Abstract
Antibodies recognizing conformational epitopes in Pfs48/45, an antigen expressed on the surface of Plasmodium falciparum gametes and zygotes, have firmly established Pfs48/45 as a promising transmission blocking vaccine (TBV) candidate. However, it has been difficult to reproducibly express Pfs48/45 in a variety of recombinant expression systems. The goal of our studies was to evaluate functional immunogenicity of Pfs48/45 using DNA vaccine format in rhesus macaques. An additional goal was to ensure that when used in combination with another malarial antigen, specific immunity to both antigens was not compromised. For testing combination vaccines, we employed Pfs25 DNA plasmids that have previously undergone evaluations in rodents and nonhuman primates. Pfs25 is expressed on the surface of parasites after fertilization and is also a lead TBV candidate. DNA plasmids based on codon-optimized sequences of Pfs48/45 and Pfs25 were administered by in vivo electroporation, followed by a final recombinant protein boost. Our studies demonstrate that Pfs48/45 encoded by DNA plasmids is capable of inducing potent transmission blocking antibody responses, and such transmission blocking immune potency of Pfs48/45 was not compromised when tested in combination with Pfs25, These findings provide the evidence in favor of further studies on Pfs48/45 and Pfs25, either alone or in combination with other known malaria vaccine candidates for developing effective vaccines capable of interrupting malaria transmission.
Keywords: Malaria. Vaccine, Transmission, DNA Vaccine, Combination Vaccine, Target Antigen, Mosquitoes
1. Introduction
Vaccines have been crucial in the control and eradication of several infectious diseases and represent one of the most effective public health tools available. Development of vaccines for malaria has focused on antigens expressed during various stages of the parasite, and malaria transmission blocking vaccines (TBVs) target antigens in sexual and mosquito midgut stage parasites. In Plasmodium falciparum these TBV target antigens include Pfs230 and Pfs48/45 expressed on circulating intra-erythrocytic male and female gametocytes and gametes, as well as Pfs25 expressed during mosquito midgut stage development (zygote to ookinete) [1]. Pfs25 has undergone extensive pre-clinical evaluation and a few phase I clinical trials as adjuvant formulated recombinant protein with mixed and varying outcomes [2–5]. Advancements with Pfs48/45 and Pfs230 have lagged, largely because of difficulties in reproducibly expressing recombinant forms of these antigens after initial success [6–8]. Moreover, the paucity of adjuvants for adequate vaccine formulations also hampers overall vaccine development efforts [9].
DNA vaccines, however, provide a single step approach for expressing the antigens in the immunized host cells and simultaneously presenting antigens to the immune system [10]. DNA vaccines encoding Pfs25 and Pvs25 (a P. vivax ortholog) have revealed highly potent immunogenicity, especially when administered using in vivo electroporation in mice and nonhuman primates [11–16]. We have recently also reported on induction of transmission-blocking antibodies in mice by DNA vaccine encoding Pfs48/45 [17]. DNA vaccines were first described in the early 1990s and generated much interest due to their simple design, manufacturability, and the ability to induce both cellular and humoral immune responses [18–23]. DNA vaccines also offer a convenient platform wherein either a single plasmid or a mixture of plasmids each encoding different antigens can be combined to develop a combination vaccine to target multiple stages or multiple species of the malaria parasite [24],[25]. Despite initial promising results, clinical development of DNA plasmid based vaccine development has been hampered, largely due to the relatively low potency seen in nonhuman primates and a few phase I clinical trials, particularly when the DNA is administered by conventional injection [26]. Exact mechanisms of poor immunogenicity of DNA vaccines in Homo sapiens are not known and few studies have systematically evaluated several approaches to enhance immune responses, including in vivo electroporation based DNA delivery, the use of genetic adjuvants, and sequence optimization for improved protein expression [25,27–29]. Additional studies evaluating these approaches individually, as well as in various combinations, are warranted, especially in nonhuman primate owing to their phylogenetic closeness to humans [30] and their presumed ability to mimic the outcomes expected in humans [26].
The primary objective of the study reported here was to investigate TBV potential of Pfs48/45 encoded by DNA plasmids in rhesus macaques. Additionally, we were able to conduct comparative immunogenicity outcome studies for two P. falciparum TBV antigens (Pfs25 and Pfs48/45, individually and in combination) in nonhuman primates, and assess relative contributions of (i) codon optimization [29], (ii) in vivo electroporation [31], (iii) DNA prime – protein boost regimen [32], and (iv) the role of N-linked glycosylation [33]. The underlying goal was to delineate factors that may catalyze further studies to facilitate development of vaccines capable of interrupting malaria transmission.
2. Materials and Methods
2.1 DNA plasmids
DNA vector VR1020 (Vical Inc. San Diego, CA) encoding codon-optimized Pfs48/45 or Pfs25, lacking signal and anchor sequences were constructed (SYN Pfs48/45 or SYN Pfs25). Additionally, an N-glycosylation mutant form of the codon-optimized Pfs48/45 sequence was developed where all seven putative N-glycosylation sites were mutated (MUT Pfs48/45). Sequence modifications to block N-linked glycosylation included N50→D, N131→D, T192→A, N204→T, N254→K, S301→A, N303→D in all NXS/T sites. Plasmid DNA (<30 EU/mg) was purified by Aldevron (Fargo, ND) and supplied at a 2.5 mg/mL concentration.
2.2 Animals
Twenty adult male and female rhesus macaques (Macaca mulatta), aged 4–13 years and weighing 4–10 kg, were utilized in this study. The macaques were housed at the Tulane National Primate Research Center (TNPRC; Covington, LA), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC000594), and were handled in accordance with protocols approved by the Institutional Animal Care and Use Committee (OLAW Assurance A4499-01). All procedures complied with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and TNPRC standards for minimizing animal distress.
2.3 Immunizations
Animals were assigned randomly to one of five immunization groups (4 per group) (Table 1). Groups 1 and 2 were immunized with SYN Pfs25 without or with electroporation (EP), respectively. Animals in groups 3 and 4 were immunized with SYN Pfs48/45 and MUT Pfs48/45, respectively, both with EP. Group 5 animals were immunized with a combination of SYN Pfs48/45 and SYN Pfs25 with EP. Plasmids (2.5 mg of each plasmid) were administered in 1.0 mL of PBS divided in equal volumes in quadriceps muscle of both legs for groups 1 to 4. Group 5 animals received a mixture of 2.5 mg of each SYN Pfs25 and SYN Pfs48/45 plasmid in 2.0 mL total volume equally divided between both legs. The animals received three repeat immunizations of respective DNA vaccines followed by a final protein boost with recombinant Pfs25 [5] (groups 1, 2), Pfs48/45 [8] (groups 3, 4), and a mixture of both rPfs25 and Pfs48/45 (group 5). Proteins were adsorbed with Alhydrogel (Brenntag Biosector) at a ratio of 1:3.2 (w/w) in PBS, pH 7.4, and each animal received 50 μg protein in a total volume of 1.0 ml. Animals were bled prior to immunization and at one month after each dose of vaccine.
Table 1.
Immunization Schedule
| Animal Group | Immunogen | EP Yes/No | Dose 1 | Dose 2 | Dose 3 | Final protein boost |
|---|---|---|---|---|---|---|
| 1 | SYN Pfs25 | No | 2.5 mg DNA | 2.5 mg DNA | 2.5 mg DNA | rPfs25 50 μg |
| 2 | SYN Pfs25 | Yes | 2.5 mg DNA | 2.5 mg DNA | 2.5 mg DNA | rPfs25 50 μg |
| 3 | SYN Pfs48/45 | Yes | 2.5 mg DNA | 2.5 mg DNA | 2.5 mg DNA | rPfs48/45 50 μg |
| 4 | Mut Pfs48/45 | Yes | 2.5 mg DNA | 2.5 mg DNA | 2.5 mg DNA | rPfs48/45 50 μg |
| 5 | SYN Pfs25 + SYN Pfs48/45 | Yes | 2.5 mg DNA of each | 2.5 mg DNA of each | 2.5 mg DNA of each | rPfs25 + rPfs4/45 50 μg each |
All the vaccinations were administered one month apart. Blood samples were collected from each animal prior to immunization and at one month after each dose of vaccine.
2.4 Antibody analysis
Sera were analyzed for antibody titers by ELISA using 96-well Immulon 4HBX plates coated with 1.5 μg/mL rPfs25 [5] or 1.5 μg/mL rPfs48/45 proteins [13]. HRP-conjugated goat anti-monkey IgG (KPL, Inc., MD) was used at 1:10,000 dilution and the plates were developed using ABTS substrate (KPL, Inc., MD) and absorbance was read at 405nm using an ELISA reader (VersaMax, Molecular Devices, CA).
Antibody avidity was determined using varying concentrations (0, 1, 2, 4, 8 M) of sodium thiocyanate (NaSCN) during an incubation (15 minutes) included in the standard ELISA protocol after primary antibody incubation, followed by the remaining ELISA steps [15]. Binding of antibody to antigen at 0 M NaSCN was considered as 100% (total) binding and avidity of antibody was expressed as NaSCN concentration giving 50% dissociation of bound antibody.
2.5 Standard membrane feeding assay (SMFA)
Mature gametocytes of P. falciparum NF54 strain were produced in vitro as described earlier [34]. Whole IgG was purified using Protein A - Sepharose (Sigma-Aldrich, MO) beads from sera of individual animals in each group. SMFAs were conducted by mixing different concentrations of purified IgG with mature P. falciparum gametocytes (0.3% gametocytemia) and human erythrocytes (50% hematocrit), and feeding the final mix to 4–5 day old Anopheles gambiae (Keele strain) mosquitoes that were starved for 4–6 hours. Blood fed mosquitoes were then maintained for 8–10 days at 26°C and 80–90% relative humidity. Mosquitoes were dissected and midguts were stained with 0.1% mercurochrome for oocyst enumeration by microscopy. Percent transmission reduction activity (TRA) is defined as percent reduction in the number of oocysts and was calculated using the following formula: percentage oocyst reduction =100 [(geometric mean number of oocysts with test sera/geometric mean number of oocysts with NMS) x 100].
2.6 Statistical analysis
All statistical tests were conducted using GraphPad Prism software (GraphPad Software Inc., CA). Antibody end point titers were defined as serum dilutions giving an absorbance higher than the average OD value at 405nm of pre-immune sera plus 3SD. Statistically significant differences in antibody responses between groups were determined using ANOVA and Mann-Whitney U test. P values of < 0.05 were considered significant. Statistical significance of MFA data was calculated using Kruskal-Wallis test (for differences in oocyst count between pre-immune and immune IgG fed mosquitoes).
3. Results
3.1 Vaccine safety
All physical exam, complete blood count, and blood chemistry results were within normal limits for all of the animals during the course of the study. No injection site or systemic vaccine reactions were observed.
3.2 Pfs48/45 antibody response after immunization with SYN Pfs48/45 and MUT Pfs48/45 DNA vaccines with EP and protein boost
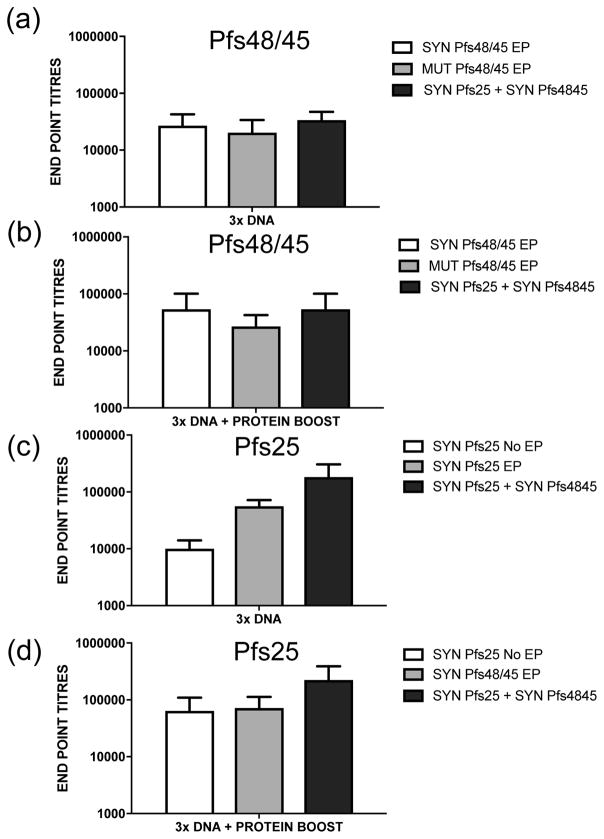
Two groups of rhesus macaques were immunized three times with SYN Pfs48/45 and MUT Pfs48/45 DNA plasmids delivered by EP followed by a final protein boost. Sera were analyzed by ELISA to compare the antibody titers elicited by DNA plasmid encoded glycosylated (SYN Pfs48/45) or unglycosylated (MUT Pfs48/45) Pfs48/45 antigens, respectively. After 3 DNA immunizations the average end point titers were not significantly different between the two immunization groups (SYN Pfs48/45 and MUT Pfs48/45) (Fig. 1a). After a final protein boost, there was a small increase in antibody titers in both groups (Fig. 1b). However the differences were not statistically significant.
Fig. 1. Analysis of antibody titers by ELISA in sera from monkeys (n=4 per group) immunized with different DNA vaccine plasmids.
Sera collected after three DNA immunizations (panels a and c) and after a final protein boost (panels b and d) were evaluated. End-point titers were defined as serum dilutions giving an absorbance (405nm) higher than that with pre-immune sera + 3SD. The error bars indicate SD.
3.3 Pfs25 antibody responses after immunization with SYN Pfs25 DNA vaccine without and with EP and protein boost
Immunogenicity of SYN Pfs25 DNA vaccine was evaluated in rhesus macaques to compare antibody titers without and with EP delivery. After 3 DNA immunizations, average end point titers for the EP group was a log higher than for the no EP group (Fig. 1c). After a final boost with rPfs25 protein, end point titers for the no EP group increased to levels comparable to those in the EP group (Fig. 1d).
3.4 Antibody responses after immunization with a combination of SYN Pfs48/45 and SYN Pfs25 DNA vaccines
To determine feasibility of immunization with a combination of two different sexual stage antigens, animals were immunized with a mixture of SYN Pfs48/45 and SYN Pfs25 DNA vaccines delivered by EP. Antibodies elicited after 3 DNA immunizations and after a protein boost were tested by ELISAs against each individual antigen (Pfs25 and Pfs48/45). As shown in Fig, 1 (panels a–d) (black bars for combination group), the antigen-specific end point titers were not very different from those elicited by single DNA plasmid immunogens (white bars, panels a–d).
3.5 Determination of antibody avidity in various immunization groups
Antibody avidity was assessed using NaSCN mediated antigen-antibody dissociation method. Individual sera from all the animals were tested to determine the qualitative differences for antibodies elicited in each immunization group. In particular we wished to compare: (i) antibodies elicited by glycosylated and unglycosylated forms of encoded Pfs48/45, (ii) antibodies against Pfs25 in no EP versus EP groups, and (iii) antibodies obtained after 3 DNA immunizations and after a protein boost. Table 2 shows the average concentration of NaSCN resulting in 50% dissociation of bound antibody to specific antigens. Higher concentrations of NaSCN were required for 50% dissociation of Pfs25 specific antibodies elicited by EP (2.3 M) as compared to conventional injection (1.4 M) and the trend was maintained after the protein boost (4.2 M with EP versus 2.9 M without EP). Antibodies elicited to Pfs48/45 following immunization with either SYN Pfs48/45 or MUTPfs48/45 DNA vaccines revealed comparable avidity index (1.7 M for SYN Pfs48/45 and 1.8 M for MUT Pfs48/45) and a protein boost did not make any further significant difference. We also analyzed sera from the group of animals immunized with a combination of SYN Pfs48/45 and SYN Pfs25 DNA vaccines with antibodies in the sera from groups receiving each DNA plasmid and the avidity values were not significantly different (Table 2).
Table 2.
Average antibody avidity index expressed as molar NaSCN concentration for 50% dissociation of bound antibodies for each group (N=4)
| Anti-Pfs25 | Anti-Pfs25 | Anti-Pfs48/45 | Anti-Pfs48/45 | |
|---|---|---|---|---|
| Vaccine Group | 3X DNA | 3X DNA + Protein | 3X DNA | 3X DNA + Protein |
| SYNPfs25 (−EP) | 1.4 | 2.3 | - | - |
| SYNPfs25 (+EP) | 2.9 | 4.2 | - | - |
| SYNPfs48/45 (+EP) | - | - | 1.7 | 1.4 |
| MutPfs48/45 (+EP) | - | - | 1.8 | 2.0 |
| SYNPfs48/45 & SYNPfs25 (+EP) | 2.0 | 2.1 | 1.2 | 2.0 |
3.6 Transmission blocking efficacy of anti-Pfs48/45 antibodies
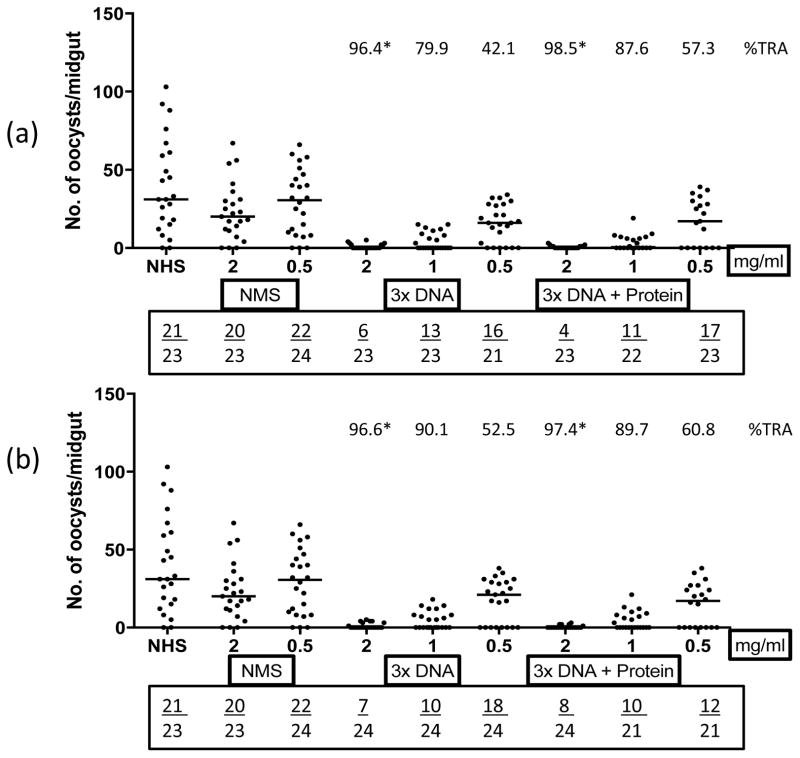
To determine transmission-blocking responses elicited by SYN Pfs48/45 and MUTPfs48/45 DNA immunizations, purified IgG from each group were tested at 0.5, 1.0 and 2 mg/mL. At 2 mg/mL, IgG from both groups revealed potent (96–97% reduction) after 3 DNA doses, and a similar reduction was revealed after protein boost. For both groups, even 1 mg/mL IgG resulted in high (80–90% after immunization with DNA and 87–90% after protein boost) transmission blocking (Fig. 2, a and b). These studies evaluating Pfs48/45 DNA vaccines in nonhuman primates demonstrate potent transmission blocking immunogenicity of codon-optimized SYN Pfs48/45. Moreover, no differences in functional responses were seen between DNA vaccines encoding glycosylated (SYN Pfs48/45 DNA) and unglycosylated (MUTPfs48/45 DNA) antigens.
Fig. 2. Membrane feeding assays using purified antibodies from SYN Pfs48/45 immunized animals.
Transmission blocking activity of purified IgG (mg/ml) from immune sera of rhesus monkey groups (a) SYN Pfs48/45 EP and (b) MUT Pfs48/45 EP. Individual dots represent number of oocysts in a mosquito. Numbers above each group represent percent reduction of oocyst numbers (%TRA) as compared to NMS (normal monkey sera) and numbers in the box below each plot represent total number of infected mosquitoes/total number of mosquitoes dissected. NHS represents normal human sera control. The horizontal bars represent the median oocyst counts. Geometric means were used to calculate percent transmission blocking. Statistical differences were analyzed by Mann Whitney U test and are indicated by an asterisk (*).
3.7 Transmission blocking efficacy of anti-Pfs25 antibodies
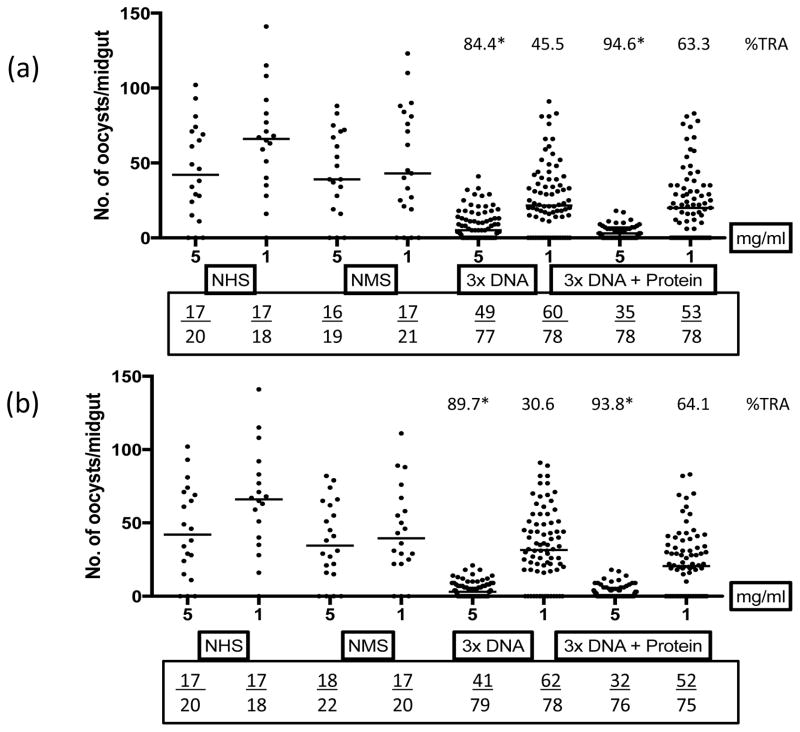
Purified IgGs from rhesus immunized with SYN Pfs25 without or with EP were tested at two different concentrations to determine functional transmission-blocking activity. IgG (5 mg/mL) from both groups after 3 DNA immunizations revealed strong transmission blocking activity (ranging from 84 to 90%) which increased marginally (ranging from 83 to 95%) after a protein boost. Transmission blocking activity at 1 mg/mL was 45–64% for IgG from no-EP group and 30–65% from the EP immunization group (Fig. 3, a and b).
Fig. 3. Membrane feeding assays using purified antibodies from SYN Pfs25 immunized animals.
Transmission blocking activity of purified IgG (mg/ml) from immune sera of rhesus groups (a) SYNPfs25 no-EP and (b) SYNPfs25 EP. Individual dots represent number of oocysts in a mosquito. Numbers above each group represent percent reduction of oocyst numbers (%TRA) as compared to NMS (normal monkey sera) and numbers in the box below each plot represent total number of infected mosquitoes/total number of mosquitoes dissected. NHS represents normal human sera control. The horizontal bars represent the median oocyst counts. Geometric means were used to calculate percent transmission blocking. Statistical differences were analyzed by Mann Whitney U test and are indicated by an asterisk (*).
3.8 Transmission blocking activity after immunization with a combination of SYN Pfs48/45 and SYN Pfs25 DNA vaccines
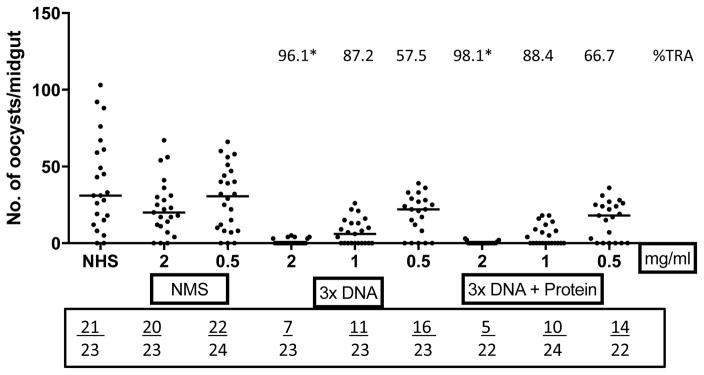
Purified IgG from animals immunized with a combination of SYN Pfs48/45 and SYN Pfs25 were also tested in SMFAs at various concentrations. These studies revealed high transmission blocking activity, ~96% after immunization with DNA, and ~98% after protein boost, at 2 mg/mL; and 88% after DNA immunizations and 89% after protein boost at 1 mg/mL. While we do not know which antibodies (anti-Pfs25 or anti-Pfs48/45) in the combination group were responsible for transmission blocking activity, these results of a first ever evaluation of functional immunogenicity of combined P. falciparum target antigens Pfs25 and Pfs48/45 rule out any immune interference by either antigen (Fig. 4).
Fig. 4. Membrane feeding assays using purified antibodies from SYN Pfs48/45 plus SYN Pfs25 immunized animals.
Transmission blocking activity of purified IgG (mg/ml) from immune sera of rhesus monkey groups (SYNPfs48/45 + SYNPfs25) with EP. Individual dots represent number of oocysts in a mosquito. Numbers above each group represent percent reduction of oocyst numbers (%TRA) as compared to NMS (normal monkey sera) and numbers in the box below each plot represent total number of infected mosquitoes/total number of mosquitoes dissected. NHS represents normal human sera control. The horizontal bars represent the median oocyst counts. Geometric means were used to calculate percent transmission blocking. Statistical differences were analyzed by Mann Whitney U test and are indicated by an asterisk (*).
4. Discussion
Malaria vaccines targeting development of parasite stages in the female Anopheles mosquitoes represent a direct approach to interrupting malaria transmission [35]. However, development of this immunization strategy has been hampered because it has been rather difficult to produce and formulate various TBV target antigens in correctly folded conformations, a key requirement for functional immunogenicity. Only Pfs25 has been studied extensively in rodent, nonhuman primate, and limited phase I human trials using various vaccine platforms such as vaccinia virus, Pichia pastoris and E. coli [2,4,5] with varying degree of success. Pfs48/45 has been less extensively evaluated owing to its larger size and the inability to reproducibly generate a full-length correctly folded protein in the monomeric form [7], [8]. These difficulties continue to underscore the need to explore alternative vaccine platforms that are technically less challenging (i.e. vaccines that are easy to produce, stable, safe, easy to store and transport), can induce effective immune responses without extensive vaccine manipulations and can also facilitate development of a multivalent combination vaccines targeting different lifecycle stages of the parasite. Many of these requirements are easily met by DNA vaccines; however further optimization approaches are needed to enhance their overall potency in larger vertebrates [10], [29]. Reduced transfection efficiency and antigen expression in larger animals [36] has been suggested as one plausible mechanism, however availability of an effective West Nile Virus DNA vaccine for horses [37] and a therapeutic melanoma DNA vaccine for dogs [38] indicates that selection of antigen and vaccine design are also key factors contributing to success of DNA vaccines. Clearly, additional studies are warranted to improve the DNA vaccine efficacy in larger animal models with more immune-physiological relatedness to humans, eg. nonhuman primates, in comparison to the commonly used laboratory rodent strains [39].
We have previously reported on the effective immunogenicity of Pfs25 DNA vaccine administered without EP and DNA prime-recombinant protein boost regimen in rhesus macaques [13], and by in vivo EP in olive baboons [15]. We have recently reported on immune potency of DNA vaccines encoding Pfs48/45, a lead TBV target antigen, in mice [17], however Pfs48/45 DNA vaccines have not been evaluated in nonhuman primates. We evaluated immunogenicity of glycosylated and unglycosylated forms of Pfs48/45 encoded by DNA vaccines delivered by in vivo EP alone or in combination with Pfs25 in rhesus macaques.
Our findings revealed overall superior immunogenicity of Pfs48/45 and Pfs25 DNA vaccine delivered with EP and a protein boost in animals immunized without EP resulted in boosting of antibody responses comparable to those in EP group. A similar boosting effect of protein was seen in a previous rhesus trial of Pfs25 DNA vaccine administered without EP [13] and in baboons employing EP delivery of unglycosylated form of Pfs25 [15]. Qualitatively, the antibodies in the EP groups revealed a trend for higher avidity of antibodies, although the differences were not statistically significant when compared with sera from non-EP groups. When comparing functional responses between groups, EP DNA delivery showed potent transmission blocking after DNA immunization, which remained high after the protein boost. Transmission blocking in the no-EP group was lower than in the EP group after DNA immunization but was higher after a protein boost. Overall, our studies confirmed earlier finding in rodent [14] and nonhuman primate studies [15] that suggest a comparative advantage for EP delivery of DNA vaccine using a codon optimized Pfs25 sequence.
The studies conducted here with Pfs48/45 DNA vaccines, show for the first time potent immunogenicity outcomes in nonhuman primates. We examined a codon- optimized, N-glycosylation site intact Pfs48/45 sequence (SYN Pfs48/45) and a codon- optimized but N-glycosylation mutant sequence (MUT Pfs48/45). The gene sequence of Pfs48/45 has seven putative N-glycosylation sites (NXS/T) and introduced mutation (N50D, N131D, T192A, N204T, N254K, S301A and N303D) were chosen based on the natural polymorphism at these sites as revealed by comparison of P48/45 sequences in other Plasmodium spp. (in alignments from OrthoMCL 5 [40] via PlasmoDB) [41]. However, results from rhesus immunizations with SYN Pfs48/45 and MUT Pfs48/45 DNA revealed no significant differences in antibody end point titers, antibody avidity and in functional transmission blocking responses. As with Pfs25 DNA vaccine EP immunizations, recombinant protein boosting did not significantly alter the antibody or blocking responses of either SYN Pfs48/45 or MUT Pfs48/45 DNA immunized groups. These findings demonstrate for the first time, the transmission reducing potential of Pfs48/45 DNA vaccines and warrant further exploration of Pfs48/45 as a TBV candidate using the DNA vaccine platform.
In the studies reported we also explored the possibility of combining multiple antigens to target different stages in the parasite’s life cycle. Pfs48/45, which is present on the gamete surface, is first expressed during maturation of erythrocytic gametocytes and is also a target of immune response during infection. On the other hand, the Pfs25 protein is expressed only on the surface of mosquito stage parasites. Antibodies obtained after immunization with both DNA plasmids, when tested against each individual antigen, showed no evidence for any interference of immune response against individual antigens. Additionally, none of the other immunogenicity parameters in the combination groups differed from the results obtained with the individual antigens. These results in nonhuman primates and our previous similar findings in mice [17] support the feasibility of combining multiple TBV antigens to improve breadth of immunity and putatively enhance vaccine outcomes.
Based on the results from these experiments, we conclude that DNA vaccines encoding Pfs25 and Pfs48/45 should be further developed as TBVs. Additional enhancement of immunogenicity could be achieved by including next-generation DNA plasmid design, use of immunomodulatory adjuvants or nanoparticle-based delivery [26], [28]. More clues might emerge from understanding of the immune correlates of protection of these transmission-blocking antigens. Considering the outcome of these studies, the potential benefits of designing TBVs targeting different parasite lifecycle stages in the mosquitoes, and the relative ease of designing cocktail DNA vaccines and combined immunization studies using Pfs25 and Pfs48/45 need to be explored further with the ultimate goal of developing effective transmission-blocking DNA vaccines that can target malaria elimination in endemic regions [42].
Acknowledgments
These studies were supported by NIH grants AI47089 and AI101427 and P51RR000164/P51OD011104
Footnotes
Author Contributions
NK and GB conceived and designed the experiments; DD, BG, DM performed experiments; DG, MP, BE and DH provided technical advice; DD, GB and NK analyzed the data; DD, GB, BG, MP, DG, BE, DH and NK participated in the preparation of manuscript; DD, GB and NK finalized the manuscript for submission.
Conflict of Interest
The authors declare no conflict of interest. BE and DH are employed by ICHOR, Medical Systems, Inc. that generously provided access to electroporator used in these studies. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu Y, Sinden RE, Churcher TS, Tsuboi T. Chapter Three-Development of Malaria Transmission-Blocking Vaccines: From Concept to Product. Advances in …. 2015 doi: 10.1016/bs.apar.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Kaslow DC, Isaacs SN, Quakyi IA, Gwadz RW, Moss B, Keister DB. Induction of Plasmodium falciparum transmission-blocking antibodies by recombinant vaccinia virus. Science. 1991;252:1310–3. doi: 10.1126/science.1925544. [DOI] [PubMed] [Google Scholar]
- 3.Kaslow DC. Transmission-blocking vaccines: uses and current status of development. Int J Parasitol. 1997;27:183–9. doi: 10.1016/s0020-7519(96)00148-8. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE. 2008;3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar R, Angov E, Kumar N. Potent malaria transmission-blocking antibody responses elicited by Plasmodium falciparum Pfs25 expressed in Escherichia coli after successful protein refolding. Infection and Immunity. 2014;82:1453–9. doi: 10.1128/IAI.01438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milek RL, Roeffen WF, Kocken CH, Jansen J, Kaan AM, Eling WM, et al. Immunological properties of recombinant proteins of the transmission blocking vaccine candidate, Pfs48/45, of the human malaria parasite Plasmodium falciparum produced in Escherichia coli. Parasite Immunol. 1998;20:377–85. doi: 10.1046/j.1365-3024.1998.00171.x. [DOI] [PubMed] [Google Scholar]
- 7.Outchkourov NS, Roeffen W, Kaan A, Jansen J, Luty A, Schuiffel D, et al. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proceedings of the National Academy of Sciences. 2008;105:4301–5. doi: 10.1073/pnas.0800459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhury DR, Angov E, Kariuki T, Kumar N. A Potent Malaria Transmission Blocking Vaccine Based on Codon Harmonized Full Length Pfs48/45 Expressed in Escherichia coli. PLoS ONE. 2009;4:e6352. doi: 10.1371/journal.pone.0006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Good MF, Doolan DL. Malaria vaccine design: immunological considerations. Immunity. 2010;33:555–66. doi: 10.1016/j.immuni.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239:62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 11.Lobo CA, Dhar R, Kumar N. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infection and Immunity. 1999;67:1688–93. doi: 10.1128/iai.67.4.1688-1693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sattabongkot J, Torii M, Tsuboi T, Kumar N. Potent immunogenicity of DNA vaccines encoding Plasmodium vivax transmission-blocking vaccine candidates Pvs25 and Pvs28—evaluation of homologous and heterologous antigen-delivery prime-boost strategy. Vaccine | ScienceDirect.com. Vaccine. 2004 doi: 10.1016/j.vaccine.2003.11.060. [DOI] [PubMed] [Google Scholar]
- 13.Coban C, Philipp MT, Purcell JE, Keister DB, Okulate M, Martin DS, et al. Induction of Plasmodium falciparum transmission-blocking antibodies in nonhuman primates by a combination of DNA and protein immunizations. Infection and Immunity. 2004;72:253–9. doi: 10.1128/IAI.72.1.253-259.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeBlanc R, Vasquez Y, Hannaman D, Kumar N. Markedly enhanced immunogenicity of a Pfs25 DNA-based malaria transmission-blocking vaccine by in vivo electroporation. Vaccine. 2008;26:185–92. doi: 10.1016/j.vaccine.2007.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar R, Nyakundi R, Kariuki T, Ozwara H, Nyamongo O, Mlambo G, et al. Functional evaluation of malaria Pfs25 DNA vaccine by in vivo electroporation in olive baboons. Vaccine. 2013;31:3140–7. doi: 10.1016/j.vaccine.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datta D, Bansal GP, Kumar R, Ellefsen B, Hannaman D, Kumar N. Evaluation of the Impact of Codon Optimization and N-Linked Glycosylation on Functional Immunogenicity of Pfs25 DNA Vaccines Delivered by In Vivo Electroporation in Preclinical Studies in Mice. Clin Vaccine Immunol. 2015;22:1013–9. doi: 10.1128/CVI.00185-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta D, Bansal GP, Gerloff DL, Ellefsen B. Immunogenicity and malaria transmission reducing potency of Pfs48/45 and Pfs25 encoded by DNA vaccines administered by intramuscular electroporation. Vaccine. 2017 doi: 10.1016/j.vaccine.2016.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–4. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 19.Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2008;58:317–24. doi: 10.1007/s00262-008-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USa. 1993;90:11478–82. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson HL, Hunt LA, Webster RG. Protection against a lethal influenza virus challenge by immunization with a haemagglutinin-expressing plasmid DNA. Vaccine. 1993;11:957–60. doi: 10.1016/0264-410x(93)90385-b. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery DL, Shiver JW, Leander KR, Perry HC, Friedman A, Martinez D, et al. Heterologous and homologous protection against influenza A by DNA vaccination: optimization of DNA vectors. DNA Cell Biol. 1993;12:777–83. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Ugen KE, Srikantan V, Agadjanyan MG, Dang K, Refaeli Y, et al. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USa. 1993;90:4156–60. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doolan DL, Hoffman SL. Multi-gene vaccination against malaria: A multistage, multi-immune response approach. Parasitol Today (Regul Ed) 1997;13:171–8. doi: 10.1016/s0169-4758(97)01040-5. [DOI] [PubMed] [Google Scholar]
- 25.Ivory C, Chadee K. Genet Vaccines Ther. 2004;2:17. doi: 10.1186/1479-0556-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulmer JB, Wahren B, Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006;12:216–22. doi: 10.1016/j.molmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Current Opinion in Immunology. 2011;23:421–9. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bins AD, van den Berg JH, Oosterhuis K, Haanen JBAG. Recent advances towards the clinical application of DNA vaccines. Neth J Med. 2013;71:109–17. [PubMed] [Google Scholar]
- 29.Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines. 2012;11:189–209. doi: 10.1586/erv.11.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy RC, Shearer MH, Hildebrand W. Nonhuman primate models to evaluate vaccine safety and immunogenicity. Vaccine. 1997 doi: 10.1016/s0264-410x(96)00277-0. [DOI] [PubMed] [Google Scholar]
- 31.van Drunen Littel-van den Hurk S, Hannaman D. Electroporation for DNA immunization: clinical application. Expert Rev Vaccines. 2010;9:503–17. doi: 10.1586/erv.10.42. [DOI] [PubMed] [Google Scholar]
- 32.Ramshaw IA, Ramsay AJ. The prime-boost strategy: exciting prospects for improved vaccination. Immunology Today. 2000;21:163–5. doi: 10.1016/S0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- 33.Jortzik E, Kehr S, Becker K. Post-translational Modifications in Apicomplexan Parasites. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. pp. 93–120. [DOI] [Google Scholar]
- 34.Ponnudurai T, Meuwissen JHET, Leeuwenberg ADEM, Verhave JP, Lensen AHW. The production of mature gametocytes of Plasmodium falciparum in continuous cultures of different isolates infective to mosquitoes. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1982;76:242–50. doi: 10.1016/0035-9203(82)90289-9. [DOI] [PubMed] [Google Scholar]
- 35.Carter R. Transmission blocking malaria vaccines. Vaccine. 2001;19:2309–14. doi: 10.1016/S0264-410X(00)00521-1. [DOI] [PubMed] [Google Scholar]
- 36.Babiuk LA, Pontarollo R, Babiuk S, Loehr B. Induction of immune responses by DNA vaccines in large animals. Vaccine. 2003 doi: 10.1016/s0264-410x(02)00574-1. [DOI] [PubMed] [Google Scholar]
- 37.Ledgerwood JE, Pierson TC, Hubka SA, Desai N, Rucker S, Gordon IJ, et al. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203:1396–404. doi: 10.1093/infdis/jir054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grosenbaugh DA, Leard AT, Bergman PJ, Klein MK, Meleo K, Susaneck S, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. 2011;72:1631–8. doi: 10.2460/ajvr.72.12.1631. [DOI] [PubMed] [Google Scholar]
- 39.Hein WR, Griebel PJ. A road less travelled: large animal models in immunological research. Nat Rev Immunol. 2003;3:79–84. doi: 10.1038/nri977. [DOI] [PubMed] [Google Scholar]
- 40.Chen F, Mackey AJ, Stoeckert CJ, Roos DS. OrthoMCL-DB: querying a comprehensive multi-species collection of ortholog groups. Nucleic Acids Res. 2006;34:D363–8. doi: 10.1093/nar/gkj123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37:D539–43. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The malERA Consultative Group on Vaccines. A Research Agenda for Malaria Eradication: Vaccines. PLoS Med. 2011;8:e1000398. doi: 10.1371/journal.pmed.1000398.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]