Epidemiology
Systemic sclerosis (SSc, scleroderma) is a connective tissue disease characterized by vasculopathy, fibrosis, and immune dysfunction with a prevalence varying from 30 to 443 per million population (1). SSc classification criteria (2) do not incorporate the gastrointestinal tract (GIT) manifestations that are often present in this disease, despite the fact that GIT involvement produces substantial morbidity and is the most commonly involved internal organ in SSc (3). The GIT is the presenting disease feature in 10% of SSc, occurs during disease course in up to 95% of individuals, and is responsible for 6–12% of mortality in SSc patients (4). Malabsorption, gastroesophageal reflux, nausea, vomiting, diarrhea, and constipation are some of the GIT complications that occur in this population, and despite varying degrees of disease severity from mouth to anus, SSc GIT involvement significantly impairs quality of life in almost all patients (5, 6). Severe GIT involvement in up to 8% of SSc patients is associated with a high morbidity and poorer outcome (7, 8).
Pathogenesis and Pathophysiology
The specific pathogenesis of GIT involvement is complex and not adequately understood, but neuropathy progressing to myopathy with eventual fibrosis has been proposed (8). The pathophysiology of GIT involvement is thought to parallel other organ involvement in SSc with fibro-proliferative vascular lesions of small arteries and arterioles, increased production of various pro-fibrotic growth factors, and alterations of innate, humoral and cellular immunity (9, 10). While the role of immune dysfunction has not been adequately characterized, environmental factors may trigger the initial endothelial cell injury, which results in release of reactive oxygen species, chemokines, and cytokines that activate and recruit chronic inflammatory cells, including T- and B-lymphocytes and macrophages (8).
Animal models for SSc described in the literature demonstrate that there are a number of induced and spontaneous systems mimicking certain inflammatory, immunologic, or fibrotic aspects of the disease, which provide contexts in which to study various aspects of this complex disorder (11). However, the most extensive GIT work has been done in the transgenic (TG) mouse strain TβRIIΔk-fib, which is characterized by ligand-dependent up-regulation of transforming growth factor-β (TGF-β) signaling. Quantitative polymerase chain reaction results of TG GIT fibroblasts showed evidence of up-regulated collagen transcription and non-canonical TGF-β signaling pathways (12).
The concept of GIT cell-mediated immunity in SSc is supported by biopsy specimens which demonstrate an increase in endothelial/lymphocyte activation leading to a pronounced increase in the CD4+/CD8+ ratio, and Type 2 helper (Th2) polarization (13). The classic Th2 cytokines interleukin (IL)-4 and IL-13 are not only pro-fibrotic, but upregulate humoral immunity by inducing immunoglobulin production (14). Of interest, immunoglobulins isolated from the serum of SSc patients interfere with cholinergic-mediated contraction of the GIT, a phenomenon which is most intense early in the disease and more extensive later in the disease, when both smooth muscle and myenteric neurons are involved (15–17). These circulating anti-muscarinic 3 receptor (M3-R) autoantibodies block cholinergic neurotransmission by inhibition of acetylcholine release and thus, the ability of the smooth muscle in the GIT to respond to stimuli. As fibroblasts become activated into myofibroblasts by transforming growth factor-β (TGF-β), excess collagen is produced, which causes structural damage and also impaired motility. The result of these processes is a dysfunctional GIT, which contributes to Barrett’s esophagus, gastroparesis, malabsorption, and fecal incontinence.
Anatomic Distribution of Involvement
Oral Cavity
Oral involvement in SSc may include perioral fibrosis, sublingual frenulum thickening, or secondary Sjogren’s syndrome, all of which can predispose patients to malnutrition due to reduction of oral aperture and intake (18, 19). Dental changes due to bone reabsorption may affect mastication and result in tooth loss (20).
Esophagus
In SSc patients, the esophagus is the most commonly affected organ of the GIT, occurring in up to 90% of patients and resulting in symptoms of heartburn, regurgitation and dysphagia (21). However, up to 30% of SSc patients may have asymptomatic esophageal involvement; thus, establishing a diagnosis of GIT involvement in an SSc patient (particularly early in the disease course) may present a challenge for the physician (22). Decreased peristalsis in the lower two-thirds of the esophagus with associated reduction of lower esophageal sphincter tone is classically defined as a patulous esophagus on imaging in SSc patients. Esophageal dysmotility is more severe in SSc patients with a longer disease duration and is associated with interstitial lung disease (ILD) due to micro-aspiration (23, 24). This latter association is particularly important to note, as chronic cough and asthma may be attributed to gastroesophageal reflux disease (GERD) and warrants assessment. Long-standing GERD is associated with both stricture formation and Barrett’s esophagus, which is a risk factor for esophageal adenocarcinoma (21).
Stomach
Stomach involvement in SSc includes gastric antral vascular ectasia (GAVE) and gastroparesis. Most patients with GAVE present with iron-deficiency anemia, however, GAVE itself may be the presenting SSc disease feature (25). While the pathogenesis of GAVE has been proposed to be similar to that of the immune-mediated development of telangiectases, further studies are needed to understand auto-antibody associations (26). In SSc, gastroparesis is due to autonomic dysfunction in the stomach, which causes impaired gastric compliance and delayed gastric emptying. Up to 50% of SSc patients complain of early satiety, nausea, bloating, and abdominal pain (27).
Small intestine
The second most commonly involved aspect of the GIT is the small intestine. Reduction in gastric acid and hypomotility of the small bowel may result in small intestinal bacterial overgrowth (SIBO), which occurs in up to 50% of SSc patients (28). Other small intestine manifestations including pneumatosis cystoides intestinalis and pseudo-obstruction are also thought to be related to motor impairment due to decreased smooth muscle contractility (29, 30). Jejunal diverticula may occur in areas of muscle atrophy.
Liver
The most common liver disease associated with SSc is primary biliary cirrhosis (PBC), which is also associated with an anti-centromere auto-antibody. The prevalence of PBC in SSc has been reported to be 2–22% and increases when anti-mitochondrial antibodies, MIT3 and gp100 are employed for diagnosis (31). The prognosis of SSc-PBC is better than that of PBC alone, with a slower progression to end stage liver disease (32). Overlap conditions with autoimmune hepatitis, idiopathic portal hypertension, intrahepatic portal hypertension due to nodular regenerative hyperplasia, and primary sclerosing cholangitis have been reported in SSc (33–35).
Colon
Colonic involvement is present in 20–50% of SSc patients (27) and is typically due to a reduction in colonic motility and prolonged transit due to an impaired gastrocolic response (36–38). Severe constipation may result in fecal impaction. Other complications of colonic involvement in SSc may include megacolon, transverse and sigmoid colonic volvulus, telangiectasia, stenosis, as well as “wide mouth” diverticula and stercoral ulceration (39). Intestinal pseudo-obstruction is a clinical syndrome that may complicate SSc; it is characterized by obstructive symptoms in the absence of a mechanical etiology and is thought to be due to impaired colonic propulsion (36).
Anus
Anorectal involvement occurs in 50–70% of SSc patients, with fecal incontinence affecting up to 40% of patients (40). Internal anal sphincter smooth muscle changes due to neuropathy or myopathy with resultant impaired inhibitory response is thought to be the etiology of fecal incontinence in SSc (41–43).
Diagnostic Evaluation
Oral Cavity
Irrespective of sicca symptoms, regular dental care is indicated as mandibular resorption, dental loss, and possible increase in tongue carcinoma have been reported in SSc (20, 44, 45). Assessment of oropharyngeal dysphagia and aspiration risk should be considered in SSc patients as the lower pharynx may be involved in SSc (46, 47).
Esophagus
Esophageal symptoms may include volume reflux, nausea, vomiting, heartburn, and dysphagia. The first line of investigation for esophageal symptoms in SSc is generally esophagogastroduodenoscopy (EGD), which can be diagnostic for esophagitis attributable to etiologies such as eosinophilic or candidiasis as well as (pre-) cancerous changes, and therapeutic for esophageal strictures (21, 48). If stricture formation is suspected, a barium swallow can identify severity. The impact of GERD on symptoms can be assessed by pH monitoring, a procedure often done on anti-reflux medications to assess therapeutic efficacy. The effect of peristalsis on symptoms can be assessed by impedance that can be performed alone or in combination with pH monitoring. Manometry is used to diagnosis motility disorders by measuring pressure profiles in the esophagus. High-resolution esophageal manometry has further enhanced the ability to study motility in much greater detail by providing pressure measurements at more levels along the esophagus (49).
Stomach
Abdominal pain and distention is often treated empirically as SIBO prior to any formal investigation due to the high costs, invasiveness, inconvenience to patients, lack of standardization and sampling error associated with testing. However, if these symptoms fail to respond to antibiotics further investigation should be pursued (21). Diagnostic tests for SIBO include culture of duodenal aspirate during EGD and the hydrogen breath test. If gastroparesis is suspected, a gastric emptying study is indicated prior to initiation of pro-kinetics (48). A SSc patient with an iron deficiency anemia requires an EGD for identification and treatment of GAVE.
Small intestine
Capsule endoscopy may also be used to evaluate the esophagus, small bowel, and colon with possible identification of occult gastrointestinal bleeding from GAVE. Additional testing of small bowel complications in SSc may include qualitative fecal fat if exocrine pancreatic insufficiency is considered or measurement of fat soluble vitamins if malabsorption is suspected (50).
Colon
An abdominal radiograph and/or computer tomography (CT) scan of the abdomen are often obtained to assess for intestinal pseudo-obstruction in patients with severe abdominal pain associated with distention. Stool studies, including testing for Clostridium Difficile, may be indicated for patients with diarrhea. Colonoscopy can identify telangiectasia and is indicated in SSc over the age of 50 for malignancy screening.
Anus
Anorectal manometry can be used to assess fecal incontinence in SSc, however, manometric changes may appear before clinical symptoms appear (51), highlighting the challenge of ordering invasive testing. Magnetic resonance (MR) defecography is a noninvasive test that uses magnetic resonance imaging to obtain images at various stages of defecation to evaluate how well the pelvic muscles are working and provide insight into rectal function. A balloon expulsion test is a procedure which places a fluid-filled balloon into the rectum in order to measure expulsion time and assess whether the rectoanal inhibitory reflex is intact, meaning that the internal anal sphincter demonstrates appropriate transient relaxation in response to rectal distention.
Malnutrition
The risk for malnutrition is reported in the range of 18–56% among SSc populations screened by questionnaire and bioelectrical impedance analysis (52, 53); thus, patients should be screened at diagnosis and annually (54). Unfortunately, traditional markers of nutritional status, including current body mass index and serum albumin do not seem to be good indicators of malnutrition in SSc (55, 56). Nonetheless, once identified, malnutrition should be closely monitored and effectively treated (54).
Physical Exam Findings
The physical exam for assessment of GIT manifestations can be rather nonspecific, however, there are a few notable findings specific to SSc that are worth highlighting. The oral exam can identify patients that will benefit from ancillary services from speech and swallow therapists as well as dentists and orthodontists (18, 19) (Figure 1). Irrespective of the GIT involvement, cutaneous telangiectases may be a clinical biomarker for pulmonary vascular disease, and thus are an important physical exam feature (58).
Figure 1.
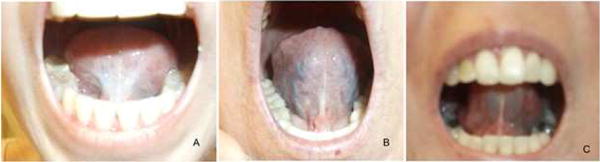
Systemic Sclerosis Oral Physical Exam Findings: (A) Frenulum thickening, (B) Telangiectasia, and (C) Reduced Oral Aperture
An abdominal exam is always important to perform in SSc patients. A low threshold for abdominal imaging is indicated for assessment of pseudo-obstruction (59). Anorectal exam may reveal rectal prolapse and direct appropriate referral.
Management of Gastrointestinal Manifestations
GIT management involves an integrated approach of patient education for lifestyle modification, medical therapies, and ancillary services for nutrition support (21). Patient questionnaires may be used to identify symptoms and assess the social and psychological contribution to symptoms; therefore, they are an important aspect of clinical decision making (60). The Patient-Reported Outcomes Measurement Information System (PROMIS) GIT symptom item bank contains 60 items that capture 8 GI-specific symptom scales, and is available at no cost with minimal respondent burden (61).
Behavioral Considerations and Interventions
Most GIT symptoms in SSc are managed through supportive therapies and symptom control, of which behavioral modification is an important component. For example, sicca syndromes in SSc patients often lead to cavities and ulcerations; thus, adequate hydration and routine dental hygiene are important factors in maintaining oral health (62). Microstomia can be managed through rehabilitation therapies, such as orofacial stretching programs. However, the long term efficacy of adaptive oral hygiene devices and orofacial exercise is unclear. Yuen, et al. explored orofacial exercise programs, which showed a 2.8 mm difference at 3 months, but no difference at 6-months. The lack of effect at 6-months may be related to poor adherence to the program, since it required about 30 min/day of orofacial exercises (63, 64).
Lifestyle modifications are an important aspect of GERD management and include maintaining a healthy weight and avoiding alcohol and tobacco products. Not eating more than three hours before reclining and elevation of the head of the bed while sleeping may also help reduce acid symptoms (65). Dietary modification to identify food intolerance associations with functional GIT symptoms may be helpful (66). In particular, if gastroparesis is present, a low residue diet with frequent small meals can reduce symptoms. Adequate hydration and minimizing constipating medications are an important aspect of constipation management. Increased fiber intake should be used cautiously in patients with concurrent SIBO (48). Behavioral therapies such as pelvic floor exercises may help patients with fecal incontinence.
Patients that have difficulty maintaining normal oral nutrition will benefit from educational support from a dietician. Multivitamin replacement should be guided by laboratory testing (48). Patients with a reduced oral aperture may benefit from education regarding mechanically soft food, as well as consultation with an oral surgeon. Patients with intestinal pseudo-obstruction often require hospitalization for bowel rest, intravenous fluids, and correction of electrolyte abnormalities. Patients with intestinal failure may require total parenteral nutrition (TPN), as post pylori jejunal tube feeding becomes more difficult with small bowel involvement (67).
Pro-motility therapeutics
Motility of the entire GI tract can be affected in SSc. Avoidance of drugs that can impair motility is a first step in management. Drugs such as anticholinergics, opiates, and non-dihydropyridine calcium channel blockers (often used for Raynaud’s management) can be responsible for dysmotility in SSc patients. Thus, an active medication administration review is an important step at all SSc patient visits. Opiate antagonists such as methylnatrexone, alvimopan, and naloxone can be used concurrently to help reverse dysmotility effects of opiates (67). If opiates must be used for pain management, tramadol has fewer effects on motility, and methadone may have a better side effect profile (67).
Metoclopramide is presently the only U.S. Food and Drug Administration (FDA) approved medication for treatment of gastroparesis. However, the FDA requires a boxed warning and risk mitigation strategy for metoclopramide-containing drugs, and warns against chronic use of these products to treat gastrointestinal disorders (68). Metoclopramide is a dopamine receptor antagonist (D2) which also activates 5-hydroxytryptamine (5HT4) receptors for the combined effect of increased peristalsis of the duodenum and jejunum, increased tone and amplitude of gastric contractions, and relaxation of the pyloric sphincter and duodenal bulb, while simultaneously increasing lower esophageal sphincter tone (67). It is a commonly used pro-kinetic, but may lead to significant neurological side effects such as tardive dyskinesia. Domperidone is another D2 antagonist with a better side effect profile related to less penetration through the blood-brain barrier. Its main adverse side effect is hyperprolactinemia. However, domperidone is not as available in the U.S. While the effect of this drug on gastric motility has been studied in diabetic gastroparesis (69), its use in SSc requires further research.
Cisapride is another 5HT-4 agonist which has been studied in reflux esophagitis in SSc, but has since been withdrawn from the market due to concern for cardiac arrhythmias (69). Cisapride increases acetylcholine release from the myenteric plexus and is thought to increase lower esophageal sphincter pressure and gastric emptying through increases in esophageal contracting amplitudes and in the number of gastric contractions (70). Another high affinity 5HT-4, prucalopride, is still being studied for pro-motility for chronic intestinal pseudo-obstruction (67), but is not yet approved by the U.S. Food and Drug administration.
Histamine (H2) blockers reduce acid production but, based on animal studies, may also stimulate gastric motility through an inhibitory effect on acetylcholinesterase which in turn increases cholinergic tone. Ranitidine demonstrates this effect to a greater extent, compared to cimetidine and famotidine. However, the degree to which this phenomenon is operative in humans is unclear. Interestingly, a study of SSc patients revealed that the lower esophageal sphincter pressure was increased significantly by both intravenous cimetidine and famotidine. However, only famotidine caused a significant esophageal pressure rise in patients without an increase of gastric motility. These findings suggest that the inhibition of lower esophageal acetylcholinesterase activity and gastric acid secretion may be involved in the mechanisms of action of cimetidine and famotidine (70).
Buspirone, which is usually prescribed as an anxiolytic, binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Because 5HT1A receptors have been found to have a strong impact on esophageal peristalsis and LES function, buspirone’s ability to augment esophageal motility was studied in a 4-week open label trial of a small group of SSc patients. This medication was found to increase LES pressure and improve GERD symptoms, but not dysphagia or chest pain (68).
Erythromycin in lower doses than those required for antibiotic effectiveness is believed to act as a motilin agonist as well as motilin mimic, thus stimulating gastric peristalsis. Accordingly, erythromycin was found to be beneficial in a case where food stasis resulted in esophagitis (71). However, it appears to have little effect on the small intestine. Moreover, tachyphylaxis can occur with prolonged administration of erythromycin. Newer motilin agonists are in development for gastroparesis (72)
In a case study of three patients with connective tissue disorders and chronic intestinal pseudo-obstruction refractory to metoclopramide, domperidone and cisapride, octreotide and antibiotics were found to be beneficial in improving intestinal motility (73). While stimulation of phase III migrating motor complexes in the intestines by octreotide was the purported mechanism of action, the concurrent treatment of SIBO for improved motility must be considered when approaching functional motility disorders in SSc patients. Similarly, a recent study found that ghrelin, which works through increasing postprandial gastric motility, stimulated increased gastric emptying in ten SSc patients (74). Of note, postprandial satiety was not improved with treatment, suggesting that there may be other factors involved with the patients’ symptoms, which again highlights the importance of co-management of other GIT issues such as SIBO.
SIBO Treatment
Treatment of SIBO involves treating the underlying disease. Dysmotility is thought to be a predisposing factor so treatment as described above may offer some relief. Nutritional modifications can also provide some benefit through elimination of simple sugars and lactose (75). Antibiotics appear to be more effective than placebo for breath test normalization in patients with symptoms attributable to SIBO, and breath test normalization may correlate with clinical response. Specific antibiotic dosing and cycling recommendations are limited by evidence based on weak and heterogeneous study designs, as well as small sample sizes (76). Several broad-spectrum systemic antibiotics such as fluoroquinolones, metronidazole, tetracycline, amoxicillin-clavulanic acid, and chloramphenicol, have been used to manage SIBO, but adverse effects are commonly reported (77, 78). Rifaximin, which is a non-absorbable antibiotic, is effective and safe for the treatment of SIBO. However, it may not be available to certain patients due to its cost and formulary restrictions (77). Of note, probiotics supplementation may effectively decontaminate SIBO and relieve abdominal pain, but has been ineffective in preventing SIBO (79). Furthermore, the association of proton pump inhibitors with SIBO warrants consideration in SSc patients (80).
Fecal incontinence management
Fecal incontinence in SSc may be related to both neurogenic factors, as well as fibrotic weakening of the muscle walls. Consequently, management may include a combination of anti-diarrheal medications and dietary interventions in order to improve stool consistency. As discussed above, consideration of concurrent SIBO is important (78). In small studies of fecal incontinence, sacral stimulation was shown to be beneficial through both temporal (81) and permanent sacral nerve stimulation (82), however, further studies are needed in SSc to define the role of these procedures.
Surgical intervention
Surgical options for fecal incontinence in SSc are often associated with both poor results and complications (81). Endoscopic intervention is necessary for management of stricture, GAVE, and feeding tube placement for nutritional support. Surgical interventions such as venting gastrostomy, gastrectomy, or gastric stimulators have a high risk of associated complications and are not recommended (48).
Liver disease
Liver disease comprises approximately 1.1% of GIT involvement in SSc, which includes autoimmune hepatitis and PBC (83). Important considerations in the management of liver disease include hepatic dosing of medications metabolized by the liver and cautious use of prednisone. In SSc patients, prednisone use increases the risk of scleroderma renal crisis. A case series of five patients with autoimmune hepatitis and SSc patients suggested that prednisone use was not associated with adverse effects (83). It is unclear what conclusions may be drawn from this small sample size, though most would agree that blood pressure education and monitoring is imperative. In patients with concurrent autoimmune hepatitis and PBC, consultation with hepatology is indicated. For patients with PBC, treatment with medications such as ursodeoxyholic acid is indicated for therapeutic relief of clinical symptoms such as itch, as well as cirrhosis prevention.
Adverse Effects of Medications
Medical therapeutics for SSc have several important considerations. Long-term consequences of acid suppression in SSc patients have not been assessed and risks of enteric infection and effects on absorption of vitamins and minerals have not been clarified in this patient population. Adverse drug reactions, such as reflux exacerbation associated with calcium channel blockers and the constipating effect of pain medications, should be considered. Adverse drug reactions related to pro-kinetic agents include development of medication tolerance with prolonged use, and as these agents may prolong the QT interval resulting in serious arrhythmias, a baseline electrocardiogram should be obtained. Of note, while erythromycin can treat gastroparesis through its effects on motilin, it can also decrease small intestinal motility. Immunosuppressive medications, particularly those which target fibrotic cytokines and intravenous immunoglobulin (IVIG), are potentially promising for treatment of SSc and warrant further study (8). However, their effects on the GIT in particular, are largely unknown.
Conclusion
Gastrointestinal tract involvement in SSc is associated with significant morbidity and mortality. An improved understanding of the pathogenesis and treatment of GIT involvement in SSc will require longitudinal, multi-center investigations that incorporate noninvasive testing as well as detailed histopathological studies and identification of biomarkers. Initiation of medical therapeutics in SSc patients requires a step-wise approach that incorporates diagnostic testing, and patient education and nutritional support are imperative in all patients with the diagnosis of SSc.
Table 1.
Systemic Sclerosis GIT involvement and Testing
| Organ | Involvement | Diagnostic Evidence | Citation |
|---|---|---|---|
| Oral Cavity | Peri-oral fibrosis Sicca Oropharyngeal dysphagia |
Perioral tethering and Sublingual frenulum thickening on exam Barium swallow with fluoroscopy Mandibular resorption on radiography |
(18, 19, 46, 47) |
| Esophagus | GERD Esophagitis Stricture Barrett’s esophagus Patulous esophagus |
EGD pH-monitoring Modified Barium Swallow Impedance monitoring Manometry |
(21, 57) |
| Stomach | GAVE Dysmotility |
EGD Gastric Emptying Study |
(36, 48) |
| Small intestine | SIBO Telangiectasia Malabsorption |
Hydrogen breath testing Capsule endoscopy Fecal fat quantification |
(27) |
| Liver | PBC | Anti-Mitochondrial Antibody Liver biopsy |
(8, 31) |
| Colon | Constipation Pseudo-obstruction |
Colonoscopy Abdominal radiograph or CT |
(27) |
| Anus | Fecal Inc ontinence Rectal Prolapse |
Manometry Defecography Balloon expulsion test |
(40) |
Key Points.
The gastrointestinal tract (GIT) is the most commonly involved internal organ in Systemic Sclerosis (SSc).
GIT management involves an integrated approach of patient education for lifestyle modification, medical therapies, and ancillary services for nutrition support.
Medical therapeutics for SSc have several important considerations that require an understanding of potential adverse effects.
Synopsis.
While classification criteria for systemic sclerosis (SSc) do not incorporate the gastrointestinal tract (GIT) manifestations that are often present in this disease, the GIT is the most common internal organ involved. Pathophysiology of GIT involvement is thought to be similar to other organs in SSc with fibro-proliferative vascular lesions of small arteries and arterioles, increased production of pro-fibrotic growth factors, and alterations of innate, humoral and cellular immunity. These processes result in neuropathy progressing to myopathy with eventual fibrosis. As such, proper diagnostics and therapeutics for SSc-GIT involvement require the treating physician to have an understanding of an integrated approach and potential medication adverse effects.
Acknowledgments
Funding source:
This work was supported by awards from the National Institutes of Health (K23AR067889) and the U.S. Department of Veterans Affairs (I01 CX001183). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors have no conflict of interest to disclose.
Contributor Information
Tracy M. Frech, University of Utah and Salt Lake Veterans Affair Medical Center, Department of Internal Medicine, Division of Rheumatology, Salt Lake City, Utah, USA.
Diane Mar, University of Colorado, Department of Internal Medicine, Denver, CO, USA.
References
- 1.Morrisroe K, Frech T, Schniering J, Maurer B, Nikpour M. Systemic sclerosis: The need for structured care. Best Pract Res Clin Rheumatol. 2016;30(1):3–21. doi: 10.1016/j.berh.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 2.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–47. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khanna D, Nagaraja V, Gladue H, Chey W, Pimentel M, Frech T. Measuring response in the gastrointestinal tract in systemic sclerosis. Curr Opin Rheumatol. 2013;25(6):700–6. doi: 10.1097/01.bor.0000434668.32150.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes A, Marie I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):iii36–9. doi: 10.1093/rheumatology/ken485. [DOI] [PubMed] [Google Scholar]
- 5.Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum. 2008;37(4):223–35. doi: 10.1016/j.semarthrit.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Malcarne VL, Hansdottir I, McKinney A, Upchurch R, Greenbergs HL, Henstorf GH, et al. Medical signs and symptoms associated with disability, pain, and psychosocial adjustment in systemic sclerosis. J Rheumatol. 2007;34(2):359–67. [PubMed] [Google Scholar]
- 7.Steen VD, Medsger TA., Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43(11):2437–44. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Singh J, Rattan S, DiMarino AJ, Cohen S, Jimenez SA. Review article: pathogenesis and clinical manifestations of gastrointestinal involvement in systemic sclerosis. Aliment Pharmacol Ther. 2017;45(7):883–98. doi: 10.1111/apt.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140(1):37–50. [PubMed] [Google Scholar]
- 10.Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE. Pathogenesis of Systemic Sclerosis. Front Immunol. 2015;6:272. doi: 10.3389/fimmu.2015.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bocchieri MH, Jimenez SA. Animal models of fibrosis. Rheum Dis Clin North Am. 1990;16(1):153–67. [PubMed] [Google Scholar]
- 12.Thoua NM, Derrett-Smith EC, Khan K, Dooley A, Shi-Wen X, Denton CP. Gut fibrosis with altered colonic contractility in a mouse model of scleroderma. Rheumatology (Oxford) 2012;51(11):1989–98. doi: 10.1093/rheumatology/kes191. [DOI] [PubMed] [Google Scholar]
- 13.Manetti M, Neumann E, Muller A, Schmeiser T, Saar P, Milia AF, et al. Endothelial/lymphocyte activation leads to prominent CD4+ T cell infiltration in the gastric mucosa of patients with systemic sclerosis. Arthritis Rheum. 2008;58(9):2866–73. doi: 10.1002/art.23806. [DOI] [PubMed] [Google Scholar]
- 14.Raja J, Denton CP. Cytokines in the immunopathology of systemic sclerosis. Semin Immunopathol. 2015;37(5):543–57. doi: 10.1007/s00281-015-0511-7. [DOI] [PubMed] [Google Scholar]
- 15.Goldblatt F, Gordon TP, Waterman SA. Antibody-mediated gastrointestinal dysmotility in scleroderma. Gastroenterology. 2002;123(4):1144–50. doi: 10.1053/gast.2002.36057. [DOI] [PubMed] [Google Scholar]
- 16.Singh J, Mehendiratta V, Del Galdo F, Jimenez SA, Cohen S, DiMarino AJ, et al. Immunoglobulins from scleroderma patients inhibit the muscarinic receptor activation in internal anal sphincter smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2009;297(6):G1206–13. doi: 10.1152/ajpgi.00286.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, Singh J, Kedika R, Mendoza F, Jimenez SA, Blomain ES, et al. Role of muscarinic-3 receptor antibody in systemic sclerosis: correlation with disease duration and effects of IVIG. Am J Physiol Gastrointest Liver Physiol. 2016;310(11):G1052–60. doi: 10.1152/ajpgi.00034.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veale BJ, Jablonski RY, Frech TM, Pauling JD. Orofacial manifestations of systemic sclerosis. Br Dent J. 2016;221(6):305–10. doi: 10.1038/sj.bdj.2016.678. [DOI] [PubMed] [Google Scholar]
- 19.Frech TM, Pauling JD, Murtaugh MA, Kendall K, Domsic RT. Sublingual Abnormalities in Systemic Sclerosis. J Clin Rheumatol. 2016;22(1):19–21. doi: 10.1097/RHU.0000000000000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung S, Martin T, Schmittbuhl M, Huck O. The spectrum of orofacial manifestations in systemic sclerosis: a challenging management. Oral Dis. 2017;23(4):424–39. doi: 10.1111/odi.12507. [DOI] [PubMed] [Google Scholar]
- 21.Hansi N, Thoua N, Carulli M, Chakravarty K, Lal S, Smyth A, et al. Consensus best practice pathway of the UK scleroderma study group: gastrointestinal manifestations of systemic sclerosis. Clin Exp Rheumatol. 2014;32(6 Suppl 86):S-214–21. [PubMed] [Google Scholar]
- 22.Thonhofer R, Siegel C, Trummer M, Graninger W. Early endoscopy in systemic sclerosis without gastrointestinal symptoms. Rheumatology International. 2012;32(1):165–8. doi: 10.1007/s00296-010-1595-y. [DOI] [PubMed] [Google Scholar]
- 23.Carlson DA, Crowell MD, Kimmel JN, Patel A, Gyawali CP, Hinchcliff M, et al. Loss of Peristaltic Reserve, Determined by Multiple Rapid Swallows, Is the Most Frequent Esophageal Motility Abnormality in Patients With Systemic Sclerosis. Clin Gastroenterol Hepatol. 2016;14(10):1502–6. doi: 10.1016/j.cgh.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson C, Agrawal R, Lee J, Almagor O, Nelson R, Varga J, et al. Esophageal dilatation and interstitial lung disease in systemic sclerosis: A cross-sectional study. Semin Arthritis Rheum. 2016;46(1):109–14. doi: 10.1016/j.semarthrit.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marie I, Ducrotte P, Antonietti M, Herve S, Levesque H. Watermelon stomach in systemic sclerosis: its incidence and management. Aliment Pharmacol Ther. 2008;28(4):412–21. doi: 10.1111/j.1365-2036.2008.03739.x. [DOI] [PubMed] [Google Scholar]
- 26.Ingraham KM, O’Brien MS, Shenin M, Derk CT, Steen VD. Gastric antral vascular ectasia in systemic sclerosis: demographics and disease predictors. J Rheumatol. 2010;37(3):603–7. doi: 10.3899/jrheum.090600. [DOI] [PubMed] [Google Scholar]
- 27.Sallam H, McNearney TA, Chen JD. Systematic review: pathophysiology and management of gastrointestinal dysmotility in systemic sclerosis (scleroderma) Aliment Pharmacol Ther. 2006;23(6):691–712. doi: 10.1111/j.1365-2036.2006.02804.x. [DOI] [PubMed] [Google Scholar]
- 28.Marie I, Ducrotte P, Denis P, Menard JF, Levesque H. Small intestinal bacterial overgrowth in systemic sclerosis. Rheumatology (Oxford) 2009;48(10):1314–9. doi: 10.1093/rheumatology/kep226. [DOI] [PubMed] [Google Scholar]
- 29.Marie I, Ducrotte P, Denis P, Hellot MF, Levesque H. Outcome of small-bowel motor impairment in systemic sclerosis–a prospective manometric 5-yr follow-up. Rheumatology (Oxford) 2007;46(1):150–3. doi: 10.1093/rheumatology/kel203. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko M, Sasaki S, Teruya S, Ozaki K, Ishimaru K, Terai E, et al. Pneumatosis Cystoides Intestinalis in Patients with Systemic Sclerosis: A Case Report and Review of 39 Japanese Cases. Case Rep Gastrointest Med. 2016;2016:2474515. doi: 10.1155/2016/2474515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assassi S, Fritzler MJ, Arnett FC, Norman GL, Shah KR, Gourh P, et al. Primary biliary cirrhosis (PBC), PBC autoantibodies, and hepatic parameter abnormalities in a large population of systemic sclerosis patients. J Rheumatol. 2009;36(10):2250–6. doi: 10.3899/jrheum.090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rigamonti C, Shand LM, Feudjo M, Bunn CC, Black CM, Denton CP, et al. Clinical features and prognosis of primary biliary cirrhosis associated with systemic sclerosis. Gut. 2006;55(3):388–94. doi: 10.1136/gut.2005.075002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaburaki J, Kuramochi S, Fujii T, Kuwana M, Tojo T, Ikeda Y, et al. Nodular regenerative hyperplasia of the liver in a patient with systemic sclerosis. Clin Rheumatol. 1996;15(6):613–6. doi: 10.1007/BF02238554. [DOI] [PubMed] [Google Scholar]
- 34.Assandri R, Monari M, Montanelli A. Development of systemic sclerosis in patients with autoimmune hepatitis: an emerging overlap syndrome. Gastroenterol Hepatol Bed Bench. 2016;9(3):211–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Skare TL, Nisihara RM, Haider O, Azevedo PM, Utiyama SR. Liver autoantibodies in patients with scleroderma. Clin Rheumatol. 2011;30(1):129–32. doi: 10.1007/s10067-010-1586-0. [DOI] [PubMed] [Google Scholar]
- 36.Ebert EC. Gastric and enteric involvement in progressive systemic sclerosis. J Clin Gastroenterol. 2008;42(1):5–12. doi: 10.1097/MCG.0b013e318042d625. [DOI] [PubMed] [Google Scholar]
- 37.Basilisco G, Barbera R, Vanoli M, Bianchi P. Anorectal dysfunction and delayed colonic transit in patients with progressive systemic sclerosis. Dig Dis Sci. 1993;38(8):1525–9. doi: 10.1007/BF01308615. [DOI] [PubMed] [Google Scholar]
- 38.Fynne L, Worsoe J, Gregersen T, Schlageter V, Laurberg S, Krogh K. Gastrointestinal transit in patients with systemic sclerosis. Scand J Gastroenterol. 2011;46(10):1187–93. doi: 10.3109/00365521.2011.603158. [DOI] [PubMed] [Google Scholar]
- 39.D’Angelo G, Stern HS, Myers E. Rectal prolapse in scleroderma: case report and review of the colonic complications of scleroderma. Can J Surg. 1985;28(1):62–3. [PubMed] [Google Scholar]
- 40.Richard N, Hudson M, Gyger G, Baron M, Sutton E, Khalidi N, et al. Clinical correlates of faecal incontinence in systemic sclerosis: identifying therapeutic avenues. Rheumatology (Oxford) 2017;56(4):581–8. doi: 10.1093/rheumatology/kew441. [DOI] [PubMed] [Google Scholar]
- 41.Thoua NM, Abdel-Halim M, Forbes A, Denton CP, Emmanuel AV. Fecal incontinence in systemic sclerosis is secondary to neuropathy. Am J Gastroenterol. 2012;107(4):597–603. doi: 10.1038/ajg.2011.399. [DOI] [PubMed] [Google Scholar]
- 42.Koh CE, Young CJ, Wright CM, Byrne CM, Young JM. The internal anal sphincter in systemic sclerosis. Dis Colon Rectum. 2009;52(2):315–8. doi: 10.1007/DCR.0b013e31819a5d59. [DOI] [PubMed] [Google Scholar]
- 43.Heyt GJ, Oh MK, Alemzadeh N, Rivera S, Jimenez SA, Rattan S, et al. Impaired rectoanal inhibitory response in scleroderma (systemic sclerosis): an association with fecal incontinence. Dig Dis Sci. 2004;49(6):1040–5. doi: 10.1023/b:ddas.0000034569.85066.69. [DOI] [PubMed] [Google Scholar]
- 44.Auluck A, Pai KM, Shetty C, Shenoi SD. Mandibular resorption in progressive systemic sclerosis: a report of three cases. Dentomaxillofac Radiol. 2005;34(6):384–6. doi: 10.1259/dmfr/14556986. [DOI] [PubMed] [Google Scholar]
- 45.Derk CT, Rasheed M, Spiegel JR, Jimenez SA. Increased incidence of carcinoma of the tongue in patients with systemic sclerosis. J Rheumatol. 2005;32(4):637–41. [PMC free article] [PubMed] [Google Scholar]
- 46.Rajapakse CN, Bancewicz J, Jones CJ, Jayson MI. Pharyngo-oesophageal dysphagia in systemic sclerosis. Ann Rheum Dis. 1981;40(6):612–4. doi: 10.1136/ard.40.6.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajapakse C. Pharyngoesophageal dysphagia: an under recognised, potentially fatal, but very treatable feature of systemic sclerosis. Intern Med J. 2016;46(11):1340–4. doi: 10.1111/imj.13243. [DOI] [PubMed] [Google Scholar]
- 48.Nagaraja V, McMahan ZH, Getzug T, Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015;1(1):82–105. doi: 10.1007/s40674-014-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khanna D, Nagaraja V, Gladue H, Chey W, Pimentel M, Frech T. Measuring response in the gastrointestinal tract in systemic sclerosis. Current Opinion in Rheumatology. 2013;25(6):700–6. doi: 10.1097/01.bor.0000434668.32150.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shawis TN, Chaloner C, Herrick AL, Jayson MI. Pancreatic function in systemic sclerosis. Br J Rheumatol. 1996;35(3):298–9. doi: 10.1093/rheumatology/35.3.298. [DOI] [PubMed] [Google Scholar]
- 51.Fynne L, Worsoe J, Laurberg S, Krogh K. Faecal incontinence in patients with systemic sclerosis: is an impaired internal anal sphincter the only cause? Scand J Rheumatol. 2011;40(6):462–6. doi: 10.3109/03009742.2011.579575. [DOI] [PubMed] [Google Scholar]
- 52.Baron M, Hudson M, Steele R, Canadian Scleroderma Research G Malnutrition is common in systemic sclerosis: results from the Canadian scleroderma research group database. J Rheumatol. 2009;36(12):2737–43. doi: 10.3899/jrheum.090694. [DOI] [PubMed] [Google Scholar]
- 53.Krause L, Becker MO, Brueckner CS, Bellinghausen CJ, Becker C, Schneider U, et al. Nutritional status as marker for disease activity and severity predicting mortality in patients with systemic sclerosis. Ann Rheum Dis. 2010;69(11):1951–7. doi: 10.1136/ard.2009.123273. [DOI] [PubMed] [Google Scholar]
- 54.Baron M, Bernier P, Cote LF, Delegge MH, Falovitch G, Friedman G, et al. Screening and therapy for malnutrition and related gastro-intestinal disorders in systemic sclerosis: recommendations of a North American expert panel. Clin Exp Rheumatol. 2010;28(2 Suppl 58):S42–6. [PubMed] [Google Scholar]
- 55.Murtaugh MA, Frech TM. Nutritional status and gastrointestinal symptoms in systemic sclerosis patients. Clin Nutr. 2013;32(1):130–5. doi: 10.1016/j.clnu.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Baron M, Hudson M, Steele R, Canadian Scleroderma Research G Is serum albumin a marker of malnutrition in chronic disease? The scleroderma paradigm. J Am Coll Nutr. 2010;29(2):144–51. doi: 10.1080/07315724.2010.10719828. [DOI] [PubMed] [Google Scholar]
- 57.Raja J, Ng CT, Sujau I, Chin KF, Sockalingam S. High-resolution oesophageal manometry and 24-hour impedance-pH study in systemic sclerosis patients: association with clinical features, symptoms and severity. Clin Exp Rheumatol. 2016;34(Suppl 100(5)):115–21. [PubMed] [Google Scholar]
- 58.Shah AA, Wigley FM, Hummers LK. Telangiectases in scleroderma: a potential clinical marker of pulmonary arterial hypertension. J Rheumatol. 2010;37(1):98–104. doi: 10.3899/jrheum.090697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valenzuela A, Li S, Becker L, Fernandez-Becker N, Khanna D, Nguyen L, et al. Intestinal pseudo-obstruction in patients with systemic sclerosis: an analysis of the Nationwide Inpatient Sample. Rheumatology (Oxford) 2016;55(4):654–8. doi: 10.1093/rheumatology/kev393. [DOI] [PubMed] [Google Scholar]
- 60.Frech TM. Understanding empirical therapeutics in systemic sclerosis gastrointestinal tract disease. Rheumatology (Oxford) 2017;56(2):176–7. doi: 10.1093/rheumatology/kew322. [DOI] [PubMed] [Google Scholar]
- 61.Spiegel BM, Hays RD, Bolus R, Melmed GY, Chang L, Whitman C, et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109(11):1804–14. doi: 10.1038/ajg.2014.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alantar A, Cabane J, Hachulla E, Princ G, Ginisty D, Hassin M, et al. Recommendations for the care of oral involvement in patients with systemic sclerosis. Arthritis Care Res (Hoboken) 2011;63(8):1126–33. doi: 10.1002/acr.20480. [DOI] [PubMed] [Google Scholar]
- 63.Yuen HK, Marlow NM, Reed SG, Mahoney S, Summerlin LM, Leite R, et al. Effect of orofacial exercises on oral aperture in adults with systemic sclerosis. Disabil Rehabil. 2012;34(1):84–9. doi: 10.3109/09638288.2011.587589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuen HK, Weng Y, Bandyopadhyay D, Reed SG, Leite RS, Silver RM. Effect of a multi-faceted intervention on gingival health among adults with systemic sclerosis. Clin Exp Rheumatol. 2011;29(2 Suppl 65):S26–32. [PMC free article] [PubMed] [Google Scholar]
- 65.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308–28. doi: 10.1038/ajg.2012.444. quiz 29. [DOI] [PubMed] [Google Scholar]
- 66.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146(1):67–75 e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 67.Paine P, McLaughlin J, Lal S. Review article: the assessment and management of chronic severe gastrointestinal dysmotility in adults. Aliment Pharmacol Ther. 2013;38(10):1209–29. doi: 10.1111/apt.12496. [DOI] [PubMed] [Google Scholar]
- 68.Camilleri M. Functional Dyspepsia and Gastroparesis. Dig Dis. 2016;34(5):491–9. doi: 10.1159/000445226. [DOI] [PubMed] [Google Scholar]
- 69.Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6(7):726–33. doi: 10.1016/j.cgh.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 70.Horikoshi T, Matsuzaki T, Sekiguchi T. Effect of H2-receptor antagonists cimetidine and famotidine on interdigestive gastric motor activity and lower esophageal sphincter pressure in progressive systemic sclerosis. Intern Med. 1994;33(7):407–12. doi: 10.2169/internalmedicine.33.407. [DOI] [PubMed] [Google Scholar]
- 71.Hara T, Ogoshi K, Yamamoto S, Kameya T, Kenmochi T, Kise Y, et al. Successful treatment of severe reflux esophagitis with erythromycin in a patient with progressive systemic sclerosis and proximal gastrectomy. Tokai J Exp Clin Med. 2006;31(2):70–2. [PubMed] [Google Scholar]
- 72.Acosta A, Camilleri M. Prokinetics in gastroparesis. Gastroenterol Clin North Am. 2015;44(1):97–111. doi: 10.1016/j.gtc.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 73.Perlemuter G, Cacoub P, Chaussade S, Wechsler B, Couturier D, Piette JC. Octreotide treatment of chronic intestinal pseudoobstruction secondary to connective tissue diseases. Arthritis Rheum. 1999;42(7):1545–9. doi: 10.1002/1529-0131(199907)42:7<1545::AID-ANR30>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 74.Ariyasu H, Iwakura H, Yukawa N, Murayama T, Yokode M, Tada H, et al. Clinical effects of ghrelin on gastrointestinal involvement in patients with systemic sclerosis. Endocr J. 2014;61(7):735–42. doi: 10.1507/endocrj.ej14-0088. [DOI] [PubMed] [Google Scholar]
- 75.Bures J, Cyrany J, Kohoutova D, Forstl M, Rejchrt S, Kvetina J, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16(24):2978–90. doi: 10.3748/wjg.v16.i24.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah SC, Day LW, Somsouk M, Sewell JL. Meta-analysis: antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2013;38(8):925–34. doi: 10.1111/apt.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gatta L, Scarpignato C. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment Pharmacol Ther. 2017;45(5):604–16. doi: 10.1111/apt.13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gyger G, Baron M. Gastrointestinal manifestations of scleroderma: recent progress in evaluation, pathogenesis, and management. Curr Rheumatol Rep. 2012;14(1):22–9. doi: 10.1007/s11926-011-0217-3. [DOI] [PubMed] [Google Scholar]
- 79.Zhong C, Qu C, Wang B, Liang S, Zeng B. Probiotics for Preventing and Treating Small Intestinal Bacterial Overgrowth: A Meta-Analysis and Systematic Review of Current Evidence. J Clin Gastroenterol. 2017;51(4):300–11. doi: 10.1097/MCG.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 80.Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11(5):483–90. doi: 10.1016/j.cgh.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Kenefick NJ, Vaizey CJ, Nicholls RJ, Cohen R, Kamm MA. Sacral nerve stimulation for faecal incontinence due to systemic sclerosis. Gut. 2002;51(6):881–3. doi: 10.1136/gut.51.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malouf AJ, Vaizey CJ, Nicholls RJ, Kamm MA. Permanent sacral nerve stimulation for fecal incontinence. Ann Surg. 2000;232(1):143–8. doi: 10.1097/00000658-200007000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.You BC, Jeong SW, Jang JY, Goo SM, Kim SG, Kim YS, et al. Liver cirrhosis due to autoimmune hepatitis combined with systemic sclerosis. Korean J Gastroenterol. 2012;59(1):48–52. doi: 10.4166/kjg.2012.59.1.48. [DOI] [PubMed] [Google Scholar]


