Summary
Staphylococcus aureus commonly colonizes the epidermis, but the mechanisms by which the host senses virulent but not commensal S. aureus to trigger inflammation remain unclear. Using a murine epicutaneous infection model, we found that S. aureus expressed phenol-soluble modulin (PSM)α, a group of secreted virulence peptides, is required to trigger cutaneous inflammation. PSMα induces the release of keratinocyte IL-1α and IL-36α, and signaling via IL-1R and IL-36R was required for induction of the pro-inflammatory cytokine IL-17. The levels of released IL-1α and IL-36α, as well as IL-17 production by γδ T cells and ILC3 and neutrophil infiltration to the site of infection were greatly reduced in mice with total or keratinocyte-specific deletion of the IL-1R and IL-36R signaling adaptor Myd88. Further, Il17a−/−f−/− mice showed blunted S. aureus-induced inflammation. Thus, keratinocyte Myd88 signaling in response to S. aureus PSMα drives an IL-17-mediated skin inflammatory response to epicutaneous S. aureus infection.
Keywords: alarmins, IL-1, IL-36, Myd88, S. aureus, skin infection, pathogen virulence
In Brief (eTOC Blurb)
Nakagawa et al. demonstrate that S. aureus produces PSMα, a group of virulence peptides, to induce keratinocyte damage and release of IL-1α and IL-36α. IL-1R and IL-36R signaling via the adaptor Myd88 induces IL-17-producing γδ T cells and ILC3, which critically mediate skin inflammation in response to epicutaneous S. aureus.
Introduction
The skin is the largest organ at the interface between the external environment and host tissues. The epidermis located on the skin surface is important in maintaining the physical and immunological barrier of the skin by protecting the host from harmful environmental stimuli including invasive microbes (Segre, 2006). Staphylococcus aureus, a Gram-positive bacterium, is a leading cause of human infection capable of invading most tissues of the human body. The superficial skin is a major infection site for S. aureus, which normally resides in 10–20% of healthy individuals (Lowy, 1998). The S. aureus causing skin infections often originate from resident bacteria that colonize the mucosal surfaces of the skin (Balasubramanian et al., 2017; Lowy, 1998). How S. aureus produces virulence factors to transform from a skin commensal to a pathogen is poorly understood. Previous studies suggested that this might be a consequence of activation of virulence gene regulatory networks in response to environmental signals (Novick and Geisinger, 2008). A major S. aureus virulence program is the accessory gene regulatory (Agr) quorum-sensing, a two-component system that responds to bacterial density (Novick, 2003). Upon activation, the agr locus induces the expression of a wide array of secreted virulence factors including toxins and enzymes that are important for the adaptation of the pathogen to the environment (Novick, 2003). Agr-regulated toxins include phenol-soluble modulins (PSMs), a group of seven different peptides divided into α- and β-type PSMs (Cheung et al., 2014). PSM peptides form amphipathic α-helical structures capable of forming pores in artificial membranes (Wang et al., 2007). PSMα peptides are highly cytotoxic to a wide variety of cells including keratinocytes (KCs) while other PSMs have very limited cytotoxic activities (Nakamura et al., 2013; Wang et al., 2007). In a mouse epicutaneous model of S. aureus infection, δ-toxin, a PSM peptide, promotes skin inflammation by inducing mast cell degranulation (Nakamura et al., 2013). However, the mechanism by which S. aureus interacts with KCs to trigger skin inflammation in vivo remains largely unknown.
KCs are the predominant cell type in the stratified epithelial layer of the epidermis. In response to environmental stimuli, KCs produce a wide variety of molecules including multiple cytokines, chemokines and anti-microbial peptides (Kennedy-Crispin et al., 2012; Nestle et al., 2009). Some of these KC molecules, including IL-1α, high-mobility group box 1 (HMGB1) protein and anti-microbial peptides, can be released upon tissue damage and function as ‘alarmins’ to activate the immune system (Rider et al., 2017; Yang et al., 2009). However, the role of KCs in S. aureus-induced inflammation in vivo is poorly understood because practically all cutaneous models of S. aureus infection rely on subepidermal inoculation or prior physical disruption of the epidermis (Miller et al., 2006; Wang et al., 2007). In the intradermal or subcutaneous model of S. aureus infection, mice deficient in Myd88, the adaptor molecule that is critical for signaling through the Toll-like receptor (TLR)/IL-1/IL-18 family of receptors, showed increased inflammation, abscess formation and epidermal ulceration which correlated with impaired S. aureus clearance (Miller et al., 2006). Furthermore, Il1r−/−, but not Tlr2−/−, mice showed increased inflammation and abscess formation in the subcutaneous infection model (Miller et al., 2006). However, the role of these signaling pathways in promoting inflammation after epidermal colonization of S. aureus remains unclear. Using conditions in which virulence genes are induced upon epidermal colonization, we show that S. aureus relies on virulent Agr-regulated PSMα peptides to trigger cutaneous inflammation. PSMα induces the release of IL-1α and IL-36 from KCs to orchestrate cutaneous inflammation via Myd88 signaling and IL-17 production.
Results
Keratinocyte Myd88 is essential for S. aureus-induced skin inflammation
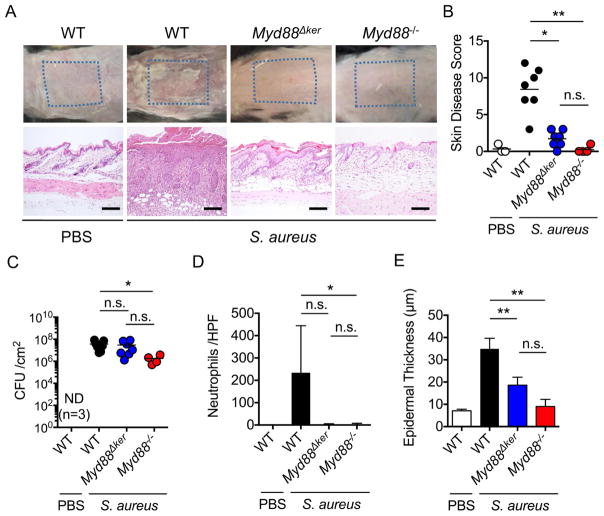
We used a recently developed model of S. aureus colonization that induces Agr-regulated virulence to investigate the mechanism by which the pathogen triggers cutaneous inflammation (Nakamura et al., 2013). In this epicutaneous model, wild-type (WT) mice infected with S. aureus (strain LAC, pulsed-field type USA300) showed severe skin disease and inflammation characterized by neutrophil infiltrates and epidermal thickening on day 7 after colonization (Figures 1A, 1B, 1D and 1E). In contrast, Myd88−/− mice showed little skin pathology when compared with WT mice (Figures 1A–1E). Unlike the epicutaneous model, Myd88−/− mice infected intradermally with S. aureus showed increased skin pathology and pathogen loads compared to that observed in WT-infected mice (Figures S1A–S1C), which is consistent with a previous report (Miller et al., 2006). In contrast, Myd88−/− mice exhibited lower S. aureus CFUs than WT mice in the epicutaneous model (Figure 1C), suggesting that Myd88-dependent inflammation promotes pathogen colonization in this model. Thus, the route of infection is critical to revealing the function of innate signaling pathways in host defense against S. aureus in the skin. To determine whether Myd88 acts within KCs, we crossed mice with a floxed exon of Myd88 (Myd88fl/fl mice) with a keratin 14 (K14)-Cre mouse deleter strain to generate K14-Cre-Myd88fl/fl mice with conditional deletion of Myd88 within KCs. Notably, mice lacking Myd88 in KCs (Myd88Δker) showed a phenotype similar to that observed in mice with whole-body deletion of Myd88 (Figures 1A, 1B and 1D). However, pathogen loads were comparable in Myd88Δker mice and WT mice (Figure 1C). Furthermore, the reduction in epidermal thickening in Myd88Δker mice was not as prominant as in Myd88−/− mice (Figure 1E). In contrast to the epicutaneous model, Myd88Δker and WT mice showed comparable skin inflammation in response to intradermal S. aureus infection (Figures S1D–S1F). These results indicate a critical role for Myd88 and particularly Myd88 in KCs for the induction of immune responses to S. aureus in the epicutaneous infection model.
Figure 1. Keratinocyte Myd88 is important for epicutaneous S. aureus-induced skin inflammation.
A, C57BL/6 (WT), K14-CreMyd88fl/fl (Myd88Δker) and Myd88−/− mice were epicutaneously colonized with S. aureus. WT mice treated with PBS are shown for comparison. Representative macroscopic images and hematoxylin and eosin (HE)-stained skin sections 7 days after colonization (n=3 to 7 mice per group). Scale bars, 100 μm. B, Day 7 skin disease scores of WT, Myd88Δker and Myd88−/− mice colonized with S. aureus. C–E, The number of S. aureus colony forming unit (CFU) (C), numbers of neutrophils per high power field (D), and epidermal thickness (E) in the skin of WT, Myd88Δker and Myd88−/− mice 7 days after colonization with S. aureus. Each dot represents a mouse (B, C). Data are presented as mean ± SD (D, E). Results shown represent combined data of 2 independent experiments. ND; not detected, n.s.; not significant, *P<0.05; **P<0.01, by Kruskal-Wallis test.
IL-1R and IL-36R are essential for induction of skin inflammation in epicutaneous S. aureus infection
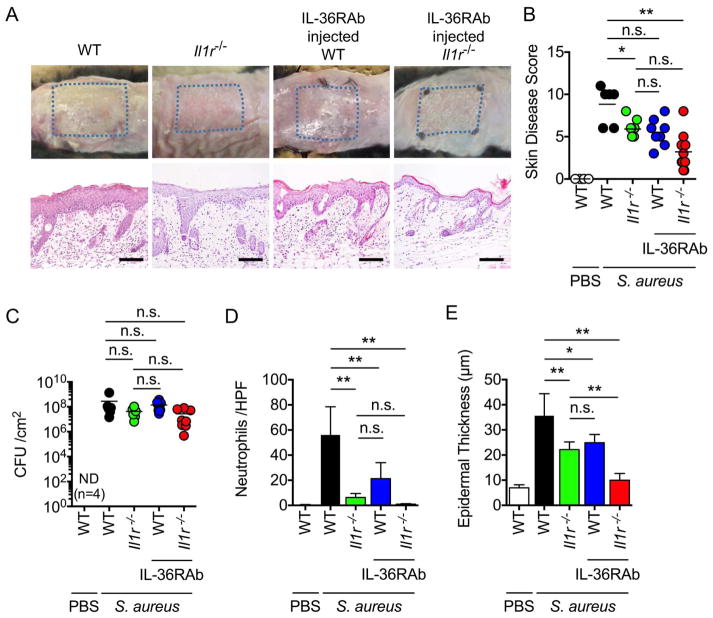
Myd88 is a critical adaptor molecule required for signaling via multiple immune receptors, including TLR/IL-1R/IL-18R/IL-36R (Palomo et al., 2015; Takeuchi and Akira, 2002). Initial experiments showed that deficiency in TLR2, TLR4 or IL-18 did not affect either skin disease, histopathology or pathogen loads in the epicutanous model of S. aureus infection (Figures S2A and S2B). In contrast, mice deficient in IL-1R, which can be stimulated by either IL-1α or IL-1β, exhibited a reduction in disease score, neutrophil infiltrates and epidermal thickening, despite similar pathogen loads when compared with WT mice (Figures 2A–2E). Two related cytokines IL-36α and IL-36γ are expressed in KCs (Gresnigt and van de Veerdonk, 2013) and activate the IL-36R that also signals via Myd88 (Towne et al., 2004). Administration of a neutralizing monoclonal antibody (Mab) against IL-36R also reduced neutrophil infiltration and epidermal thickening induced by epicutaneous S. aureus infection without affecting pathogen loads (Figures 2C–2E). Treatment of Ilr1−/− mice with the neutralizing anti-IL-36R Mab further reduced the disease score, neutrophil infiltration and epidermal thickening when compared WT mice (Figures 2A, 2B, 2D and 2E). These results indicate that both IL-1R and IL-36R signaling contribute to skin inflammation induced by S. aureus infection.
Figure 2. Both IL-1R and IL-36R contribute to skin inflammation induced by epicutaneous S. aureus colonization.
A, WT, Il1r−/−, WT mice treated with IL-36R blocking antibody (IL-36RAb), and Il1r−/− mice treated with IL-36RAb were epicutaneously colonized with S. aureus for 7 days. Representative macroscopic images and HE-stained skin sections of mice colonized with S. aureus or treated with PBS (n=4 to 8 mice per group). Scale bars, 100 μm. B–E, Day 7 skin disease scores (B), S. aureus CFU in the skin (C), the number of neutrophils in the skin (D) and epidermal thickness (E) of WT, Il1r−/−, WT mice treated with IL-36RAb, and Il1r−/− mice treated with IL-36RAb (n=4 to 8 mice per group). WT mice treated with PBS are shown for comparison. Each dot represents a mouse (B, C). Data are presented as mean ± SD (D, E). Data represent combined results from 3 independent experiments. ND; not detected, n.s.; not significant, *P<0.05 and **P<0.01, by Kruskal-Wallis test (B, C) or by one-way ANOVA test with Bonferroni’s correction (D, E).
S. aureus induces IL-1α and IL-36α expression via Myd88 in keratinocytes
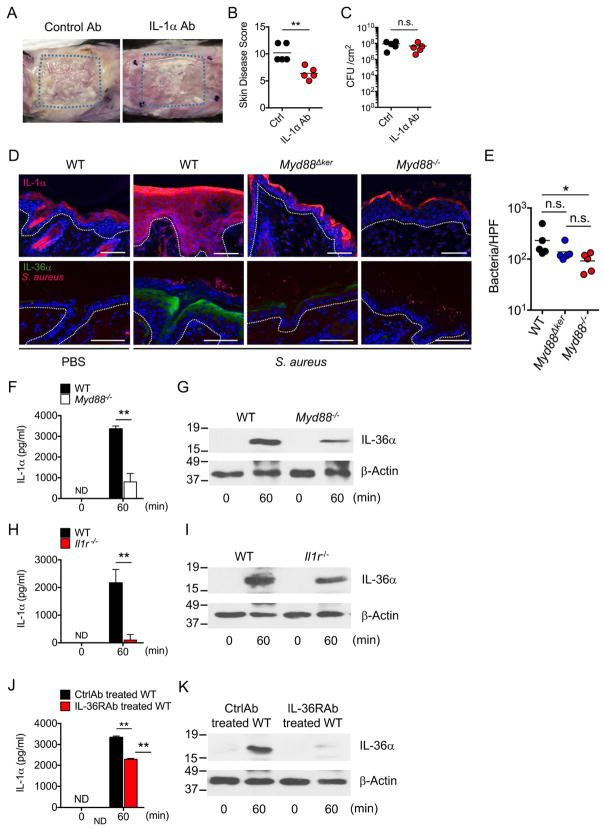
Given that IL-1R signaling is important for S. aureus-induced inflammation, we asked whether IL-1α or IL-1β mediates skin pathology in the epicutaneous infection model. Infection with S. aureus showed comparable pathology, disease scores and pathogen loads in WT and Il1β−/− mice (Figure S2C). In contrast, neutralization of IL-1α with a Mab reduced skin pathology without affecting pathogen loads, compared with mice treated with isotype-matched control Mab (Figures 3A–3C). Epicutaneous infection of S. aureus in WT mice substantially enhanced the expression of IL-1α and IL-36α in the upper area of the epidermis, which was reduced in Myd88Δker and Myd88−/− mice (Figures 3D and S3). Consistent with results showed in Figure 1C, pathogen loads were reduced in the skin of Myd88−/− mice compared to WT and Myd88Δker (Figure 3E). To determine whether S. aureus produces molecules that induce IL-1α and IL-36α release from KCs, primary KCs from WT and Myd88−/− mice were cultured under conditions that promote their terminal differentiation (Bikle et al., 2012) and then incubated with supernatants of S. aureus cultures. There was marked release of IL-1α and IL-36α after 60 min incubation of WT KCs with S. aureus culture supernatants (Figures 3F and 3G). Notably, the release of IL-1α and IL-36α was reduced in KCs from Myd88−/− mice (Figures 3F and 3G). Furthermore, the KC release of IL-1α was markedly reduced and that of IL-36α was partially affected in KC deficient in IL-1R (Figures 3H and 3I), suggesting that IL-1α release enhances IL-1α and IL-36α production via IL-1R signaling in KCs. In addition, IL-36α release by S. aureus was inhibited by neutralization of IL-36R whereas that of IL-1α was only minimally affected (Figures 3J and 3K). Collectively these results indicate that IL-1α and IL36α production are regulated by S. aureus stimulation via Myd88 signaling in KCs.
Figure 3. IL-1α and IL-36α are induced by S. aureus via Myd88 signaling in keratinocytes.
A, WT mice treated with IL-1α blocking antibody (IL-1αAb) or isotype-matched control Ab were epicutaneously colonized with S. aureus. Representative macroscopic images of mice colonized with S. aureus (n=5 mice per group). B–C, Skin disease scores (B) and S. aureus CFU in the skin (C) of WT mice treated with IL-1α Ab or control Ab. Each dot represents a mouse. D, Skin tissues of WT, and Myd88Δker and Myd88−/− mice colonized with S. aureus or treated with PBS were stained with Hoechst stain (blue) and antibody against IL-1α (red) or Hoechst stain (blue) and antibodies against S. aureus (red) and IL-36α (green). Scale bars, 50 μm. E, The numbers of S. aureus per high power field are (HPF) are shown. Each dot represents average results from an individual mouse. Data are representative of 2 independent experiments. F–K, IL-1α (F, H, J) and IL-36α (G, I, K) release of differentiated primary KCs isolated from WT and Myd88−/− mice (F, G), WT and Ilr1−/− mice (H, I), or WT mice in the presence of anti-IL-36 neutralizing Mab or isotype-matched control Mab (J, K) and stimulated with culture supernatants of S. aureus for indicated time. IL-1α and IL-36α were detected by ELISA assay and immunoblotting, respectively. β-actin in whole cell lysates is shown as loading control. Data are presented as mean±SD. Data are presented as mean ± SD. Data are representative of at least 2 independent experiments. ND; not detected, n.s.; not significant, *P<0.05 and **P<0.01, by unpaired two-tailed Mann-Whitney U test (B, C, F, H, J) or Kruskal-Wallis test (E).
IL-17 is critical for S. aureus-induced skin inflammation
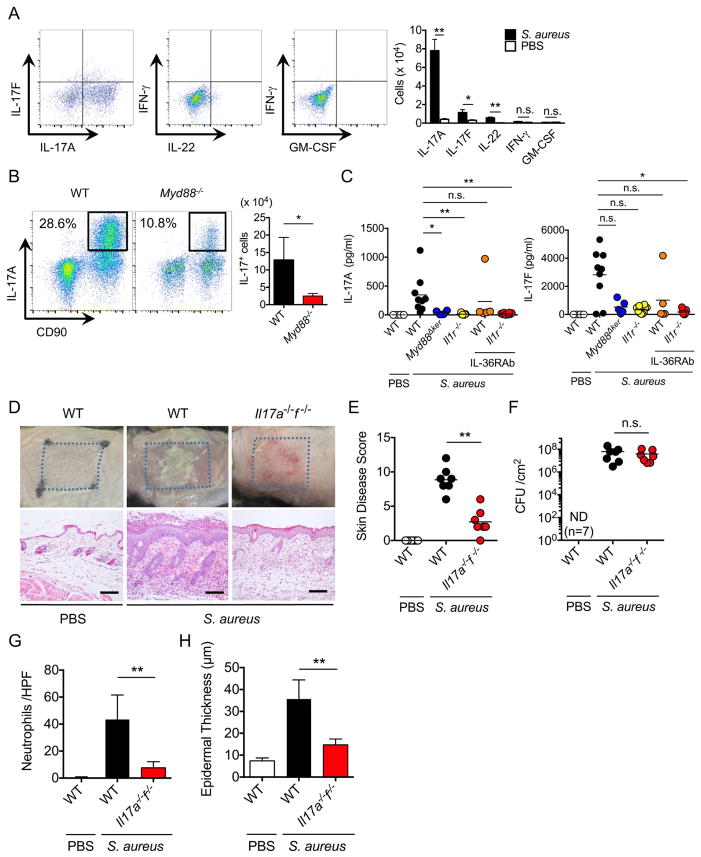
Members of the IL-1 family of cytokines are important for the induction of cellular immune responses and production of several cytokines including IL-17 and IL-22 (Sonnenberg et al., 2011; Villarino and Laurence, 2015). To determine which cytokines are induced upon epicutaneous infection with S. aureus, we assessed the production of multiple cytokines by skin immune cells at the peak of inflammation. Total skin cells were gated on hematopoietic CD45+CD90+ cells and the number of CD45+CD90+ cells producing IL-17A, IL-17F, IL-22, interferon-γ (IFN-γ) and GM-CSF was measured by flow cytometry. The analysis revealed that epicutaneous S. aureus infection induces a marked increase in the number of IL-17A-producing cells and a modest increase in cells producing IL-17F and IL-22 while there was minimal or no induction of IFN-γ and GM-CSF-producing cells (Figure 4A). The number of leukocytes infiltrating the skin including IL-17A-producing cells, was reduced in Myd88−/− mice (Figure 4B). Furthermore, the production of IL17A was reduced in the skin of Myd88Δker and Il1r−/− mice injected with IL-36R neutralizing Mab (Figure 4C). The production of IL-17F was also reduced in Il1r−/− mice injected with IL-36R neutralizing Mab compared to WT mice (Figure 4C). To determine if IL-17 was important for the induction of skin inflammation, WT mice and mice doubly deficient in IL-17A and IL-17F were infected epicutaneously with S. aureus. We found that Il17a−/−f−/− mice exhibited greatly reduced skin disease scores and neutrophil infiltration, but normal pathogen loads, compared with WT mice (Figures 4D–4H). In contrast, WT and Il22−/− mice showed comparable skin phenotype after S. aureus infection (Figure S2D). These results indicate that S. aureus induces IL-17 via Myd88 signaling which is critical for inflammatory pathology in the skin.
Figure 4. IL-17 is critical for skin inflammation induced by epicutaneous S. aureus colonization.
A, Production of IL-17A, IL-17F, IL-22, IFN-γ and GM-CSF by skin cells isolated from WT mice colonized with S. aureus or treated with PBS. Intracellular cytokine production was assessed in gated CD45+CD90+ cells on day 7 after pathogen colonization by flow cytometry. Representative flow cytometry profiles (left panels) and the number of cytokine-producing cells (right panel). Results in right panel represent mean ± SD of 2 experiments. B, Production of IL-17A by CD45+ cells in the skin of WT and Myd88−/− mice 7 days after epicutaneous infection. Representative flow cytometry profiles (left panels) and the number of IL-17A-producing cells (right panel). Results in right panel represent mean ± SD of 2 experiments. C, IL-17A and IL-17F production in skin tissues of WT mice, Myd88Δker mice, Il1r−/− mice, WT mice treated with IL-36RAb and Il1r−/− mice treated with IL-36RAb 7 days after pathogen colonization. WT mice treated with PBS are shown for comparison. Each dot represents a mouse. Data represent combined data of 3 independent experiments. D WT and Il17a−/−f−/− mice were epicutaneously colonized with S. aureus or treated with PBS. Representative macroscopic images and HE-stained skin sections 7 days after colonization (n=7 mice per group). Scale bars, 100 μm. E–H, Skin disease scores (E), S. aureus CFU in the skin (F), the numbers of neutrophils (G) and epidermal thickness (H) of WT and Il17a−/−f−/− mice colonized with S. aureus. WT mice treated with PBS are shown for comparison. Each dot represents a mouse (C, E, F). Data are presented as mean ± SD (A, B, G, H). Results shown represent combined data of 3 independent experiments. ND; not detected, n.s.; not significant, *P<0.05; **P<0.01, by unpaired two-tailed Mann-Whitney U test (A, B, E, F, G, H) or Kruskal-Wallis test (C).
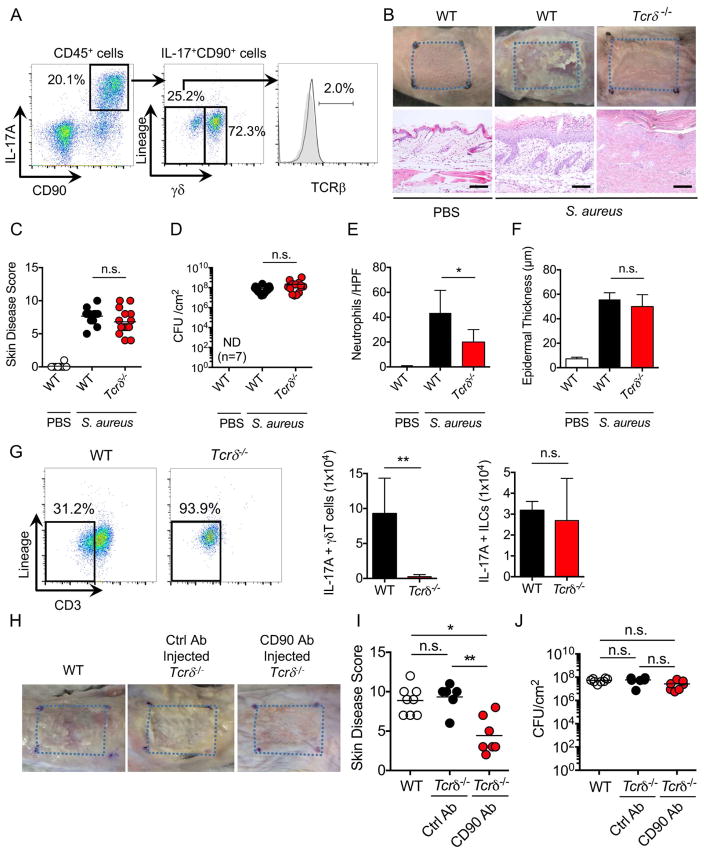
γδ T cells and type 3 innate lymphoid cells are the main producers of IL-17 in response to skin infection
We next asked which immune cell population(s) produces IL-17 in response to epicutaneous S. aureus infection. Gating on CD45+ cells revealed two populations of IL-17+CD90+ cells in the skin of mice epicutaneouly infected with S. aureus (Figure 5A). These included γδintermediate dermal γδ T cells and lineage-negative CD45+CD90+ immune cells which marks type 3 innate lymphoid cells (ILC3) (Figure 5A). To determine whether γδ T cells contribute to skin inflammation in response to S. aureus, we infected WT and Tcrδ−/− mice with the pathogen and assessed the pathology and disease scores in the skin. We found comparable disease scores, but reduced neutrophil infiltration in Tcrδ−/− mice compared with WT mice (Figures 5B–5F). As expected, IL-17-producing γδ T cells were absent in the skin of infected Tcrδ−/− mice, but the population of IL-17-producing ILC was unchanged in Tcrδ−/− mice (Figure 5G). To determine whether ILC3 contribute to skin inflammation, we treated Tcrδ−/− mice with anti-CD90 Mab to deplete the ILC3 population (Figure S4) and infected the treated mice epicutaneously with S. aureus. Treatment with anti-CD90 Mab significantly reduced skin disease scores without affecting pathogen loads in Tcrδ−/− mice when compared with mutant mice treated with control Mab (Figures 5H–5J). These results indicate that both ILC3 and γδ T cells contribute to skin inflammation in response to epicutaneous S. aureus infection.
Figure 5. Both γδ T cells and ILC3 contribute to skin inflammation after S. aureus colonization.
A, IL-17A-producing γδ T cells, ILC3 and αβ T cells were evaluated by flow cytometric analysis after epicutaneous S. aureus colonization. Flow cytometric analysis of lineage (B220, CD11b, CD11c, Gr-1, NK1.1)-negative cells and γδ T cells was performed on gated CD45+CD90+IL-17A+ skin cells. Data are representative of 3 independent experiments. B, WT and Tcrδ−/− mice were epicutaneously colonized with S. aureus. WT mice treated with PBS are shown for comparison. Representative macroscopic images and HE-stained sections of mouse skin on day 7 after colonization (n=10 to 15 mice per group). Scale bars, 100 μm. C–E, Day 7 skin disease scores (C), S. aureus CFU in the skin (D), neutrophil numbers in the skin (E) and epidermal thickening (F) of WT and Tcrδ−/− mice colonized with S. aureus. Each dot represents a mouse (C, D). Data are presented as mean ± SD (E, F). Results represent combined data of 4 independent experiments. G, IL-17A-producing γδ T cells and ILC3 were evaluated by flow cytometric analysis in WT and Tcrδ−/− mice after S. aureus colonization. Representative flow cytometric profiles of CD3 and lineage labeling on CD45+CD90+IL-17A+ skin cells (left panels). The number of IL-17A+ γδ T cells and ILC3 in WT and Tcrδ−/− mice (right panels). Results on right panels represent mean ± SD of 3 experiments. H, WT mice and Tcrδ−/− mice treated with control Ab and anti-CD90 Ab were epicutaneously colonized with S. aureus. Representative macroscopic images and HE-stained sections of mouse skin on day 7 after colonization (n=7 mice per group). Scale bars, 100 μm. I–J, Skin disease scores (I) and S. aureus CFU in the skin (J) of WT mice and Tcrδ−/− mice treated with control Ab and anti-CD90 Ab. Each dot represents a mouse. Results represent combined data of 2 independent experiments. ND; not detected, n.s.; not significant, *P<0.05 and **P<0.01, by using unpaired two-tailed Mann-Whitney U test (C–G) or by one-way ANOVA test with Bonferroni’s correction (I, J).
S. aureus PSMα induces keratinocyte IL-1α and IL-36α release to mediate skin inflammation
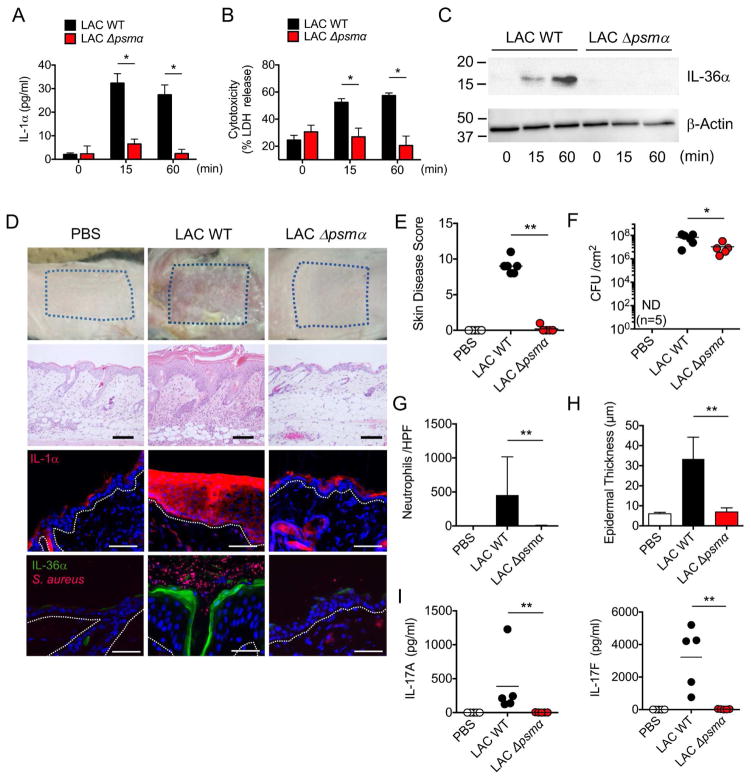
We next assessed which factor(s) released by S. aureus is important for eliciting the release of IL-1α and IL-36α from KCs and thereby triggering IL-17-dependent skin inflammation. PSMs including the peptides PSMα1 through PSMα4 induce robust cell death of mouse KCs in vitro (Nakamura et al., 2013). Because the Agr virulence system that controls the production of PSMα is activated during epicutaneous S. aureus infection in our model (Nakamura et al., 2013), we asked whether PSMα is important for eliciting skin inflammation. We first assessed whether PSMα is important for the release of IL-1α and IL-36α by incubating primary mouse KCs with culture supernatants from WT or isogenic S. aureus mutant strains deficient in PSMα1–4 (psmα) or PSMβ1–2 (psmβ). Incubation with the culture supernatant from WT and mutant psmβ, but not mutant psmα, strain induced cell death and release of IL-1α and IL-36α (Figures 6A–6C, S5A–S5B). In addition to mouse KCs, human KCs showed a marked release of IL-1α and enhanced cytotoxicity after treatment with supernatants from the WT and mutant psmβ strains, but not mutant psmα (Figures S6A–S6B). Complementation of mutant Δpsmα S. aureus with psmα plasmid restored cytotoxicity and cytokine release in both mouse and human KCs (Figures S5F–S5G, S6C–S6D). In addition, stimulation of human KCs with synthetic PSMα3 peptide induced IL-1α release and cytotoxicity (Figures S6E–S6F). Furthermore, pre-treatment with anakinra, an IL-1R antagonist, reduced IL-1α release induced by PSMα3 peptide without affecting cytotoxicity (Figures S6G–S6H). To determine whether PSMα is important for the induction of skin inflammation, we infected WT mice with WT and mutant psmα and psmβ S. aureus strains using the epicutaneous model. Histological analysis revealed that the ability of the psmα mutant strain to induce skin disease, epidermal thickening and neutrophil infiltration were impaired compared with the WT and psmβ strains (Figures 6D, 6E, 6G, 6H, S5C and S5D). There were reduced pathogen loads and IL-17 production in the skin of mice infected with the psmα mutant strain when compared to the WT bacterium (Figures 6F, 6I, and S5E). These results suggest that PSMα from S. aureus is a key virulence factor for triggering S. aureus-induced skin inflammation.
Figure 6. PSMα peptides induce the release of keratinocyte IL-1α and IL-36α to mediate skin inflammation.
A–B, IL-1α release (A) and cytotoxicity (B) of primary KCs from WT mice stimulated with culture supernatant of WT and Δpsma S. aureus (LAC strain) for indicated time. Data are presented as mean ± SD. C, IL-36α release from primary KCs stimulated with culture supernatant of WT or Δpsma S. aureus for indicated time. IL-36α was detected by immunoblotting. β-actin in whole cell lysates is shown as loading control. D, Representative macroscopic images (top panels) and HE-stained sections (middle upper panels), and sections stained with Hoechst stain (blue) and antibody against IL-1α (red) (middle lower panels) and stained with Hoechst stain (blue) and antibodies against S. aureus (red) and IL-36α (green) (bottom panels) of the skin from WT mice colonized with WT and Δpsma S. aureus or treated with PBS (n=5 to 8 per group) 7 days post infection. Epidermis/dermis border is marked by dotted white line. Scale bars, 100 μm (middle upper panels), 50 μm (middle lower panels) and 25 μm (bottom panels). E–H, Day 7 skin disease scores (E), S. aureus CFU in the skin (F), quantification of neutrophils in the lesional skin (G) and epidermal thickening (H) of WT mice colonized with WT and Δpsma S. aureus or treated with PBS (n=5 to 8 per group). Each dot represents a mouse (E, F, I). Data are presented as mean ± SD (G, H). I, The amounts of IL-17A and IL-17F in the lesional skin of WT mice colonized with WT or Δpsma S. aureus or WT mice treated with PBS for 7 days. Each dot represents a mouse. Results represent combined data of 3 independent experiments. Data are representative of at least 2 independent experiments. ND; not detected, n.s.; not significant, *P<0.05 and **P<0.01, by unpaired, two-tailed Mann-Whitney U test.
Discussion
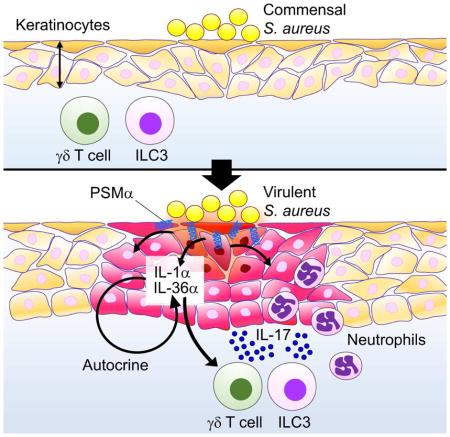
The mechanism by which S. aureus triggers skin inflammation in response to epidermal colonization has remained largely unknown. The paucity of knowledge is largely explained by the lack of a model of epidermal infection that mimics the virulent lifestyle of the pathogen. Using conditions in which S. aureus colonizes the epidermis and induces virulence (Nakamura et al., 2013), we found that Agr-regulated PSMα peptides trigger the release the KC alarmins IL-1α and IL-36α to elicit skin inflammation. These observations indicate that the host senses the pathogen indirectly through the induction of KC damage triggered by cytotoxic virulence factors. Because expression of aggressive, cytolytic PSMs appears to be limited mostly to S. aureus in comparison to S. epidermidis (Cheung et al., 2010), these observations suggest a model in which the host immune system can discriminate a virulent pathogen from commensals by sensing KC damage. Because PSMα peptides have been shown to induce chemokines from neutrophils (Kretschmer et al., 2010; Wang et al., 2007), it is possible that PSMα also contribute to skin inflammation via KC-independent mechanisms. Previous work showed that epidermal colonization of some commensals can induce IL-17-producing CD8+ cells in the dermis (Naik et al., 2015; Naik et al., 2012). However, colonization by commensals does not trigger overt inflammation and skin pathology including epidermal thickening and neutrophil recruitment which is observed after S. aureus infection (Naik et al., 2015). This model also implies that the host senses not the bacterium per se, but the virulent state of the pathogen through PSMα-induced release of KC alarmins. S. aureus lacking PSMα peptides accumulate at lower numbers than the WT bacterium after epicutaneous colonization. These results suggest that the pathogen benefits from PSMα production, at least in part, by inducing the release of nutrients or other factors from damaged KCs.
These studies have revealed a critical role for Myd88 signaling within KCs in orchestrating cutaneous inflammation induced by epicutaneous S. aureus infection. In mice with deletion of Myd88 in KCs, there was reduction of skin inflammation which was associated with impaired production of IL-1α and IL-36α by KCs. Studies in vivo and in vitro revealed that IL-1α and IL-36α expression is enhanced by S. aureus stimulation and this is impaired in Myd88−/− KCs. Furthermore, induction of IL-1α and IL-36α by S. aureus was reduced by IL-1R defiency and IL-36R neutralization, respectively. These results suggest that IL-1α and IL-36α act in a positive feedback loop that regulates their own expression via Myd88 signaling in KCs. These studies do not rule out a role for Myd88 on cells other than KCs to regulate the inflammatory response in the skin. IL-1R and IL-36R are expressed on immune cells and regulate the differentiation of IL-17-producing immune cells including γδ T cells, Th17 cells and ILC3 (Klose and Artis, 2016; Tortola et al., 2012; Vigne et al., 2011). Therefore, it is likely that Myd88 also acts critically on immune cells via IL-1R and IL-36R stimulation to regulate the induction of IL-17-producing cells in response to S. aureus epidermal infection. The phenotype of Myd88−/− mice is in contrast to that observed in the intradermal S. aureus inoculation model in which Myd88 deficiency is associated with increased pathogen loads and tissue pathology (Miller et al., 2006). The opposite roles of Myd88 in the two models suggest a different function of the immune system in the presence and absence of pathogen invasion. In the epicutaneous model, the epidermal barrier is not physically breached, and epidermal thickening and the recruitment of neutrophils are induced via Myd88 function to prevent pathogen invasion. In contrast to the role of Myd88 signaling in sensing pathogen on the epidermal surface in the epicutaneous model, in the dermal/subcutaneous model Myd88 signaling appears more critical in promoting killing of S. aureus by immune cells within in the dermis and subcutaneous tissues (Miller et al., 2006).
Our findings may have relevance to the inflammatory response associated with some forms of dermatitis in humans. Over 90% of atopic dermatitis (AD) patients are colonized in the lesional epidermis by S. aureus, which is increased during disease flares (Kong et al., 2012; Rudikoff and Lebwohl, 1998). In adult AD, lesional skin has been associated with Th2, Th22 and Th17 immune polarization, but strong Th17 polarization is characteristic of new-onset pediatric AD (Esaki et al., 2016). Previous work revealed the expression of S. aureus Agr virulence factors that produce PSMs in the lesional skin in AD (Nakamura et al., 2013). Furthermore, δ-toxin promoted allergic skin disease through the activation of mast cells that induces Th2 type inflammation in the epicutaneous S. aureus model (Nakamura et al., 2013). Together, these results indicate that different PSMs contribute to S. aureus-induced skin IL-17-driven inflammation via different mechanisms. However, a role for PSMα-induced IL-1α and IL-36 production in the pathogenesis of AD remains to be investigated. Although there is no direct evidence of S. aureus colonization in psoriasis, both psoriasis vulgaris and generalized pustular psoriasis are associated with increased production of IL-1 and IL-36 cytokines (D’Erme et al., 2015; Johnston et al., 2016). The importance of IL-36 in generalized pustular psoriasis is highlighted by the observation that missense loss-of-function mutations of the IL-36R antagonist leading to unrestrained IL-36 activity, which is associated with the disease (Marrakchi et al., 2011). Furthermore, neutralization of IL-17 is highly effective in the treatment of psoriasis (McInnes et al., 2015; Papp et al., 2012). Thus, IL-17 appears to promote protective immunity against pathogens, but if its production is excessive can also contribute to inflammatory disease. Our observations suggest that identifying pathogenic stimuli that trigger KC damage would help unravel the mechanisms underlying the induction of pathogenic IL-17 responses often associated with psoriasis.
STAR METHODS
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Armenian hamster monoclonal anti-IL-1α (clone ALF-161) | BioXCell | Cat# BE0243 |
| Armenian hamster polyclonal IgG | BioXCell | Cat# BE0091 |
| Goat polyclonal anti-IL-36α | R&D Systems | Cat# AF2297; RRID: AB_355216 |
| Mouse monoclonal anti-S. aureus (clone 704) | Abcam | Cat# ab37644; RRID: AB_778082 |
| Rat monoclonal anti-CD16/32 (clone 93) | BioLegend | Cat# 101302; RRID: AB_312801 |
| Rat monoclonal anti-CD90 (clone T24/31) | BioXCell | Cat# BE0212 |
| Rat monoclonal anti-IL-36 receptor (clone M616) | Amgen | MTA |
| Rat monoclonal IgG2a isotype control (clone 2A3) | BioXCell | Cat# BE0089 |
| Rat monoclonal IgG2b isotype control (clone LTF-2) | BioXCell | Cat# BE0090 |
| Alexa Fluor 488 rabbit polyclonal anti-goat IgG | Life Technologies | Cat# A11078; RRID: AB_10584486 |
| Alexa Fluor 647 goat polyclonal anti-mouse IgG | Invitrogen | Cat# A-21463; RRID: AB_2535869 |
| APC anti-CD45 | eBioscience | Cat# 17-0451-82; RRID: AB_469392 |
| APC anti-IL-17A | eBioscience | Cat# 17-7177-81; RRID: AB_763580 |
| Brilliant Violet 421 anti-CD3ε | BioLegend | Cat# 100341; RRID: AB_2562556 |
| Brilliant Violet 421 anti-γδ TCR | BioLegend | Cat# 118120; RRID: AB_2562566 |
| FITC anti-CD90.2 | eBioscience | Cat# 11-0902-85; RRID: AB_465154 |
| FITC anti-GM-CSF | BioLegend | Cat# 505403; RRID: AB_315379 |
| Pacific Blue anti-CD90.2 | BioLegend | Cat# 140305; RRID: AB_10645335 |
| PE anti-B220 | eBioscience | Cat# 12-0452-83; RRID: AB_465671 |
| PE anti-CD11b | BioLegend | Cat# 101208; RRID: AB_312791 |
| PE anti-CD11c | BioLegend | Cat# 117307; RRID: AB_313776 |
| PE anti-γδ TCR | eBioscience | Cat# 12-5711-81; RRID: AB_465934 |
| PE anti-Gr1 | BD Biosciences | Cat# 553128 |
| PE anti-IFN-γ | Thermo Fisher Scientific | Cat# RM9004; RRID: AB_10376293 |
| PE anti-IL-1α | BioLegend | Cat# 503203; RRID: AB_315281 |
| PE anti-IL-17F | eBioscience | Cat# 12-7471-80; RRID: AB_1210742 |
| PE anti-NK1.1 | BioLegend | Cat# 108707; RRID: AB_313394 |
| PECy7 anti-CD45 | BioLegend | Cat# 103113; RRID: AB_312978 |
| PerCP5.5 Armenian hamster IgG | BioLegend | Cat# 400931 |
| PerCP5.5 anti-CD45 | BD Biosciences | Cat# 561869; RRID: AB_394003 |
| PerCP5.5 anti-TCRβ | BioLegend | Cat# 109227; RRID: AB_1575176 |
| PerCP-eFluor 710 anti-IL-22 | eBioscience | Cat# 46-7221-80; RRID: AB_10598646 |
| Bacterial and Virus Strains | ||
| Staphylococcus aureus LAC (USA300) wild-type | Dr. Michael Otto (Wang et al., 2007) | N/A |
| Staphylococcus aureus LAC (USA300) Δpsmα | Dr. Michael Otto (Wang et al., 2007) | N/A |
| Staphylococcus aureus LAC (USA300) Δpsmβ | Dr. Michael Otto (Wang et al., 2007) | N/A |
| Staphylococcus aureus LAC (USA300) Δpsmα with plasmid pTXΔpsmα | Dr. Michael Otto (Wang et al., 2007) | N/A |
| Staphylococcus aureus LAC (USA300) Δpsmα with plasmid pTXΔ16 | Dr. Michael Otto (Wang et al., 2007) | N/A |
| Biological Samples | ||
| Human primary keratinocytes obtained from hair follicle | Chiba University Hospital (Nakano et al., 2016) | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Accutase Cell Detachment Solution | CELLnTEC | Cat# CnT-ACCUTASE-100 |
| Blocking One | Nacalai Tesque | Cat# 03953-95 |
| Brefeldin A | BD Biosciences | Cat# 555029 |
| Collagenase type 2 | Worthington | Cat# LS004176 |
| Dispase | CELLnTEC | Cat# CnT-DNP-10 |
| Dispase II | Sigma-Aldrich | Cat# D4693 |
| DNase I | Sigma-Aldrich | Cat# DN25 |
| Fixable Viability Dye eFluor 780 | eBioscience | Cat# 65-0865-14 |
| Fluorescent mounting medium | Dako | Cat# S3023 |
| Hoechst 33342 | Invitrogen | Cat# H3570 |
| Human IL-1 receptor antagonist | Wako | Cat# 093-05991 |
| Hyaruronidase | Sigma-Aldrich | Cat# H3506 |
| Ionomycin | Sigma-Aldrich | Cat# I9657 |
| Keratinocyte Growth Medium 2 | PromoCell | Cat# C20011 |
| Mannitol salt agar | Nissui | Cat# 05236 |
| PCT Epidermal Keratinocyte Medium | CELLnTEC | Cat# CnT-07 |
| Percoll | GE Healthcare | Cat# 17-0891-01 |
| Phorbol 12-myristate 13-acetate | Sigma-Aldrich | Cat# 79346 |
| Proteinase-inhibitor | Thermo Fisher Scientific | Cat# 78430 |
| Recombinant formylated phenol-soluble modulin α3 | Invitrogen | Cat# N/A |
| RIPA buffer | Wako | Cat# 182-02451 |
| Target retrieval solution pH 9 | Dako | Cat# S2031 |
| Critical Commercial Assays | ||
| CytoTox96® Non-Radioactive Cytotoxicity Assay | Promega | Cat# G1780 |
| Human IL-1α ELISA kit | R&D Systems | Cat# DLA50 |
| Mouse IL-1α ELISA kit | eBioscience | Cat# 88-5019 |
| Mouse IL-17A Flex set | BD Biosciences | Cat# 560283 |
| Mouse IL-17F Flex set | BD Biosciences | Cat# 562174 |
| Experimental Models: Cell Lines | ||
| Murine primary keratinocyte obtained from C57BL/6J mice | In this paper | N/A |
| Murine primary keratinocyte obtained from Il1r−/− mice | In this paper | N/A |
| Murine primary keratinocyte obtained from Myd88−/− mice | In this paper | N/A |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J | The Jackson Laboratory (CLEA Japan) | JAX: 000664 |
| Mouse: Il1r−/−: B6.129S7-Il1r1tm1Imx/J | The Jackson Laboratory | JAX: 003245 |
| Mouse: Il1β−/− | Dr. Yoichiro Iwakura (Horai et al., 1998) | MGI: 2157396 |
| Mouse: Il17a−/−f−/− | Dr. Shinobu Saijo (Ishigame et al., 2009) | MGI: 3830057 |
| Mouse: Il18−/− | Dr. Shizuo Akira (Takeda et al., 1998) | MGI: 2136769 |
| Mouse: Il22−/− | Genentech (Zheng et al., 2007) | MGI: 3699310 |
| Mouse: K14-Cre: B6N.Cg-Tg(KRT14-cre)1Amc/J | The Jackson Laboratory | JAX: 018964 |
| Mouse: Myd88−/− | Dr. Shizuo Akira (Adachi et al., 1998) | MGI: 2385681 |
| Mouse: Myd88fl/fl | Dr. Xiaoxia Li (Hou et al., 2008) | MTA |
| Mouse: Tcrδ−/−: B6.129P2-Tcrdtm1Mom/J | The Jackson Laboratory | JAX: 002120 |
| Mouse: Tlr2−/−4−/− | Dr. Shizuo Akira (Hoshino et al., 1999, Takeuchi et al., 1999) | MGI: 2178675 |
| Recombinant DNA | ||
| pTXΔpsmα | Wang et al., 2007 | N/A |
| pTXΔ16 | Wang et al., 2007 | N/A |
| Software and Algorithms | ||
| Axio vision | Zeiss | https://www.zeiss.com/microscopy/int/products/microscope-software/axiovision.html |
| BZ-H3A | Keyence | N/A |
| FCAP Array™ Software | BD Bioscience | https://www.bdbiosciences.com/documents/BD_FCAP_Array_Software_Guide.pdf |
| FlowJo | N/A | https://www.flowjo.com |
| Gen5™ | BioTek | https://www.biotek.com/products/software-robotics-software/gen5-microplate-reader-and-imager-software/ |
| Prism (GraphPad Software) | N/A | https://www.graphpad.com |
CONTACT FOR REAGENT AND RESOURCING SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Gabriel Nunez (gabriel.nunez@umich.edu).
EXPERIMENTAL MODELS AND SUBJECTS DETAILS
Animals
C57BL/6 mice were purchased from CLEA Co. (Tokyo, Japan) or the Jackson Laboratory and expanded in our mouse Facility. K14-Cre and Tcrδ−/− were purchased from the Jackson Laboratory. Tlr2−/−4−/−, Myd88−/−, Il1r−/−, Il1β−/−, Il18−/− and Il17a−/−f−/− mice on C57BL/6 background have been described previously (Adachi et al., 1998; Franchi et al., 2012; Horai et al., 1998; Hoshino et al., 1999; Ishigame et al., 2009; Takeda et al., 1998; Takeuchi et al., 1999). Myd88fl/fl mice on C57BL/6 background were a gift from Dr. Xiaoxia Li (The Cleveland Clinic)(Hou et al., 2008). Il22−/− mice on C57BL/6 background were obtained from Genentech. All mice were maintained under specific pathogen-free conditions and were used at 8 to 12 weeks of age. Female mice were used in the majority of the experiments. All animal studies were performed according to approved protocols by Chiba University or the University of Michigan Review Board for Animal Care.
Isolation of murine primary keratinocytes
Skins isolated from neonatal (day 1–3 after birth) WT, Il1r−/− and Myd88−/− mice were treated with 5 mg/ml dispase (CELLnTEC) overnight and then separated into dermis and epidermis. The epidermis sheets were treated with accutase (CELLnTEC) for 30 min and single-cell suspensions prepared from the epidermal sheets were cultured in CnT-07 medium (CELLnTEC) for 5–6 days. After confluency, terminal differentiation of KCs was induced by addition of 1.2 mM CaCl2 for the last 2 days. To block IL-36R, mouse KCs were treated with anti-IL-36R Ab (30 μg/ml) or its isotype control Ab (clone: 2A3)(BioXCell) before addition of CaCl2.
Preparation of human primary keratinocytes
Human primary KCs were established as previously described (Nakano et al., 2016). 1×104 cells in 24-well plate were cultured in Keratinocyte Growth Medium 2 (PromoCell) for 4–7 days. After confluency, terminal differentiation of KCs was induced by an addition of 1.8 mM CaCl2 for last 2 days. To block human IL-1 receptor, human primary KCs were treated with human IL-1 receptor antagonist, Anakinra (50 μg/ml) (Wako), every 12 hrs from 48 hrs before stimulation. The collection of skin samples and the use of primary KC samples was approved by the ethics committee of Chiba University Graduate School of Medicine (No. 519).
METHOD DETAILS
Antibody treatment
For antibody-mediated depletion, antibodies were purchased from BioXCell. To deplete ILCs, mice were treated with anti-CD90 antibody (200 μg) (clone: T24/31) subcutaneously on day -4, -2, 0, 2, 4, and 6 of S. aureus infection. Mice were treated with anti-IL-1α antibody (100 μg) (clone: ALF-161) subcutaneously on a daily base starting from day -1 of infection. To neutralize IL-36R, mice were injected intradermally on day 0 and intraperitoneally on day 1, 3 and 5 with an anti-IL36R Mab (50 μg) (clone: M616) provided by Amgen.
Cell isolation and flow cytometric analysis
For isolation of skin mononuclear cells, skin tissues were treated with 0.5% dispase II (Sigma) and then digested with 1.6 mg/ml collagenase type 2 (Worthington), 100 μg/ml deoxyribonuclease I (DNase I; Sigma) and 1.2 mg/ml hyaruronidase (Sigma). The cells were resuspended on a Percoll gradient (75%/40%) (GE Healthcare) and centrifuged at 2000 rpm for 20 min at 25°C. Single-cell suspensions were stimulated with 100 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma) plus 1 μM ionomycin (Sigma) for 4 hrs in the presence of brefeldin A (BD Biosciences) and then stained with the following fluorochrome-conjugated antibodies purchased from BD Biosciences, Biolegend, or eBioscience: Allophycocyanin (APC)-conjugated anti-CD45 (30-F11); brilliant violet 421-conjugated anti-CD3ε (145-2C11) and anti-γδ TCR (GL3); Fixable Viability Dye eFluor 780; fluorescein isothiocyanate (FITC)-conjugated anti-CD90.2 (53-2.1); pacific blue-conjugated anti-CD90.2 (53-2.1); peridinin chlorophyll protein complex-cyanin 5.5 (PerCP5.5)-conjugated Armenian Hamster IgG (HTK888), anti-CD45 (30-F11) and anti-TCRβ (H57-597); phycoerythrin (PE)-conjugated anti-B220 (RA-6B2), anti-CD11b (M1/70), anti-CD11c (HL3), anti-γδ TCR (GL3), anti-Gr1 (RB6-8C5) and anti-NK1.1 (PK136) ; PE-Cy7 conjugated anti-CD45 (30-F11). The cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences) before intracellular staining with APC-conjugated anti-IL-17A (TC11-18H10), FITC-conjugated anti-GM-CSF (MP1-22E9), PerCP-eFluor710-conjugated anti-IL-22 (1H8PWSR) and PE-conjugated anti-IFN-γ (XMG1.2) and anti-IL-17F (eBio18F10) and then analyzed on a LSRII flow cytometer (BD Biosciences).
Cytometric bead assay
Supernatants from crude suspension of epidermis and dermis were collected from colonized skin and then protein level of IL-17A and IL-17F was measured by BD™CBA Flex Sets according to the manufacturer’s instructions (BD Biosciences). The results were generated using CBA analysis software (BD Bioscience-PharMingen).
Cytotoxicity Assay
Cytotoxicity were measured by LDH release assay with CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega). Primary KCs were stimulated with various S. aureus culture supernatants and LDH release assay was performed according to manufacturers’ instructions.
Enzyme-linked immunosorbent assay
Mouse IL-1α was detected with IL-1 alpha ELISA Ready-Set-Go! Kit (eBioscience). Purified IL-1α capturing antibody was added to a coated Nunc-Immuno™ plate (Thermo scientific). After blocking with blocking buffer provided in the kit, supernatants from primary KCs were added and the cells were incubated at room temperature for 2 h. The binding of IL-1α was detected by HRP-conjugated detection antibody and TMB solution (eBioscience). Human IL-1α was detected with Quantikine® ELISA Human IL-1α/IL-1F1 Immunoassay (R&D). IL-1α measurement was performed according to manufacturers’ instructions.
Western blotting
Supernatants were concentrated with an Amicon® Ultra-0.5 centrifuge filter device. Primary KCs were lysed in the presence of proteinase-inhibitor (Thermo Scientific) and RIPA buffer (Wako) was added. The eluted samples were separated on 15% SDS-PAGE, transferred to polyvinyldifluoride membranes by electorophoresis and then analyzed by immunoblot with anti-mouse IL-36α antibody (R&D systems) followed by HRP-conjugated secondary antibody.
Immunofluorescent staining
Sections of skins fixed in 10% paraformaldehyde were treated with Target Retrieval Solution pH 9 (Dako) for IL-36α immunofluorescent staining, and frozen sections of skins were fixed with aceton for IL-1α immunofluorescent staining. The skin sections were treated with polyclonal antibodies against mouse IL-36α (R&D systems) and S. aureus (abcam) at 4°C overnight and then stained with Alexa Fluor 488-labeled anti-goat IgG antibodies (Life technologies) and Alexa Fluor 647 anti-mouse IgG antibodies (Invitrogen), respectively. For IL-1α and nuclear staining, PE-conjugated anti-IL-1α (ALF-161, Biolegend) and Hoechst (Invitrogen) were used, respectively. Images of stained sections were viewed with fluorescence microscope Axio Observer (Carl Zeiss) for IL-36α and BZ-X700 (Keyence) for IL-1α.
S. aureus colonization
The methicillin-resistant Staphylococcus aureus strain USA300 (LAC) and the isogenic Δpsma, Δpsmα pTXΔ16, Δpsmα pTXΔα and Δpsmβ mutant strains have been described (Wang et al., 2007). For colonization, bacteria were grown for 4 hrs in tryptic soy broth with shaking at 37°C. Mice were colonized on the shaved dorsal skin of mice by applying a 1 cm2 sterile gauze containing 1 × 108 CFUs of S. aureus which was covered with occlusive plastic dressing. In some experiments, mice were intradermally injected with 1 × 106 CFUs of S. aureus. To determine bacterial numbers in the colonized skin, skin was collected from individual mice, homogenized in cold PBS and plated at serial dilution onto Mannitol-salt agar containing 10% egg yolk. The number of CFU was determined after 24 hrs of incubation at 37°C. Mice were sacrificed on day 7 day after colonization, and skins were assessed in a blinded fashion using a scoring system described previously (Nakamura et al., 2013). Briefly, 4 points were used to denote the severity of erythema (0, none; 1, mild; 2, moderate; 3, severe), scaling (0, none; 1, mild; 2, moderate; 3, severe), erosion (0, none; 1, mild; 2, moderate; 3, severe), edema (0, none; 1, mild; 2, moderate; 3, severe), and thickness (0, none; 1, mild; 2, moderate; 3, severe). Skin samples were fixed in 10% formalin and processed for HE staining or frozen sections were obtained for immunohistochemistry.
QUANTIFICATION AND STATISTICAL ANALYSES
Statistical analyses were performed using GraphPad Prism software version 5.0 (GraphPad Software Inc.). Differences between two groups were evaluated using Student’s t test (parametric) or Mann-Whitney U test (non-parametric). For multiple comparisons, statistical analysis was performed using one-way ANOVA (parametric) or Kruscal-Wallis test (nonparametric), and then Bonferroni test for parametric samples, or Dunn’s test for nonparametric samples as a post-hoc test. Differences at P<0.05 were considered significant.
Supplementary Material
HIGHLIGHTS.
S. aureus virulence factor PSMα induces the release of keratinocyte IL-1α and IL-36α
Myd88 signaling in keratinocytes is required for IL-1α and IL-36α production
IL-1R and IL-36R signaling is critical for the induction of IL-17-producing cells.
Mice deficient in IL17A/F show blunted S. aureus-induced skin inflammation.
Acknowledgments
The authors thank the University of Michigan Flow Cytometry Core for Flow cytometry analysis, A. Oikawa in Chiba University for histology analysis, N. Saito in Chiba University for Enzyme-linked immunosorbent assay analysis and Melody Zeng for review of the manuscript. This work was supported by JSPS KAKENHI; Grant Number 26713038 (Y.N.), 16H06252 (Y.N.), the Naito Foundation (Y.N.), the Uehara Memorial Foundation (M.M.), Mochida Memorial Foundation for Medical and Pharmaceutical Research (M.M.) and NIH grant AR069303 (G.N.). Y.N. and S.N. were supported by Institute for Global Prominent Research, Chiba University. S.N. was supported by the Leading Graduate School Program of Chiba University (Nurture of Creative Research Leaders in Immune System Regulation and Innovative Therapeutics). M.O. was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, US National Institutes of Health (grant number ZIA AI000904-16).
Footnotes
Author Contributions
Designed experiments, S.N., M.M., G.N. and Y.N.; Performed experiments, S.N., M.M., Y.K., R.O., S.W., T.N., S.S. and Y.N.; Provided critical reagents and scientific insight, M. O.; Analyzed data, S.N., M.M., N.I., H.M., G.N. and Y.N.; S. N., M. M., G.N. and Y.N. wrote the paper.
Competing Financial Interests
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Balasubramanian D, Harper L, Shopsin B, Torres VJ. Staphylococcus aureus pathogenesis in diverse host environments. Pathog Dis. 2017 doi: 10.1093/femspd/ftx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Xie Z, Tu CL. Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metab. 2012;7:461–472. doi: 10.1586/eem.12.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Joo HS, Chatterjee SS, Otto M. Phenol-soluble modulins--critical determinants of staphylococcal virulence. FEMS Microbiol Rev. 2014;38:698–719. doi: 10.1111/1574-6976.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR, Whitney AR, Teintze M, DeLeo FR, Otto M. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog. 2010;6:e1001133. doi: 10.1371/journal.ppat.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Erme AM, Wilsmann-Theis D, Wagenpfeil J, Holzel M, Ferring-Schmitt S, Sternberg S, Wittmann M, Peters B, Bosio A, Bieber T, et al. IL-36gamma (IL-1F9) is a biomarker for psoriasis skin lesions. J Invest Dermatol. 2015;135:1025–1032. doi: 10.1038/jid.2014.532. [DOI] [PubMed] [Google Scholar]
- Esaki H, Brunner PM, Renert-Yuval Y, Czarnowicki T, Huynh T, Tran G, Lyon S, Rodriguez G, Immaneni S, Johnson DB, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol. 2016;138:1639–1651. doi: 10.1016/j.jaci.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Franchi L, Kamada N, Nakamura Y, Burberry A, Kuffa P, Suzuki S, Shaw MH, Kim YG, Nunez G. NLRC4-driven production of IL-1beta discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat Immunol. 2012;13:449–456. doi: 10.1038/ni.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresnigt MS, van de Veerdonk FL. Biology of IL-36 cytokines and their role in disease. Semin Immunol. 2013;25:458–465. doi: 10.1016/j.smim.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Johnston A, Xing X, Wolterink L, Barnes DH, Yin Z, Reingold L, Kahlenberg JM, Harms PW, Gudjonsson JE. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy-Crispin M, Billick E, Mitsui H, Gulati N, Fujita H, Gilleaudeau P, Sullivan-Whalen M, Johnson-Huang LM, Suarez-Farinas M, Krueger JG. Human keratinocytes’ response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. J Invest Dermatol. 2012;132:105–113. doi: 10.1038/jid.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, Program NCS, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, Schoneberg T, Rabiet MJ, Boulay F, Klebanoff SJ, et al. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, Zribi J, Bal E, Cluzeau C, Chrabieh M, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, van der Heijde D, Landewe R, Conaghan PG, Gottlieb AB, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Villaruz AE, Cheung GY, McGavin MJ, Travers JB, et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503:397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Kamada N, Suehiro K, Oikawa A, Shibata C, Nakamura Y, Matsue H, Sasahara Y, Hosokawa H, Nakayama T, et al. Establishment of a new three-dimensional human epidermal model reconstructed from plucked hair follicle-derived keratinocytes. Exp Dermatol. 2016;25:903–906. doi: 10.1111/exd.13066. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76:25–37. doi: 10.1016/j.cyto.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- Rider P, Voronov E, Dinarello CA, Apte RN, Cohen I. Alarmins: Feel the Stress. J Immunol. 2017;198:1395–1402. doi: 10.4049/jimmunol.1601342. [DOI] [PubMed] [Google Scholar]
- Rudikoff D, Lebwohl M. Atopic dermatitis. Lancet. 1998;351:1715–1721. doi: 10.1016/S0140-6736(97)12082-7. [DOI] [PubMed] [Google Scholar]
- Segre JA. Epidermal barrier formation and recovery in skin disorders. J Clin Invest. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. [Google Scholar]
- Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr Top Microbiol Immunol. 2002;270:155–167. doi: 10.1007/978-3-642-59430-4_10. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Tortola L, Rosenwald E, Abel B, Blumberg H, Schafer M, Coyle AJ, Renauld JC, Werner S, Kisielow J, Kopf M. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest. 2012;122:3965–3976. doi: 10.1172/JCI63451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne JE, Garka KE, Renshaw BR, Virca GD, Sims JE. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J Biol Chem. 2004;279:13677–13688. doi: 10.1074/jbc.M400117200. [DOI] [PubMed] [Google Scholar]
- Vigne S, Palmer G, Lamacchia C, Martin P, Talabot-Ayer D, Rodriguez E, Ronchi F, Sallusto F, Dinh H, Sims JE, et al. IL-36R ligands are potent regulators of dendritic and T cells. Blood. 2011;118:5813–5823. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- Villarino AV, Laurence A. IL-1 watches the watchmen. Nat Immunol. 2015;16:226–227. doi: 10.1038/ni.3105. [DOI] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Yang D, de la Rosa G, Tewary P, Oppenheim JJ. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30:531–537. doi: 10.1016/j.it.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.