Monitoring Control Group
3.4 Primary Substance Use Outcomes
For low DERS, the days of primary substance use outcome (Figure 3) was significantly lower for RPT relative to COPE, M difference = −1.15, 95% CI [−2.15, −0.15], t(957) = 2.26, p=.02, and RPT relative to AMCG, M difference = −2.35, 95% CI [−3.49, −1.20], t(957) = 4.02, p<.001, but not significantly different between COPE and AMCG. For high DERS, days of primary substance use at end of treatment was lower for each of the treatment groups relative to control, COPE-AMCG = −1.46, 95% CI [−2.70, −0.21], t(957) = 2.30, p = .02, RPT-AMCG=−1.29, 95% CI [−2.56, −0.02], t(957) = 1.99, p = .047, but not significantly different between COPE and RPT. For participants in the low DERS group, the statistically significant difference between RPT and COPE represented a reduction of 1.15 additional days of use per week (16%) by end-of-treatment.
Figure 3.
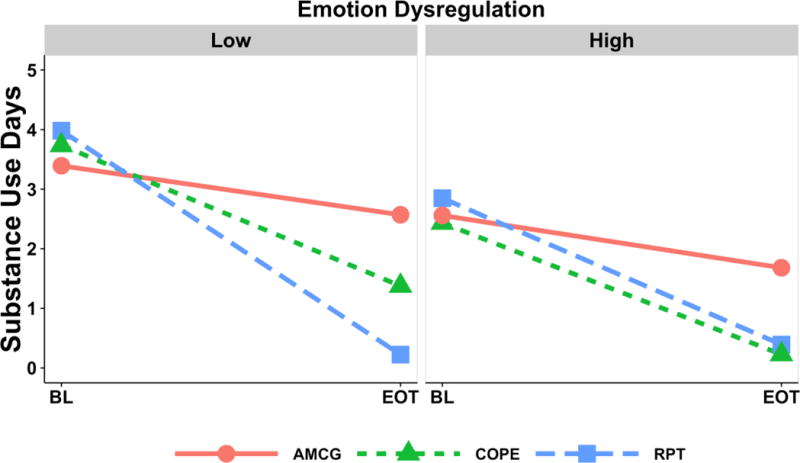
Substance use outcome (last 7 days of use) for each of the three groups at low and high emotion dysregulation. BL = baseline measure, EOT = end-of-treatment. COPE = Concurrent Treatment of PTSD and SUD using Prolonged Exposure; RPT = Relapse Prevention Therapy; AMCG = Active Monitoring Control Group
Discussion
4.1 Overview of Findings
Disruptions in emotion regulation are evident in both PTSD and SUD and have been shown to complicate treatment response for each disorder (Fox, Hong, & Sinha, 2008; Price et al., 2006). For many patients, ED is one of the more significant clinical challenges to manage and represents the underlying convergence of biological, developmental and interpersonal components of this comorbidity (e.g., Lanius et al., 2010; Norman et al., 2012). The present secondary analysis demonstrates that individual differences in baseline ED significantly moderated the outcomes of therapies designed to help patients learn cognitive and behavioral skills to reduce their substance use and PTSD symptoms. Furthermore, the current study provides indications for treatment matching by degree of emotion regulation difficulties. Without taking baseline level of ED into consideration, Ruglass et al. (2017) showed that those who received either COPE or RPT demonstrated statistically significant improvements in both PTSD and SUD outcomes when compared to the control group. The present study’s examination of baseline level of ED as a moderator revealed indications for differential treatment selection. In line with our hypothesis, ED moderated treatment effects for both the PTSD and SUD outcomes, but in different ways depending upon outcome.
4.2 Findings for PTSD outcomes
By the end of treatment, intervention group differences were not observed in the PTSD outcome among those with low ED. In contrast, participants with high ED who received COPE showed greater reduction in PTSD symptoms than those with high ED who received RPT or AMCG. These findings run counter to the reported concerns of applying processing therapies to emotionally dysregulated clients (e.g., Ford et al., 2012; Zlotnick et al., 1997). Our results suggest that treatments like COPE, which focus on processing trauma-related memories and emotions and reducing avoidance behaviors, may be critical in helping such patients decrease PTSD. COPE may have provided more targeted treatment for patients with self-regulation difficulties by offering specific skill building approaches to address their ED. COPE sessions focused on therapeutic activities such as identifying PTSD symptoms clusters (hyperarousal, avoidance and numbing, and trauma memory re-experiencing) and then directly linking PTSD symptoms to substance use triggers. Such labeling and psychoeducation may have increased awareness and understanding of emotions to the high ED group. Similarly, conducting in-vivo and imaginal exposure involved the routine practice of distress tolerance. In contrast, those with low ED did not show a differential treatment effect between COPE and RPT. This may be explained by COPE and RPT sharing the common emotion regulation skill-building components of self-monitoring and enhancing cognitive and behavioral strategies for emotional management. Also, those with low ED may not have needed extensive emotion regulation skill training, as they may have had higher functioning to begin with. This speculation is consistent with the fact that those with low ED had fewer PTSD symptoms at baseline. These data further support the benefit of RPT on PTSD symptoms for individuals who self-report adaptive emotion regulation strategies.
4.3 Findings for SUD outcomes
With regard to substance use outcomes, a different picture emerged. Whereas those with high ED did not differ in their days of substance use by active treatment type (COPE vs. RPT), participants in the RPT group with low ED showed significantly fewer days of use than those in the COPE group with low ED (although on average the reduction was 1.15 days of use). For those with comorbid PTSD and adaptive emotion regulation strategies, RPT may be considered a reasonable choice to impact substance use outcomes. This finding is supported by existing literature on treatment for substance use (without taking PTSD comorbidity into consideration). Pervasive difficulties with emotion regulation have been documented in individuals seeking SUD treatment (e.g., Fox, Hong, & Sinha, 2008; McDermott et al., 2009). When ED was examined as a moderator of outcomes, pre-treatment level of ED was significantly associated with SUD treatment response. Those individuals with lower ED at baseline benefited more from treatment targeting their addictions than those with higher ED. For those with higher ED, adjunctive interventions (e.g., pharmacotherapy or emotion regulation skills training like Dialectical Behavior Therapy) could be important precursors of substance abuse treatment to allow patients to achieve maximum benefit from their PTSD+SUD treatments.
4.4 Clinical Implications
Taken together, the findings that baseline difficulties with emotion regulation moderated treatment effects informs the direction of ongoing efforts to individualize and optimize treatment selection for individuals with PTSD+SUD. Despite the fact that the majority of participants presented with complex trauma (i.e., early and multiple childhood sexual and physical abuse, ongoing lifetime interpersonal violence, severe substance use, and other psychiatric comorbidities), baseline ED level still differentiated how participants improved in terms of best treatment type. The indications provided by this study suggest that for patients with PTSD+SUD who present at baseline with few difficulties in emotion regulation, a standard cognitive behavioral therapy without an exposure-based component (RPT) could be effective in alleviating SUD and PTSD symptoms. In turn, for those with significant emotion regulation deficits, COPE, which introduces an emotional processing/PE component, could be the better treatment choice. During assessment and treatment planning stages, our study results underscore the clinical importance of attunement to the variation in regulatory capacities available to patients with PTSD+SUD.
4.5 Relationships between ED and PTSD+SUD severity
ED had a moderate positive association with baseline PTSD severity (R=.44, p<.001) and a weak negative association with baseline days of substance use (R=−.21, p=.03). These complex relationships suggest that COPE differentially benefited participants with high ED, high PTSD symptoms, and low substance use on the PTSD outcome. In turn, RPT differentially benefited participants with low ED, low PTSD severity, and high substance use on the substance outcome. Since baseline values of the outcome measures were included in the models, results revealed that variation in baseline ED accounted for a moderation of the treatment effect over and above variation in the baseline differences in PTSD or substance use severity.
4.6 Study Limitations and Future Directions
One of the strengths of the present study was its relative heterogeneity in PTSD+SUD symptomatology, trauma exposure, gender, and race/ethnicity. This heterogeneity affords substantial ecological validity for a clinical population often excluded from RCTs. Several limitations also bear mentioning. Emotion regulation was assessed through self-report alone. It was thus susceptible to both generic self-report issues (such as response bias) and limitations more specific to ED (such as difficulties in the ability to recognize or describe emotional experiences). Nevertheless, the study used the DERS which has robust psychometric properties and is one of the most widely employed ED measures in contemporary research (Seligowski et al., 2015). As a next step, future investigations should consider integrating behavioral and physiologic assessment for a more comprehensive record of emotion regulation. We also did not collect participant ED data prior to the onset of their PTSD and SUD, so we were unable to determine whether ED was a preexisting vulnerability or a product of trauma and addiction. Fine-grained, temporal analysis of the relationship between trauma exposure, substance use, and self-regulation, measured ideally with psychophysiologic measures in addition to self-report, remains critical to furthering our knowledge of PTSD+SUD.
Study attrition was high yet in line with other PTSD+SUD and SUD-only treatment studies (e.g., Resko & Mendoza, 2012). Since those who remained in treatment may have been more motivated than non-completers, our findings may not generalize to individuals who dropped out. Sample size and attrition rates also affected study power and scope. Factors like trauma type or substance use class that may have interacted with ED and influenced outcomes were not examined. These, along with the role of specific ED dimensions and the impact of treatment upon them, are valuable avenues for future work.
Participants showed a broad range of DERS total scores. In contrast to a moderate and positive relationship with baseline PTSD severity, we found a weak, negative correlation between participants’ self-report of ED and their baseline substance use. In this sample of individuals with chronic SUD and comorbid PTSD, less difficulties with emotion regulation was associated with more substance use in the seven days prior to treatment. This findings appears discrepant with evidence in support of a positive relationship between deficits in self-regulation and substance abuse problems (Aldao, Nolen-Hoeksema, & Schweizer, 2010). This may be indicative of a clinical phenomenon specific to PTSD+SUD wherein the self-perception of possessing adaptive emotion regulation strategies is associated with more problematic use. Alternately, this inverse relationship may be an artifact of measurement; the current study employed a count of use days in the past seven to represent SUD severity. A larger window of time or other measures of SUD severity such as quantity of substance use or SCID symptom count/severity may have provided us with more data to corroborate this finding. In conclusion, the post hoc nature of these interpretations warrants further, direct investigation of the dynamics of self-regulation and addiction. Nonetheless, the study’s findings indicate that despite the possibility of an inverse relationship with SUD at baseline, the availability of emotion regulation skills is significant for how PTSD+SUD treatments affect PTSD and substance misuse.
4.7 Conclusion
The present study provides evidence that advances the field of PTSD+SUD treatment by demonstrating that baseline individual differences in ED moderated treatment outcomes differentially by treatment type and symptom domain. This is a critical step for the development of treatment-matching protocols and other personalized medicine strategies. Our secondary analysis suggests that taking difficulties in emotion regulation into consideration can facilitate efforts to individualize and optimize treatment pathways for PTSD+SUD.
Highlights.
Emotion dysregulation (ED) has been associated with PTSD and substance use disorders (SUD).
ED moderated response to integrated, prolonged exposure treatment for PTSD/SUD (COPE, Concurrent treatment of PTSD and Substance Use Disorders Using Prolonged Exposure, Back et al., 2017).
Those with high ED showed more PTSD improvement in COPE compared with relapse prevention for SUD.
Those with low ED showed more SUD improvement in relapse prevention for SUD compared with COPE.
Findings suggest ED may serve as a treatment matching variable for PTSD/SUD.
Acknowledgments
This study was supported by a grant from the National Institute on Drug Abuse (NIDA; R01DA10843; PI: Denise A. Hien, Ph.D.);
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Registration: clinicaltrials.gov Identifier: NCT01365247
Conflict of Interest Disclosures: Drs. Hien, Lopez-Castro, Mr. Papini, Ruglass & Gorman declare they have no conflicts of interest regarding the publication of this paper.
References
- Aiken LS, West SG, Reno RR. Multiple Regression: Testing and Interpreting Interactions. SAGE Publications; 1991. http://doi.org/10.1080/00031305.1972.10478934. [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review. 2010 doi: 10.1016/j.cpr.2009.11.004. http://doi.org/10.1016/j.cpr.2009.11.004. [DOI] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- Back SE, Foa EB, Killeen TK, Mills KL, Teesson M, Cotton BD, Brady KT. Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE): Therapist Guide. New York: Oxford University Press; 2014. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Boden MT, Westermann S, McRae K, Kuo J, Alvarez J, Kulkarni MR, Bonn-Miller MO. Emotion regulation and posttraumatic stress disorder: A prospective investigation. Journal of Social and Clinical Psychology. 2013;32(3):296–314. http://doi.org/10.1521/jscp.2013.32.3.296. [Google Scholar]
- Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry. 2005 Feb;162:214–227. doi: 10.1176/appi.ajp.162.2.214. http://doi.org/10.1093/clipsy/bpg024. [DOI] [PubMed] [Google Scholar]
- Bryant RA. The complexity of complex PTSD. American Journal of Psychiatry. 2010;167(8):879–881. doi: 10.1176/appi.ajp.2010.10040606. http://doi.org/10.1176/appi.ajp.2010.10040606. [DOI] [PubMed] [Google Scholar]
- Bryant RA. Psychological interventions for trauma exposure and PTSD. Post-Traumatic Stress Disorder. 2011:171–202. http://doi.org/10.1002/9781119998471.ch5.
- Carroll KM. Relapse prevention as a psychosocial treatment: A review of controlled clinical trials. Experimental and Clinical Psychopharmacology. 1996;4(1):46–54. http://doi.org/10.1037/1064–1297.4.1.46. [Google Scholar]
- Carroll KM. A Cognitive Behavioral Approach: Treating Cocaine Addiction. Vol. 1. Rockville, MD: National Institute on Drug Abuse; 1998. [Google Scholar]
- Cloitre M, Courtois CA, Charuvastra A, Carapezza R, Stolbach BC, Green BL. Treatment of complex PTSD: Results of the ISTSS expert clinician survey on best practices. Journal of Traumatic Stress. 2011;24(6):615–627. doi: 10.1002/jts.20697. http://doi.org/10.1002/jts.20697. [DOI] [PubMed] [Google Scholar]
- Cloitre M, Miranda R, Stovall-McClough KC, Han H. Beyond PTSD: Emotion regulation and interpersonal problems as predictors of functional impairment in survivors of childhood abuse. Behavior Therapy. 2005;36(2):119–124. http://doi.org/10.1016/S0005–7894(05)80060–7. [Google Scholar]
- Cloitre M, Stolbach BC, Herman JL, Van Der Kolk B, Pynoos R, Wang J, Petkova E. A developmental approach to complex PTSD: Childhood and adult cumulative trauma as predictors of symptom complexity. Journal of Traumatic Stress. 2009;22(5):399–408. doi: 10.1002/jts.20444. http://doi.org/10.1002/jts.20444. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. The American Journal of Psychiatry. 2008;165(2):179–87. doi: 10.1176/appi.ajp.2007.06111851. http://doi.org/10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Ehring T, Quack D. Emotion regulation difficulties in trauma survivors: The role of trauma type and PTSD symptom severity. Behavior Therapy. 2010;41(4):587–598. doi: 10.1016/j.beth.2010.04.004. http://doi.org/10.1016/j.beth.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG. The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. Behaviour Therapist. 1993;16:161–162. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Foa E, Hembree E, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Foa E, Yusko D, McLean C, Suvak M, Bux D, Oslin D, Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: A randomized clinical trial. JAMA. 2013;310(5):488–495. doi: 10.1001/jama.2013.8268. http://doi.org/10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. http://doi.org/10.1016/0022–3956(75)90026–6. [DOI] [PubMed] [Google Scholar]
- Ford JD, Steinberg KL, Hawke J, Levine J, Zhang W. Randomized trial comparison of emotion regulation and relational psychotherapies for PTSD with girls involved in delinquency. Journal of Clinical Child and Adolescent Psychology. 2012;41(1):27–37. doi: 10.1080/15374416.2012.632343. http://doi.org/10.1080/15374416.2012.632343. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Sinha R. Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Addictive Behaviors. 2008;33(2):388–394. doi: 10.1016/j.addbeh.2007.10.002. http://doi.org/10.1016/j.addbeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Bradley B, Ressler KJ, Powers A. Associations between posttraumatic stress disorder, emotion dysregulation, and alcohol dependence symptoms among inner city females. Journal of Clinical Psychology. 2017;73(3):319–330. doi: 10.1002/jclp.22332. http://doi.org/10.1002/jclp.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment. 2004;26:41–54. http://doi.org/10.1023/B:JOBA.0000007455.08539.94. [Google Scholar]
- Gratz KL, Tull MT, Matusiewicz AM, Breetz AA, Lejuez CW. Multimodal examination of emotion regulation difficulties as a function of co-occurring avoidant personality disorder among women with borderline personality disorder. Personality Disorders. 2013;4(4):304–14. doi: 10.1037/per0000020. http://doi.org/10.1037/per0000020. [DOI] [PubMed] [Google Scholar]
- Grubaugh AL, Magruder KM, Waldrop AE, Elhai JD, Knapp RG, Frueh BC. Subthreshold PTSD in primary care: Prevalence, psychiatric disorders, healthcare use, and functional status. The Journal of Nervous and Mental Disease. 2005;193(10):658–664. doi: 10.1097/01.nmd.0000180740.02644.ab. http://doi.org/10.1097/01.nmd.0000180740.02644.ab. [DOI] [PubMed] [Google Scholar]
- Hien DA, Campbell ANC, Ruglass LM, Saavedra L, Mathews AG, Kiriakos G, Morgan-Lopez A. Maximizing effectiveness trials in PTSD and SUD through secondary analysis: Benefits and limitations using the National Institute on Drug Abuse Clinical Trials Network “Women and Trauma” study as a case example. Journal of Substance Abuse Treatment. 2015;56:23–33. doi: 10.1016/j.jsat.2015.04.001. http://doi.org/10.1016/j.jsat.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Litt LC, Cohen LR, Miele GM, Campbell A. Trauma Services for Women in Substance Abuse Treatment: An Integrated Approach. American Psychological Association; 2009. [Google Scholar]
- Jerud AB, Zoellner LA, Pruitt LD, Feeny NC. Changes in emotion regulation in adults with and without a history of childhood abuse following posttraumatic stress disorder treatment. Journal of Consulting and Clinical Psychology. 2014;82(4):721–30. doi: 10.1037/a0036520. http://doi.org/10.1037/a0036520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048. doi: 10.1001/archpsyc.1995.03950240066012. http://doi.org/10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kraemer HC. Messages for clinicians: Moderators and mediators of treatment outcome in randomized clinical trials. American Journal of Psychiatry. 2016;173(7):672–679. doi: 10.1176/appi.ajp.2016.15101333. http://doi.org/10.1176/appi.ajp.2016.15101333. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Christian S, Bremner JD, Spiegel D. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry. 2010;167(6):640–647. doi: 10.1176/appi.ajp.2009.09081168. http://doi.org/10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological methods. 2002;7(1):19. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York, NY: Guilford Press; 2005. [Google Scholar]
- McDermott MJ, Tull MT, Gratz KL, Daughters SB, Lejuez CW. The role of anxiety sensitivity and difficulties in emotion regulation in posttraumatic stress disorder among crack/cocaine dependent patients in residential substance abuse treatment. Journal of Anxiety Disorders. 2009;23(5):591–599. doi: 10.1016/j.janxdis.2009.01.006. http://doi.org/10.1016/j.janxdis.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles SR, Smith TL, Maieritsch KP, Ahearn EP. Fear of losing emotional control is associated with cognitive processing therapy outcomes in US Veterans of Afghanistan and Iraq. Journal of traumatic stress. 2015;28(5):475–479. doi: 10.1002/jts.22036. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Hien D. Helping vulnerable populations: A comprehensive review of the treatment outcome literature on substance use disorder and PTSD. Journal of Clinical Psychology. 2013;69(5):433–479. doi: 10.1002/jclp.21980. http://doi.org/10.1002/jclp.21980. [DOI] [PubMed] [Google Scholar]
- Norman SB, Myers US, Wilkins KC, Goldsmith AA, Hristova V, Huang Z, Robinson SK. Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology. 2012;62(2):542–551. doi: 10.1016/j.neuropharm.2011.04.032. http://doi.org/10.1016/j.neuropharm.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryan EM, McLeish AC, Kraemer KM, Fleming JB. Emotion regulation difficulties and posttraumatic stress disorder symptom cluster severity among trauma-exposed college students. Psychological Trauma: Theory, Research, Practice, and Policy. 2015;7(2):131. doi: 10.1037/a0037764. [DOI] [PubMed] [Google Scholar]
- Price JL, Monson CM, Callahan K, Rodriguez BF. The role of emotional functioning in military-related PTSD and its treatment. Journal of Anxiety Disorders. 2006;20(5):661–674. doi: 10.1016/j.janxdis.2005.04.004. http://doi.org/10.1016/j.janxdis.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Resick PA, Miller MW. Posttraumatic stress disorder: Anxiety or traumatic stress disorder? Journal of Traumatic Stress. 2009;22:384–390. doi: 10.1002/jts.20437. http://doi.org/10.1002/jts. [DOI] [PubMed] [Google Scholar]
- Resko SM, Mendoza NS. Early attrition from treatment among women with cooccurring substance use disorders and PTSD. Journal of Social Work Practice in the Addictions. 2012;12(4):348–369. http://doi.org/10.1080/1533256X.2012.728104. [Google Scholar]
- Ruglass LM, Lopez-Castro T, Papini S, Killeen TK, Back SE, Hien DA. Concurrent treatment with prolonged exposure for co-occurring full or subthreshold posttraumatic stress disorder and substance use disorders: A randomized clinical trial. Psychotherapy and Psychosomatics. 2017;86:150–161. doi: 10.1159/000462977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruglass LM, Papini S, Trub L, Hien DA. Psychometric properties of the Modified Posttraumatic Stress Disorder Symptom Scale among women with posttraumatic stress disorder and substance use disorders receiving outpatient group treatments. Journal of Traumatic Stress Disorders & Treatment. 2014;4(1):1–7. doi: 10.4172/2324-8947.1000139. http://doi.org/10.4172/2324-8947.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiya T, Lopez-Castro T. Ashamed and afraid: A scoping review of the role of shame in post-traumatic stress disorder (PTSD) Journal of Clinical Medicine. 2016;5(11):94. doi: 10.3390/jcm5110094. http://doi.org/10.3390/jcm5110094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligowski AV, Lee DJ, Bardeen JR, Orcutt HK. Emotion regulation and posttraumatic stress symptoms: A meta-analysis. Cognitive Behaviour Therapy. 2015;44(2):87–102. doi: 10.1080/16506073.2014.980753. http://doi.org/10.1080/16506073.2014.980753. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Kring AM. Measuring changes in emotion during psychotherapy: Conceptual and methodological issues. Clinical Psychology: Science and Practice. 2007 http://doi.org/10.1111/j.1468-2850.2007.00092.x.
- Stroup WW. Generalized Linear Mixed Models: Modern Concepts, Methods and Applications. CRC press; 2012. [Google Scholar]
- Tull MT, Bardeen JR, DiLillo D, Messman-Moore T, Gratz KL. A prospective investigation of emotion dysregulation as a moderator of the relation between posttraumatic stress symptoms and substance use severity. Journal of Anxiety Disorders. 2015;29:52–60. doi: 10.1016/j.janxdis.2014.11.003. http://doi.org/10.1016/j.janxdis.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tull MT, Barrett HM, McMillan ES, Roemer L. A preliminary investigation of the relationship between emotion regulation difficulties and posttraumatic stress symptoms. Behavior Therapy. 2007;38(3):303–313. doi: 10.1016/j.beth.2006.10.001. http://doi.org/10.1016/j.beth.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Tull MT, Gratz KL, Coffey SF, Weiss NH, McDermott MJ. Examining the interactive effect of posttraumatic stress disorder, distress tolerance, and gender on residential substance use disorder treatment retention. Psychology of Addictive Behaviors. 2013;27(3):763–73. doi: 10.1037/a0029911. http://doi.org/10.1037/a0029911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tull MT, Gratz KL, McDermott MJ, Bordieri MJ, Daughters SB, Lejuez CW. The role of emotion regulation difficulties in the relation between ptsd symptoms and the learned association between trauma-related and cocaine cues. Substance Use & Misuse. 2016;51(10):1318–1329. doi: 10.3109/10826084.2016.1168445. http://doi.org/10.3109/10826084.2016.1168445. [DOI] [PubMed] [Google Scholar]
- van Dam D, Vedel E, Ehring T, Emmelkamp PMG. Psychological treatments for concurrent posttraumatic stress disorder and substance use disorder: A systematic review. Clinical Psychology Review. 2012;32:202–214. doi: 10.1016/j.cpr.2012.01.004. http://doi.org/10.1016/j.cpr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JRT. Clinician-administered PTSD scale: A review of the first ten years of research. Depression and Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. http://doi.org/10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Weiss NH, Tull MT, Viana AG, Anestis MD, Gratz KL. Impulsive behaviors as an emotion regulation strategy: Examining associations between PTSD, emotion dysregulation, and impulsive behaviors among substance dependent inpatients. Journal of Anxiety Disorders. 2012;26(3):453–458. doi: 10.1016/j.janxdis.2012.01.007. http://doi.org/10.1016/j.janxdis.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N, Tull MT, Anestis MD, Gratz KL. The relative and unique contributions of emotion dysregulation and impulsivity to posttraumatic stress disorder among substance dependent inpatients. Drug and Alcohol Dependence. 2013;128(1–2):45–51. doi: 10.1016/j.drugalcdep.2012.07.017. http://doi.org/10.1016/j.drugalcdep.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Hufford C, Najavits LM. Weekly Substance Use Inventory (Unpublishe) Boston, MA: Harvard Medical School; 1995. [Google Scholar]
- Westphal M, Aldao A, Jackson C. Emotion dysregulation in comorbid posttraumatic stress disorder and substance use disorders: A narrative review. Military Psychology. 2017;29(3):216–233. http://doi.org/10.1037/mil0000157. [Google Scholar]
- Wolff S, Holl J, Stopsack M, Arens EA, Höcker A, Staben KA, CANSAS Study Group, the C. S. Does emotion dysregulation mediate the relationship between early maltreatment and later substance dependence? Findings of the CANSAS study. European Addiction Research. 2016;22(6):292–300. doi: 10.1159/000447397. http://doi.org/10.1159/000447397. [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Shea MT, Recupero P, Bidadi K, Pearlstein T, Brown P. Trauma, dissociation, impulsivity, and self-mutilation among substance abuse patients. American Journal of Orthopsychiatry. 1997;67(4):650–654. doi: 10.1037/h0080263. http://doi.org/10.1037/h0080263. [DOI] [PubMed] [Google Scholar]


