Abstract
Objective
To describe a study of medical monitoring methods and lessons learned in detecting health outcomes in U.S. plants producing toluene diisocyanate (TDI).
Methods
A multidisciplinary team implemented a medical and environmental monitoring program in three TDI plants.
Results
Of 269 eligible workers, 197 (73%) participated and 42 (21%) met symptom and/or lung function criteria that would trigger evaluation for possible asthma over five years of data collection. Subsequent evaluation was delayed for most, and a web-based data collection system improved timeliness.
Conclusions
Medical monitoring of TDI workers identified workers triggering further assessment per study protocol. Systems and/or personnel to ensure rapid follow-up are needed to highlight when triggering events represent potential cases of asthma needing further evaluation. Implementation of a research protocol requires resources and oversight beyond an occupational health program.
Keywords: Occupational asthma, Toluene diisocyanate, Medical monitoring
Workplace exposures to diisocyanates may have adverse respiratory health effects including the potential for work-related occupational asthma. Occupational asthma (OA) is defined as asthma caused by exposure to an agent specific to a workplace.1 It is a subset of work-related asthma (WRA) which includes pre-existing asthma made worse by workplace exposures (work-exacerbated asthma).1 Diisocyanates are known sensitizers and have long been recognized as capable of inducing asthma.2–7 Toluene diisocyanate (TDI) is a commonly used diisocyanate and a known cause of OA. This highly reactive monomer has a diversity of applications in flexible polyurethane foam, coatings, and elastomers. Workplace exposure to TDI may occur during the manufacturing process or in the production or application of TDI-containing polyurethane products.7 However, evidence from state-based and occupational health surveillance systems in other countries and workplace studies suggests that occurrences of OA due to diisocyanates in general and TDI in particular are declining, despite increases in the total number of workplaces using diisocyanates.8–12 This downward trend in diisocyanate-induced OA is probably related to reduced workplace exposures attributed to improved engineering controls, increased usage of respiratory protection, and/or effective medical surveillance.12
Medical monitoring programs implemented in workplaces producing or using diisocyanates may be successful at detecting isocyanate asthma, reducing the severity of asthma, and improving prognosis when workers are removed early from further exposures.12–14 For example, a medical monitoring program introduced in Ontario, Canada in 1983, likely contributed to a reduction in accepted compensation claims for OA, earlier detection of diisocyanate-induced OA, and a less severe health outcome among those diagnosed.15,16 Companies with medical monitoring programs diagnose OA after symptom onset an average of one year earlier than companies without such programs.15
Medical surveillance programs for occupational asthma ideally involve a multidisciplinary team approach to surveillance comprising medical and nursing professionals, epidemiologists, and industrial hygienists. They have included a pre-placement baseline examination followed by a periodic (usually annual) health encounter of workers having potential relevant exposure, with more frequent health encounters during the first two years of exposure.13 Pre-placement exams and health encounters typically include questionnaire items regarding jobs, exposures, and work-related upper and lower respiratory symptoms;13 lung function measurements, i.e. spirometry;17 and immunological tests as appropriate and feasible to detect a pre-disease state.13,16,17 However, the effectiveness of medical surveillance programs for occupational asthma warrants further evaluation.
This paper reports on lessons learned from the planning and conduct of a complex study to evaluate the effectiveness of a structured monitoring program designed to facilitate rapid standardized clinical evaluation of potential cases of asthma. Three companion papers report on exposure assessment, health outcomes and their relationship to TDI exposures, and lung function of the cohort over time.18–20
Background
In 2003, the National Institute for Occupational Safety and Health (NIOSH), part of the Centers for Disease Control (CDC); member companies of the American Chemistry Council (ACC) Diisocyanates Panel; and the International Chemical Workers Union Council (ICWUC) established a collaborative research and development program to improve the management of health risks associated with occupational exposure to diisocyanates. The objectives of the program were to:
characterize workplace TDI environmental concentrations based on use of standardized industrial hygiene monitoring and assessment procedures;
monitor employee health through questionnaires and spirometry, using approaches that help ensure maximal participation;
investigate potential cases of OA using a standardized medical evaluation process and evaluation of historical exposure experience;
create a registry of OA cases, if any, occurring among workers with potential exposure to TDI in the production environment;
evaluate the effectiveness of the program methods, including the standardized health and environmental monitoring procedures; and
communicate the program findings to study participants, plant management, and the scientific community in a manner consistent with CDC and ACC guidelines for assuring the quality, objectivity, utility, and integrity of the information presented.
As part of the program, all U.S. TDI production plants planned to enroll all employees with potential TDI exposure into a structured medical and environmental monitoring program during 2006 through 2012. Contractors were not included. While all participating plants had on-going medical monitoring programs for TDI-exposed workers, this project aimed to standardize medical monitoring, clinical evaluation, and reporting across the TDI production plants in order to generate consistent data that could be analyzed in aggregate. This paper describes the standardized medical monitoring program, the cohort enrolled, outcomes, program successes and limitations, and approaches implemented to improve data collection and decision-making at the level of the plant occupational health facilities.
The medical surveillance program had three aims:
define and test a practical, science-based model program for medical monitoring of employees working with diisocyanates and evaluate the operating characteristics (sensitivity and specificity) of various components of the program in identifying cases consistent with work-aggravated, work-related, and TDI-induced asthma;
assess the effectiveness of specific methods (e.g., questionnaire, spirometry) and protocol (e.g., frequency, timing) of medical monitoring programs, using monitoring and follow-up data obtained during clinical evaluation of TDI-production employees; and
determine to what extent utilization of a standardized medical monitoring program facilitates early detection of TDI-induced asthma cases and leads to a favorable outcome after cessation of exposure.
METHODS
Study Design and Participants
Representatives of five companies comprising four TDI production plants and two plants using TDI participated in the initial project planning. One of the germinal concepts for this study was to layer data collection on top of each participating company’s medical surveillance and exposure assessment programs. Each participating company and each participating plant had its own medical surveillance program of its potentially exposed TDI workers. These programs pre-dated this project and continued following completion of the data collection phase of this project. While the company medical programs were similar, they were not identical. Much time and effort was expended prior to data collection in developing a detailed study protocol prior to enrolling participants and initiating data collection. This was done so that spirometry measurements and clinical classifications could be treated as equivalent between the different sources and could legitimately be pooled for analysis.
Before the study started, the two plants using TDI and one of the TDI producing plants ceased operation and became ineligible to participate prior to the start of data collection. One of the plants that ceased operation was represented by the ICWUC, who thereafter ceased participation in the study due to its diminished level of direct engagement and interest. A representative of the International Union of Operating Engineers (IUOE) subsequently joined the study oversight group. Ultimately, three U.S. plants (with an estimated eligible workforce of 300) participated between 2006 and 2012, and one of these plants ceased production of TDI in 2010 but continued the medical surveillance through 2012 according to protocol.
Eligible workers, designated by a study coordinator at each site, were those who performed tasks in areas of potential TDI exposure (averaging over 10 hours per week throughout the year) or who performed tasks with a high potential for direct contact with TDI (averaging at least 24 times per year). More than one task with potential TDI exposure could be counted during a single shift. Eligible employees included those who had previously worked with other diisocyanates, or had concurrent exposures to other diisocyanates. Eligible workers had to be enrolled in the plants’ existing medical surveillance program. Contract workers were not eligible, as they were not enrolled in the plants’ existing, on-site medical surveillance programs. Recruitment of eligible workers by occupational health and safety staff occurred during health and safety meetings, medical surveillance program visits, and at the time of any reported exposure or health concern potentially related to TDI. No incentives to participate were offered.
Eligible workers completed a Participant Election Form that included the worker’s acknowledgement of informed consent. Workers who agreed to participate completed their first Periodic Questionnaire (including asthma symptoms). Participants then completed a more detailed Intake Questionnaire and baseline spirometry within six months and subsequent Periodic Questionnaires and spirometry each year thereafter (Figure 1). Those electing not to participate were asked to record whether they had any respiratory symptoms and if they were working with TDI.
Figure 1.
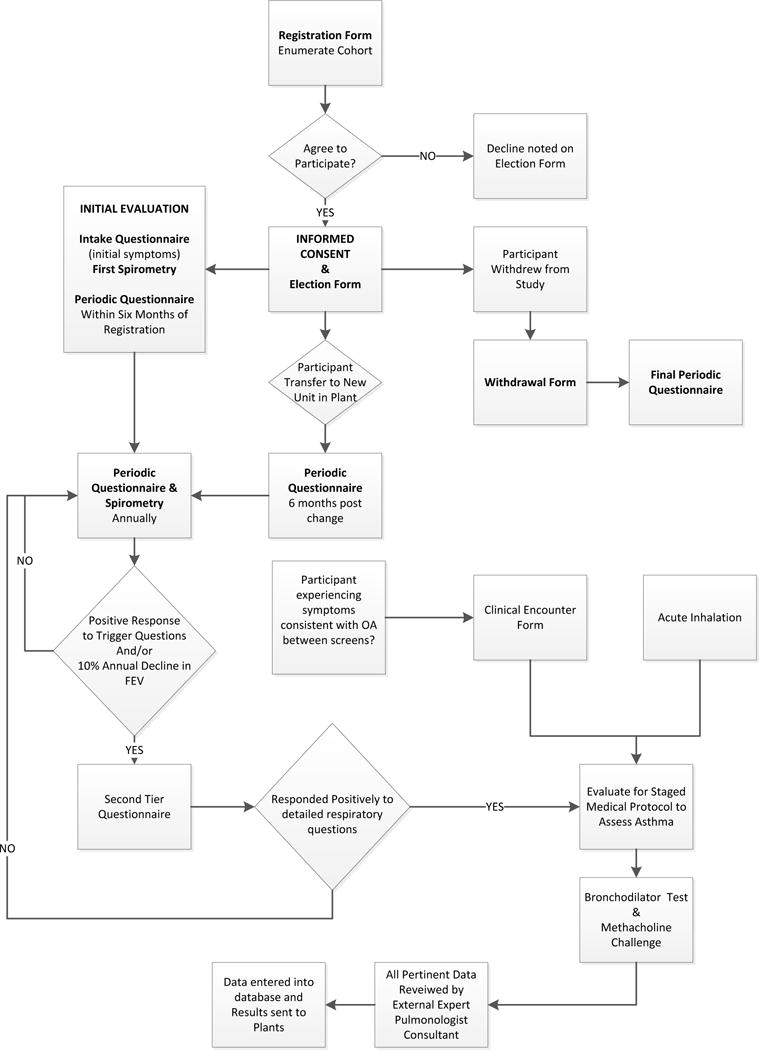
Schematic of cohort enrollment into the study and participation in the medical monitoring program for TDI workers including questionnaires, spirometry, and implementation of medical evaluation.
At any time, participants could withdraw from the study and were asked to complete both a form to indicate the reason for their withdrawal and a final Periodic Questionnaire (Figure 1). Study participants who were transferred out of a job category with potential exposure to TDI to a new unit at the plant were asked to continue in the study. Approval for the study was obtained from both the NIOSH and Dow Chemical Company Institutional Review Boards.
Spirometry
NIOSH personnel visited each participating plant and provided spirometry training to plant occupational health personnel and furnished each plant with a spirometer (SensorMedics dry-rolling seal volume spirometer) and computer software (The Occupational Marketing Inc. (Houston, TX) (OMI) spirometry software) to assure consistency across plants. Spirometry results were sent to NIOSH for quality review before submission to the study database. NIOSH provided regular reports to the plant spirometry technicians regarding test quality.
Referral for Medical Evaluation of Possible Asthma
According to the protocol, participants who reported specific symptoms on the Periodic Questionnaire occurring at least four times within the past 12 months at the time of the questionnaire (wheezing; chest tightness or shortness of breath with wheezing; being awakened by cough, wheeze, or chest tightness; tightness in chest for longer than a minute; or a combination of chills, fever, cough, and muscle aches) or who demonstrated a specified loss of forced expiratory volume in one second (FEV1) (350 ml or 10% decline over one year or less) were to complete a more detailed Second Tier Questionnaire (See Appendix). Additionally, participants who presented with new respiratory symptoms or following a TDI exposure event were also asked to complete the Second Tier Questionnaire. If indicated, based on Second Tier responses, (i.e., TDI was one of the exposure agents, respiratory symptoms were present, and TDI-induced asthma could not be excluded) a worker was to be referred for clinical testing with serial peak flow and FEV1 while at work on normal duties with potential exposure to TDI, while restricted from TDI, and post-shift spirometry with pre- and post-bronchodilator measurements. A methacholine challenge could be added to the clinical evaluation if previous tests were not conclusive. Upon completion of this evaluation, all pertinent records were to be reviewed by a university-based, consulting pulmonologist.
The consulting pulmonologist made a determination for each case to be consistent with asthma, other respiratory disease, or normal respiratory status. If consistent with asthma, then a category was specified:
consistent with non-work-related asthma;
- consistent with work-related asthma
-
○consistent with TDI-induced asthma,
-
○consistent with Irritant-induced asthma/Reactive Airways Dysfunction Syndrome (RADS),
-
○consistent with asthma induced by other agent(s),
-
○indeterminate regarding agent; or
-
○
indeterminate regarding work-relatedness
These classifications reflect a determination based on the study protocol, not clinical diagnoses. Medicolegal liabilities of each participating party were carefully considered during planning. Consequently, a core design concept was to avoid shifting responsibilities between the companies, NIOSH, and the ACC. Each company had its own, separate responsibility to its workers to prevent occupational disease and disability in its own workforce. The protocol was developed with an intent to avoid shifting any of the responsibilities for early detection, diagnosis, and removal from future exposure. The opinions of the occupational pulmonary disease expert supporting the study were shared with the respective plant medical departments. None-the-less, ultimate responsibility for diagnosis and clinical management remained with the employer’s medical staff serving each participating worker. At the conclusion of the study in 2012, follow-up with workers continued through the companies’ medical monitoring protocols.
Data Management
In the original data collection procedures, plants faxed study forms to a vendor external to NIOSH and the participating companies for manual data entry into a database. The vendor created a unique de-identified ID for each participant. The vendor shared periodic reports of surveillance activities with the medical surveillance task group (the plants’ medical directors and NIOSH staff). The group identified several limitations with this process: (1) plants did not consistently fax forms to the vendor in a timely manner; (2) feedback to the data sources was delayed by weeks and was not consistent; (3) participants who should complete a Second Tier Questionnaire were not identified at the time they reported symptoms or other triggering events resulting in untimely evaluation; and (4) the plants were not receiving regular and timely reports of participants who were due for monitoring.
In 2010, a quality control evaluation was conducted. The study team hired a project manager and implemented a real time, HIPAA compliant, Internet-based data collection tool created using the Research Electronic Data Capture (REDCap) system to improve the efficiency, quality, and functionality of the original data collection and management protocol.21 REDCap provided a stream-lined process for rapidly building a database, an intuitive interface for collecting data with data validation, automated export procedures for data downloads, and other advanced features. The potential advantages to updating the system included timely access to data and improved feedback to plant medical personnel regarding next steps required to comply with the study protocol. Participants completed forms using a computer or paper form and the plant nurse entered results directly into REDCap and reviewing medical staff submitted the survey. Real time reports were generated to alert the plant personnel if follow-up was indicated and when participants were due for monitoring. The project manager ran standard automated queries to identify instances where follow-up was indicated and worked with plant personnel to ensure the protocol was followed.
Worker Evaluation Survey
To further evaluate the program, a 10-item Worker Evaluation Questionnaire was mailed to 164 participants in early 2011. The survey items used a Likert scale to rate the barriers and facilitators of study participation and also included a section for open ended comments.
RESULTS
Program Implementation
The three plants registered 269 eligible workers. As shown in Figure 2, 14 (5.2%) did not sign the Election Form required to enroll. Of the 255 who completed an Election Form, 193 initially agreed to participate. Of the 62 who initially declined, eight later consented to participate, yielding a total of 201 participants (74.7%). Of the 201 who elected to participate, 79.6% completed the Intake or a Periodic Questionnaire and spirometry within six months. The initial participation rate, defined as the percentage of workers eligible who completed a questionnaire and spirometry within six months of registration was 59.5% (160/269). An additional 37 workers completed these after six months and were included in the analysis, resulting in 197 participants.
Figure 2.
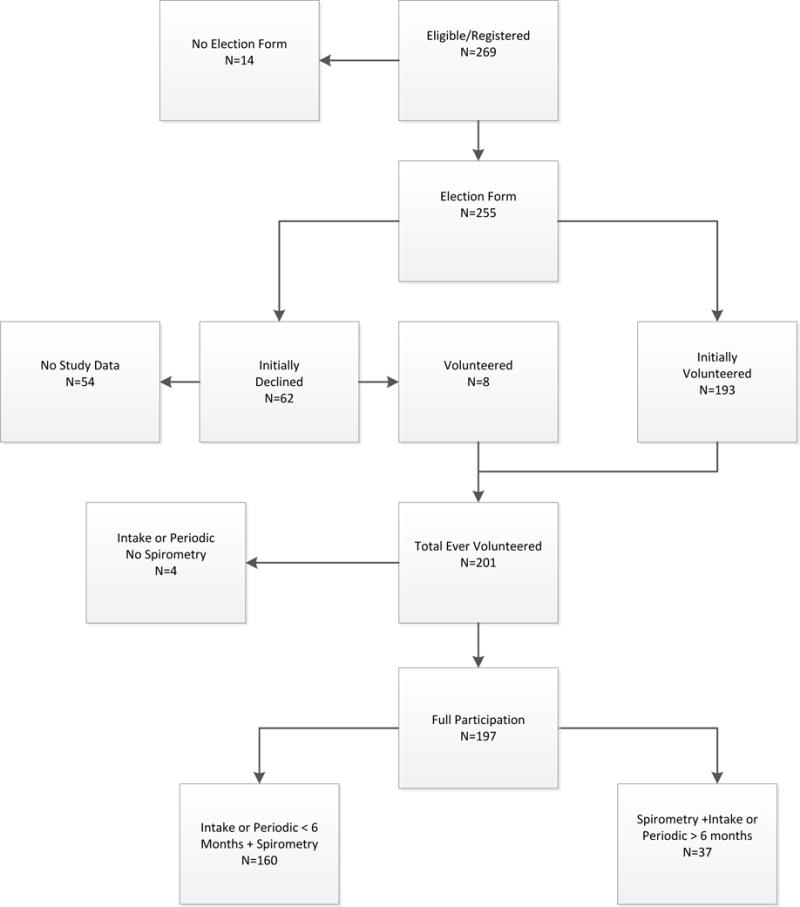
Eligible TDI workers who volunteered to participate, both initially and after data collection began, and who did not volunteer to participate or did not provide data
Table 1 summarizes the level of participation of the cohort, 121 (47.5%) participated until the end of the study, 67 (26.3%) volunteered and then withdrew, eight initially declined and then enrolled and one person volunteered, withdrew and then volunteered again. Of the 67 who withdrew for reasons not specified, 46.5% were terminated, 28.2% were transferred out of the unit, and 19.7% decided not to participate (data not shown). Almost 50% of the 201 who elected to participate completed at least five Periodic Questionnaires.
Table 1.
Participation Status of Cohort of TDI Workers in a Medical Surveillance Program (n = 255)
| Participation Status | N (%) |
|---|---|
| Never | 54 (21.2) |
| Participated until study end | 121 (47.5) |
| Volunteered then withdrew | 67 (26.3) |
| Initially declined then enrolled | 8 (3.1) |
| Volunteered, withdrew, volunteered | 1 (0.4) |
| Volunteered, questionnaire, no spirometry | 4 (1.6) |
TDI, toluene diisocyanate
The demographic characteristics did not vary between the TDI workers who agreed to participate (n = 201) and did not participate (n = 54) (P > 0.05) (Table 2). In both groups, the majority of workers were male (93.5% participants, 87.0% non-participants) and White (67.7% participants, 64.8% non-participants). The majority of participants and non-participants were between 36 and 50 years of age (54.2% and 59.3%, respectively). The mean and standard deviation years of employment at the time of enrollment was 11.8 ± 10.1 for participants and 13 ± 9.9 for non-participants (Table 2). Four non-participants (7.4%) and 32 participants (15.9%) had been employed for less than one year. Ten non-participants (18.5%) were employed for less than two years and 49 participants (24.3%) were employed for less than two years (data not shown).
Table 2.
Demographic Characteristics of TDI Workers who Agreed to Participate (n = 201) and Did Not Participate (n = 54)*
| Characteristic | Participants (n = 201) | Non-Participants (n = 54) |
|---|---|---|
| Gender (%) | ||
| Male | 188 (93.5) | 47 (87.0) |
| Female | 13 (6.5) | 7 (13.0) |
| Race (%) | ||
| African American | 34 (16.9) | 8 (14.8) |
| Caucasian | 136 (67.7) | 35 (64.8) |
| Hispanic | 29 (14.4) | 10 (18.5) |
| Other | 2 (1.0) | 1 (1.9) |
| Age group (%) | ||
| 21–35 Years | 51 (25.4) | 12 (22.2) |
| 36–50 Years | 109 (54.2) | 32 (59.3) |
| 51–65 Years | 41 (20.4) | 10 (18.5) |
| Mean years employed (SD) | 11.8 (10.1) | 13 (9.9) |
P > 0.05 for all comparisons between the Participants and Non-Participants
SD, standard deviation; TDI, toluene diisocyanate
Prior to 2010, 23 participants were eligible for a Second Tier Questionnaire; however, only four completed it the same day as called for in the protocol. The average time until completing the Second Tier was 14.3 months. After implementing REDCap, two participants eligible for a Second Tier Questionnaire completed it on the same day (and did not meet criteria for further evaluation).
During the course of the study, 42 participants were eligible for a Second Tier Questionnaire. Records of the 23 participants identified by questionnaire without timely completion of the Second Tier Questionnaire and the eight participants identified by decline in FEV1 during the 2010 quality review of data, were reviewed by the consulting pulmonologist. During data analysis, 11 additional participants were identified as having a FEV1 decline; however their records were not sent to the consulting pulmonologist because data collection had ended. There was no automated method to identify FEV1 decline in real time during the study. Of the 42 participants who met criteria for further evaluation, no participants completed serial peak flow, one completed spirometry with bronchodilator testing, and another individual completed methacholine testing.
Potential Health Outcomes
Of the 31 workers whose records were sent to the consulting pulmonologist, nine (29%) were determined to have an evaluation consistent with asthma, one was consistent with other non-specified respiratory disease, and 21 were considered to have normal respiratory status as shown in Table 3. These classifications reflect a determination based on the study protocol, not clinical diagnoses. Of the nine with results consistent with asthma, seven (23%) were consistent with TDI-induced asthma, and two were indeterminate regarding work-relatedness. The one who was consistent with other respiratory disease was unspecified. Review of the study records indicated that four participants identified as having a condition consistent with TDI-induced asthma had been transferred from the TDI environment to a different location, two with specific TDI restrictions and two for non-specified reasons. There was no documentation of transfer or restrictions for the other three and they continued in the monitoring program of the study.
Table 3.
Determination by the Consulting Pulmonologist for 31 TDI Workers
| Determination | N (%) |
|---|---|
| Consistent with Asthma | 9 (29%) |
| Consistent with Non-Work-Related | 0 (0%) |
| Consistent with TDI-induced | 7 (78%) |
| Indeterminate Regarding Work-Relatedness | 2 (22%) |
| Consistent with Other Respiratory Disease | 1 (3%) |
| Normal Respiratory Status | 21 (68%) |
TDI, toluene diisocyanate
Of the 54 who never participated, none had reported symptoms at the time they declined and 38 (84.4%) were working with TDI. Of the 67 who withdrew from the study after enrolling, only one reported symptoms at the time of withdrawal and that person was one of the participants restricted from working with TDI.
Effectiveness of Methods to Identify Referrals for Medical Evaluation
Participants met criteria for further evaluation through five triggering events in the protocol. Table 4 shows the criteria met for further evaluation and the results from the consulting pulmonologist for the 31 eligible participants identified as of the study end date. Some participants were identified through more than one mechanism. Of the 31, 10 were identified through report of an acute inhalation event (3 of which were TDI exposures), four through a clinical encounter, seven through the Intake Questionnaire, nine through the Periodic Questionnaire and eight through decline in lung function (Table 4).
Table 4.
Events Prompting Completion of a More Detailed Respiratory Questionnaire for 31 TDI Workers, Overall and by Determination by the Pulmonologista
| Overall (n = 31) | Determination by Pulmonologist
|
|||
|---|---|---|---|---|
| Consistent with Asthma (n = 9) | Other Respiratory Disease (n = 1) | Normal Respiratory Status (n = 21) | ||
| Event | N | N | N | N |
| Acute Inhalation Event | 6 (19.4%) | 1 (11.1%) | 0 (0.0%) | 5 (23.8%) |
| Clinical Encounter | 2 (6.5%) | 2 (22.2%) | 0 (0.0%) | 0 (0.0%) |
| Acute Inhalation and Clinical Encounter | 1 (3.2%) | 1 (11.1%) | 0 (0.0%) | 0 (0.0%) |
| Intake Questionnaire | 4 (12.9) | 2 (22.2%) | 1 (100%) | 1 (4.8%) |
| Intake Questionnaire and Acute Inhalation | 1 (3.2%) | 0 (0.0%) | 0 (0.0%) | 1 (4.8%) |
| Periodic Questionnaire | 5 (16.1%) | 1 (11.1%) | 0 (0.0%) | 4 (19.0%) |
| Periodic Questionnaire and Acute Inhalation | 1 (3.2%) | 1 (11.1%) | 0 (0.0%) | 0 (0.0%) |
| Periodic Questionnaire, Acute Inhalation, and Clinical Encounter | 1 (3.2%) | 0 (0.0%) | 0 (0.0%) | 1 (4.8%) |
| Periodic Questionnaire and Intake Questionnaire | 2 (6.5%) | 0 (0.0%) | 0 (0.0%) | 2 (9.5%) |
| Decline in FEV1 | 8 (25.8%) | 1 (11.1%) | 0 (0.0%) | 7 (33.3%) |
a13 workers had results that were not sent to the pulmonologist, 11 met the criteria for further evaluation by the pulmonologist but were identified after the study ended, and two were prompted to complete a more detailed questionnaire but did not meet criteria for further evaluation by the pulmonologist.
FEV1, forced expiratory volume in one second; TDI, toluene diisocyanate
Five of the participants with findings consistent with TDI-induced asthma met criteria for further evaluation based on events outside of scheduled monitoring. Three reported an acute inhalation event and two others sought care for respiratory symptoms. Of the three with inhalation events, one was with TDI and two were with phosgene. Only two had an indication for further evaluation identified only on a scheduled questionnaire (data not shown).
In this study, spirometry methods and equipment were standardized, and quality assurance was ongoing. The longitudinal design allowed multiple spirograms for each individual during the five year study. The loss of FEV1 in any given year did not lead to the timely evaluation of individual workers for possible asthma as called for in the study protocol due to lack of a mechanism to flag these changes. While it is possible that follow-up did occur without documentation as part of the study, this information is not available to the study investigators. Therefore, the utility of the spirometry performed for early identification of occupational asthma cannot be evaluated.
Experience of Participants
The Worker Evaluation Survey was mailed to 164 participants and 82 (50%) completed it. Those responding selected responses indicating that it was easy to arrange their schedule for medical screenings (93%) and to complete the Periodic Questionnaire (91%). The majority selected the response that they “felt better knowing that the management of their plant was concerned about their health” (90%). Most also reported that the doctor or health professional staffing their plant’s medical unit listened carefully to what they have to say (99%) and answered all of their questions (96%). Eighty-seven percent mildly or strongly agreed that the study spirometry equipment was no different than the routine spirometry equipment used by the plant. Twenty-three percent thought that “participating in the study was too much trouble for what they got out of it” and 18% reported worrying that if they were sent for additional medical testing it would affect their employment status. Overall, 91% reported they got their breathing test results back within 30 days of the test. Overall, 76% reported that they received the results of their exposure monitoring for the study.
DISCUSSION
This paper reports on a longitudinal medical monitoring program offered to all potentially exposed non-contract TDI production workers in the U.S. Representatives from the ACC, NIOSH, engaged labor unions, and all the primary producers of TDI in the U.S. worked together to design a prospective study to investigate the association between TDI exposure and OA. A detailed protocol including TDI exposure assessment, medical monitoring, criteria for identifying workers requiring further medical evaluation, and a structured clinical evaluation was developed. The results presented here focus on implementation of the medical monitoring protocol and initial findings.
A short, self-administered questionnaire and screening spirometry of TDI workers triggered criteria for further testing in 21% over a 5 year period when administered twice annually. Plant medical staff found it challenging to reliably apply the complicated, multistage medical protocol which delayed the further evaluations called for. What, if any, clinical impact resulted is unknown. This experience indicates that if data collected as a part of routine, on-going medical surveillance of potentially exposed workers are to be relied on to assess the safety and healthfulness of a workplace in scientific literature, rigorous methods to assure timely and complete follow-up to document each worker’s occupational health are essential. Even experienced, highly committed occupational health practitioners produce data of limited scientific value for measuring asthma incidence and effectiveness of medical surveillance programs without detailed, timely program management throughout data collection.
The study team faced specific challenges while implementing the standardized protocol across multiple production plants. The study team hypothesized feasibly achieving a 75% participation rate because most eligible employees were already included in their plant’s medical surveillance program. However, recruiting and retaining such a high participation rate proved challenging, and the initial participation rate was 59.5%. Because three plants ceased TDI production before the study began, the eligible study size was reduced to a total of 269 workers from the three remaining U.S. plants eligible to participate. Of that reduced number, 54 never enrolled during the study period. Non-participants were not prompted for their reasons to opt out of the study, but they had similar demographic characteristics and durations of employment as participants and none reported symptoms at the time they declined. Most of the enrolled workers (n = 67) who discontinued participation did so because they were either transferred out of the unit (28.2%) or terminated employment (46.5%). Overall 121 workers remained in the study throughout its duration and completed periodic screening sessions with some workers completing up to eight sessions.
Overall, the study identified 42 workers who were eligible for evaluation with the more detailed questionnaire due to an acute inhalation event, reporting to the clinic with symptoms, responses to symptom questions, or a decline in lung function. However, methods to ensure timely implementation of the protocol for participants identified for further evaluation proved to be essential. Only six participants out of 44 completed the Second Tier Questionnaire according to protocol. Review of Figures 1 and 2 provide a picture of the complexity of the protocol which the study team designed and then attempted to implement. The need for a study coordinator to monitor implementation of the protocol was not recognized until several years into data collection.
Implementation of the web-based data collection system enhanced application of the study protocol process, however, this system was not implemented until the last 18 months of the study. It seems reasonable to hypothesize that data acquisition with such a data collection system from study onset could have improved this study and should be considered in the design of future studies of on-going, medical monitoring programs of production workers.
Because there was a significant delay in completing most Second Tier evaluations, the investigators conservatively sent records of all potential cases identified prior to the protocol revision to the consulting pulmonologist for review (n = 31). A total of 19 workers were identified with FEV1 decline that met criteria for further evaluation, 11 after the study ended.
Despite the lack of implementation of the medical protocol specified in the study to quickly identify asthma developing in this population, 29% of the possible cases reviewed by the pulmonologist were thought to have developed asthma and 23% were thought to have developed isocyanate-induced asthma. This suggests that the criteria for further evaluation had utility. While this study does not provide enough detailed data to evaluate the efficacy of each component of the monitoring system, it does suggest that access to healthcare providers well versed in recognizing occupational asthma is critical for workers potentially exposed to isocyanates, as more than half of the cases presented for evaluation outside of the scheduled monitoring activities.
Monitoring programs that include spirometry should include a mechanism to immediately compare each test to prior tests in order to assess potential declines over time.22 Research studies should include a time after the termination of periodic monitoring to follow-up on participants who trigger an evaluation towards the end of the program.
Maintaining uniformity of procedures and ensuring high quality data collection and management is essential for data aggregation across plants and subsequent data analyses regarding the diagnosis and prevention of TDI-induced asthma. Design of the original data collection, entry, and management procedures focused on assuring confidentiality of the participating workers and their respective plants. One result was that the protocol lacked provisions for immediate, i.e. before the participant left the plant medical unit, feedback to the plant nurses and physicians. Many challenges occurred while collecting and aggregating paper forms via fax from the plants. This meant that notifying plant staff of missing questionnaires, spirograms, or failed escalation of the multistep, medical evaluation process was inefficient.
Upon evaluation of the data collection and management procedures in 2010, the study team hired a project manager and implemented the REDCap data collection system to improve the efficiency, quality, and functionality of the original data collection and management protocol. Advantages of entering data into REDCap included relieving the burden of faxing the forms to the vendors and likely reduced the potential for error resulting from performing manual data entry. The REDCap system included automated quality control checks with skip logic and context sensitivity to streamline data collection. After implementing the REDCap system, the staff in the plant medical units gained more control of the process and were better able to schedule and recall the study participants as called for by the study protocol. This system allowed for timely feedback to the plants, immediate identification of participants who should be entered into the staged medical protocol, and regular and timely reports of participants who were due for screening.
The experience of the study team has implications for both medical monitoring for respiratory disease as well as scientific analysis of the data produced from that monitoring among workers exposed to agents known to cause asthma. Rapid recognition of early signs of possible asthma requires timely clinical follow-up of reported symptoms, reported exposure events, and changes in spirometry measurements. REDCap is a research tool not routinely available in the occupational health practice setting. However, emerging technologies provide opportunities to implement clinical decision support mechanisms which could assist with prompt recognition of “triggers” for further evaluation. Systems exist to provide immediate visual displays of excessive longitudinal lung function decline for individual workers as well as groups of workers (https://www.cdc.gov/niosh/topics/spirometry/spirola.html).
The Worker Evaluation Survey suggests that overall the participants viewed the program positively. The majority who responded felt that the management cared about their health, the health professional listened to their concerns and that participation was not troublesome. There was some stated concern that if they were sent for additional testing it would affect their employment. The survey revealed that workers were not receiving their exposure monitoring results which led to adjustments in communications to remedy this problem.
CONCLUSION
Medical monitoring programs were implemented by all the U.S.-based producers of TDI at the plant locations prior to this study. This study built upon this base of long-standing isocyanate medical surveillance programs to standardize a medical monitoring program across plants for a prospective epidemiological study. Although the majority of the participants meeting the criteria for further evaluation were identified through questionnaires, additional methods in the surveillance program such as spirometry and respiratory exposure events identified additional potential cases.
Repeated breakdowns in follow-up steps called for by the study protocol indicate that timely means to assure evaluation and feedback to personnel involved in data collection is essential to the success of medical monitoring programs among workers. Systems, either automated or manual, are needed to help ensure complete and consistent application of the detailed study protocol requirements to pre-existing, plant-specific medical surveillance programs. Additionally, when conducting research in such settings, a study coordinator is most likely needed to fully utilize these protocol compliance monitoring systems. Without a real-time mechanism to ensure full compliance with the study protocol, conclusions drawn from data generated in the course of on-going medical monitoring programs should be viewed with caution. While data collected in this study does not provide conclusive evidence as to the utility of any one component of the medical surveillance program, the study demonstrates the importance of immediate feedback to occupational health personnel conducting such monitoring to ensure appropriate follow-up.
Acknowledgments
We thank individuals who registered the workers, enrolled them, and those who conducted questionnaire/spirometry. We also thank Janie De Jesus and Jaime Salazar (deceased) from Dow Chemical Company; Donna Barbay, Michelle Anderson, Mary Broussard from BASF Geismar; and Courtney Meyer and Don Molenaar from Covestro LLC (formerly Bayer MaterialScience LLC) Baytown. Kristin Cummings and Cara Halldin of NIOSH provided many substantive comments that were used to improve the paper.
The authors would also like to acknowledge the original study team including Edward L. Petsonk, Sue Englehart, Mei Lin Wang, Paul Middendorf, Brent Doney from NIOSH; Pat Conner, William Robert, Gerald Ott, L. Tanner Martinez from BASF Geismar; Jim Chapman, Don Molenaar, Raffie Sessa, Raj Dharmarajan, Barbara Cummings from Covestro LLC; Jean Kasakevich, James J. Collins, John Cikalo, Jaime Salazar (deceased) from Dow Freeport; Liz McDaniel and Athena Jolly from Huntsman; Emmett Russell from the International Union of Operating Engineers; and Sahar Osman-Sypher from the American Chemistry Council.
This study was funded by the American Chemistry Council Diisocyanates Panel.
Support for this work was obtained from the National Occupational Research Agenda, NIOSH, CDC.
Appendix: Criteria for Second Tier Questionnaire
Items on the Periodic Questionnaire that triggered administration of a Second Tier Questionnaire included having answered positively to experiencing any of the following symptoms at least four times within the past 12 months: (1) Had wheezing or whistling in the chest, apart from when you have a cold?; (2) Had an attack of chest tightness or shortness of breath with wheezing or whistling in the chest?; (3) Been awakened by coughing wheezing or chest tightness?; (4) Felt tightness in your chest for longer than a minute?; and (5) Had an illness with chills, fever, cough and muscle aches?
If a worker answered positively for questions items 1–5 above on the Intake Questionnaire, he or she was eligible for a Second Tier Questionnaire. In addition, participants enrolled in the study could visit the clinic to report symptoms consistent with asthma from a TDI exposure. These symptoms were recorded on the clinical encounter form, and the protocol dictated that further evaluation of eligibility for the staged medical protocol to assess asthma would be implemented. Non-routine exposure episodes were identified through a compilation of Acute Inhalation Worksheets, indicating the occurrence of such exposure incidents and the severity of symptoms associated with those incidents. These workers were also referred for medical evaluation to assess eligibility for the staged medical protocol.
Footnotes
Brent Doney, Laura Kurth, Mei Lin Wang, and Eileen Storey declare no conflicts of interest. Laura Cassidy and Carrie Redlich were consultants to the ACC. Patrick Conner was an employee of BASF, James Collins and Michael Carson were employees of Dow, and Don Molenaar was an employee of Bayer.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- 1.Santos MS, Jung H, Peyrovi J, Lou W, Liss GM, Tarlo SM. Occupational asthma and work-exacerbated asthma: factors associated with time to diagnostic steps. Chest. 2007;131:1768–1775. doi: 10.1378/chest.06-2487. [DOI] [PubMed] [Google Scholar]
- 2.Bello D, Herrick CA, Smith TJ, et al. Skin exposure to isocyanates: reasons for concern. Environ Health Perspect. 2007;115:328–335. doi: 10.1289/ehp.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs S, Valade P. Clinical and experimental study of some cases of poisoning by desmodur T (1-2-4 and 1-2-6 di-isocyanates of toluene) Arch Mal Prof. 1951;12:191–196. [PubMed] [Google Scholar]
- 4.Ott MG, Klees JE, Poche SL. Respiratory health surveillance in a toluene di-isocyanate production unit, 1967–97: clinical observations and lung function analyses. Occup Environ Med. 2000;57:43–52. doi: 10.1136/oem.57.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redlich CA, Karol MH. Diisocyanate asthma: clinical aspects and immunopathogenesis. Int Immunopharmacol. 2002;2:213–224. doi: 10.1016/s1567-5769(01)00174-6. [DOI] [PubMed] [Google Scholar]
- 6.Woodbury JW. Asthmatic syndrome following exposure to tolylene diisocyanate. Ind Med Surg. 1956;25:540–543. [PubMed] [Google Scholar]
- 7.Vandenplas O. Occupational asthma: etiologies and risk factors. Allergy Asthma Immunol Res. 2011;3:157–167. doi: 10.4168/aair.2011.3.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diller WF. Frequency and trends of occupational asthma due to toluene diisocyanate: a critical review. Appl Occup Environ Hyg. 2002;17:872–877. doi: 10.1080/10473220290107075. [DOI] [PubMed] [Google Scholar]
- 9.McDonald JC, Keynes HL, Meredith SK. Reported incidence of occupational asthma in the United Kingdom, 1989-97. Occup Environ Med. 2000;57:823–829. doi: 10.1136/oem.57.12.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meredith S, Nordman H. Occupational asthma: measures of frequency from four countries. Thorax. 1996;51:435–440. doi: 10.1136/thx.51.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malo JL, Zunzunegui MV, L’Archeveque J, Cardinal S, Ghezzo H. Direct costs of occupational asthma due to sensitization in Quebec (1988 to 2002): revisited. Can Respir J. 2011;18:e1–5. doi: 10.1155/2011/329805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ott MG, Diller WF, Jolly AT. Respiratory effects of toluene diisocyanate in the workplace: a discussion of exposure-response relationships. Crit Rev Toxicol. 2003;33:1–59. doi: 10.1080/713611031. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson PJ, Cullinan P, Taylor AJ, Burge PS, Boyle C. Evidence based guidelines for the prevention, identification, and management of occupational asthma. Occup Environ Med. 2005;62:290–299. doi: 10.1136/oem.2004.016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labrecque M, Malo JL, Alaoui KM, Rabhi K. Medical surveillance programme for diisocyanate exposure. Occup Environ Med. 2011;68:302–307. doi: 10.1136/oem.2010.055129. [DOI] [PubMed] [Google Scholar]
- 15.Tarlo SM, Liss GM, Yeung KS. Changes in rates and severity of compensation claims for asthma due to diisocyanates: a possible effect of medical surveillance measures. Occup Environ Med. 2002;59:58–62. doi: 10.1136/oem.59.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarlo SM, Malo JL. Fourth Jack Pepys Workshop on Asthma in the Workplace Participants. An official American Thoracic Society proceedings: work-related asthma and airway diseases. Presentations and discussion from the Fourth Jack Pepys Workshop on Asthma in the Workplace. Ann Am Thorac Soc. 2013;10:S17–24. doi: 10.1513/AnnalsATS.201305-119ST. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein DI, Jolly A. Current diagnostic methods for diisocyanate induced occupational asthma. Am J Ind Med. 1999;36:459–468. doi: 10.1002/(sici)1097-0274(199910)36:4<459::aid-ajim7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Middendorf PJ, Miller W, Feeley T, Doney B. Toluene diisocyanate exposure: exposure assessment and development of cross-facility similar exposure groups among TDI production plants. J Occup Environ Med. doi: 10.1097/JOM.0000000000001117. in press. [DOI] [PubMed] [Google Scholar]
- 19.Collins JJ, Anteau S, Conner PR, et al. The incidence of occupational asthma and exposure to toluene diisocyanate in the US production industry. J Occup Environ Med. doi: 10.1097/JOM.0000000000000890. accepted with revisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang ML, Storey E, Cassidy LD, et al. Longitudianl and cross-sectional analyses of lung function in toluene diisocyanate production workers. J Occup Environ Med. doi: 10.1097/JOM.0000000000001124. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redlich CA, Tarlo SM, Hankinson JL, et al. Official American Thoracic Society technical standards: spirometry in the occupational setting. Am J Respir Crit Care Med. 2014;189:983–993. doi: 10.1164/rccm.201402-0337ST. [DOI] [PubMed] [Google Scholar]


