Abstract
Cancer is the second leading cause of death among American Indians and Alaskan Natives (AIAN); although cancer survival information in this population is limited, particularly among urban AIAN. In this retrospective cohort study, we compared all-cause and prostate, breast, lung, and colorectal cancer-specific mortality among AIAN (n=582) and non-Hispanic Whites (NHW) (n=82,696) enrollees of Kaiser Permanente Northern California (KPNC) diagnosed with primary invasive breast, prostate, lung, or colorectal cancer from 1997–2015. Tumor registry and other electronic health records provided information on sociodemographic, comorbidities, tumor, clinical, and treatment characteristics. Cox regression models were used to estimate adjusted survival curves and hazard ratios (HR) with 95% confidence intervals (CI). AIAN had a significantly higher comorbidity burden compared to NHW (p < 0.05). When adjusting for patient, disease characteristics and Charlson comorbidity scores, all-cause mortality and cancer-specific mortality were significantly higher for AIAN than NHW patients with breast cancer (HR= 1.47, 95% CI: 1.13, 1.92) or with prostate cancer (HR = 1.87, 95% CI: 1.14, 3.06) but not for AIAN patients with lung and colorectal cancer. Despite approximately equal access to preventive services and cancer care in this setting, we found higher mortality for AIAN than NHW with some cancers, and a greater proportion of AIAN cancer patients with multiple comorbid conditions. This study provides severely needed information on the cancer experience of the 71% of American Indians/Alaskan Natives who live in urban areas and access cancer care outside of the Indian Health Services, from which the vast majority of AIAN cancer information comes.
Keywords: AIAN, American Indians, health disparities, cancer survival
INTRODUCTION
American Indians and Alaskan Natives (AIANs) are reported to have the lowest 5-year cancer survival (55.5%) in the United States (US) when compared to all other races/ethnicities (white, black, Hispanic, Asian/Pacific Islander)(1). Cancer is the leading cause of death in AIAN women and the second leading cause of death in AIAN men (2). Despite this, research on the drivers of these poorer cancer outcomes among AIANs remains understudied.
Most information regarding cancer among AIANs is from population-based cancer registries (e.g., the National Cancer Institute’s Surveillance, Epidemiology and End Results [SEER] Program), in which underestimation due to racial misclassification is a significant problem (3–7), even when these data are linked with Indian Health Service (IHS) data (8). Importantly, these data are largely generated by IHS hospitals and clinics located on or adjacent to reservation lands, primarily in rural areas. Moreover, access to IHS facilities can be a challenge for AIANs who do not either live near an IHS facility or qualify for services. Approximately 71% of AIANs live in urban settings and only members of federally-recognized AIAN tribes are eligible for IHS services (9). Taken together, up to 80% of AIANs do not have access to IHS (10,11).
Accordingly, previous studies on the cancer experience of AIANs have been limited by available information. Cancer treatment is not available at all IHS facilities (6) and, outside of IHS, AIANs have the lowest rate of private health insurance (41%) of any racial/ethnic group (10,12). As a consequence, data on cancer diagnoses and survival among AIANs living in urban geographic areas is sparse (6,13). To address the gap in knowledge about cancer survival among urban dwelling AIAN and to extend findings from previous studies, we leveraged electronic medical record data from a large, multi-specialty health care system serving more than 3.9 million people in Northern California. California is home to the largest total number of AIANs compared to any other state, estimated at over 720,000 individuals (14,15). We examined cancer mortality among AIAN members and a comparison cohort of Non-Hispanic white (NHW) members diagnosed over a 20-year period. We hypothesized that, in a setting where AIANs and NHWs have similar access to health care services, the all-cause and cancer-specific mortality would be greater for AIANs than NHWs, although the disparity might be less than in other settings.
MATERIALS AND METHODS
Data Source and Study Population
Data for our study was obtained from Kaiser Permanente Northern California (KPNC), which includes over 3.9 million currently active members. KPNC membership comprises approximately one-third of the population of California’s San Francisco Bay Area and Central Valley. The KPNC tumor registry (KPTR) was used to identify AIAN and NHW health plan members diagnosed with a new primary cancer between January 1, 1997 and December 31, 2015. The KPTR records information on all new primary cancers, except non-melanoma skin cancer, diagnosed among KPNC members. The KPTR provided data on diagnosis, age at diagnosis, tumor size, tumor grade, stage, year and age at diagnosis, and first course cancer treatment (e.g. surgery, radiation, chemotherapy, and hormone). The study population was restricted to individuals who were members of KPNC for at least 12 months prior to cancer diagnosis. Cancer patients were restricted to individuals with a first primary invasive cancer of the breast, prostate, colorectum or lung. Patients with a prior cancer at any site were excluded. The final study population included 83,278 patients (582 AIANs and 82,696 NHWs). This Institutional Review Board of KPNC approved this study. This study was conducted in accordance with the U. S. Common Rule ethical guidelines and informed written consent from patients was waived.
Race/ethnicity
Information on race/ethnicity was obtained from KPNC demographic files. Race/ethnicity is primarily obtained through patient self-report during outpatient visits or hospitalizations. KPNC members are generally comparable to the underlying population in terms of race/ethnicity (16). The predominant American Indian groups in California are Mexican American Indian, Cherokee, Apache, Navajo, and Choctaw (17). We did not have access to information regarding Indian Health Service eligibility or tribal membership.
Covariates
We included covariates with known association with cancer mortality, including sex, age, stage, treatment type, and Charlson score as covariates in our models. The Charlson Comorbidity Index categorizes comorbidities based on adjusted 1-year risk of mortality and resource usage. Each comorbidity is assigned a weighted score, which is summed to provide a total score; a higher total score predicts greater mortality risk. KPNC diagnosis and procedure files were used to identify comorbidities present from within 2 years prior to the date of cancer diagnosis. This information was used to create the Charlson Comorbidity Index. We also examined potential confounding by economic status (based on census tract income). Because income did not materially impact survival or hazard ratio (HR) estimates, we did not include this variable in final models.
Outcomes
Information on mortality, including date and cause of death, was obtained from KPNC death files. These files come from linkage of the KPNC cancer registry (i.e., vital status), KPNC membership, the California State Mortality file, and the Social Security Administration Death Master file. For each of the four cancer sites included, we used the SEER cause-specific death variable to classify deaths due to cancer, based on methods previously described (18). Briefly, cause-specific deaths due to cancer included cancer deaths attributed to the same cancer site, cancer deaths within the general organ system, deaths from site-specific diseases, cancer deaths from other malignant cancers, and a death from AIDS with cancer.
Statistical Analysis
Descriptive statistics were calculated for all participant sociodemographic, tumor, and clinical characteristics. For survival analyses, follow-up time started at cancer diagnosis and ended at death, discontinuation of membership, second cancer diagnosis, or end of the study period (March 31, 2017), whichever came first. In analyses of all-cause mortality, all deaths were considered events. For analyses of cause-specific mortality, only those deaths classified as due to the first primary cancer were included, as described above. Deaths due to other causes were censoring events.
To estimate adjusted survival, we fit Cox proportional hazards regression models. Cox proportional hazards models were run separately for each cancer site (prostate, breast, lung, and colorectal). The final Cox proportional hazards models were adjusted for age, sex, stage, treatment type, and Charlson comorbidity index score. These covariates were chosen a priori. To assess the influence of comorbidities, we also ran adjusted Cox proportional hazards models with all the confounding variables except the Charlson comorbidity index score. We report the HR and corresponding 95% confidence intervals (CIs) comparing survival for AIANs and NHWs. We investigated possible violations of the proportional hazards assumption statistically by adding interactions with time to the model observing the p-values and visually by graphically observing plots of negative log survival against log time. No violations of the assumption were observed.
The a priori significance level was considered an alpha level of 0.05. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Patient characteristics and comorbidity status
Baseline demographic and clinical characteristics of the 83,278 participants (582 AIAN and 82,696 NHW) are shown in Table 1, stratified by race. A higher proportion of AIANs had colorectal cancer (i.e., 23% AIANs vs. 17% NHWs, p < 0.001), were younger (p < 0.001) and had diabetes (20% AIANs vs. 13% NHWs, p < 0.001) than NHWs. The difference in median follow-up time between AIAN and NHW was statistically significant; median follow-up time in AIAN was 3.4 years (interquartile range [IQR]: 1.3, 6.8) compared to 4.3 years (IQR: 1.5, 8.7) in NHWs (Table 1). The two most common comorbidities at diagnosis were chronic obstructive pulmonary disease and diabetes. Compared to NHWs, a slightly greater proportion of AIANs with any cancer had one or more comorbid conditions compared to NHWs (49.5% AIAN vs. 44.5% NHW, p < 0.05).
Table 1.
Demographic and Clinical Characteristics of Study Sample by Race/Ethnicity from Kaiser Permanente Northern California, 1997–2015 (N=83,278)
| Demographic/Clinical Characteristic | American Indian and Alaskan Natives (582) |
Non-Hispanic White (82,696) |
||
|---|---|---|---|---|
| n | % | n | % | |
| Follow-up time, years Median (IQR) | 3.4 | (1.3–6.8) | 4.3 | (1.5–8.7) |
| Age at diagnosis, years | ||||
| < 45 | 41 | 7.0 | 2,942 | 3.6 |
| 45–54 | 88 | 15.1 | 10,385 | 12.6 |
| 55–64 | 197 | 33.9 | 22,593 | 27.3 |
| 65–74 | 162 | 27.8 | 26,269 | 31.8 |
| ≥75 | 94 | 16.2 | 20,507 | 24.8 |
| Sex | ||||
| Female | 310 | 53.3 | 41,192 | 49.8 |
| Male | 272 | 46.7 | 41,504 | 50.2 |
| Common Cancers | ||||
| Prostate | 147 | 25.3 | 26,205 | 31.7 |
| Breast | 179 | 30.8 | 25,548 | 30.9 |
| Lung | 122 | 21.0 | 16,933 | 20.5 |
| Colorectal | 134 | 23.0 | 14,010 | 16.9 |
| Common Comorbidities | ||||
| Chronic obstructive pulmonary disease | 145 | 24.9 | 18,700 | 22.6 |
| Diabetes (all) | 114 | 19.6 | 11,062 | 13.4 |
| Renal disease | 35 | 6.0 | 4,684 | 5.7 |
| Cerebrovascular disease | 33 | 5.7 | 4,711 | 5.7 |
| Congestive heart disease | 32 | 5.5 | 4,623 | 5.6 |
| Charlson Comorbidity Indexa | ||||
| 0 | 294 | 50.5 | 45,880 | 55.5 |
| 1 | 112 | 19.2 | 17,228 | 20.8 |
| 2–5 | 133 | 22.9 | 15,611 | 18.9 |
| 6–18 | 43 | 7.4 | 3,977 | 4.8 |
Within 2-years prior to date of cancer diagnosis
Proportions may not equal 100 due to rounding
Staging and Treatment
The localized cancer stage distributions between AIANs and NHWs were similar across prostate, breast, lung and colorectal cancer (Table 2). AIANs had slightly lower proportions of localized breast cancer (63% AIAN vs. 67% NHW) and nearly the same proportion of localized prostate cancer (81% AIAN vs. 82% NHW). However, AIANs had slightly higher proportion of localized lung and colorectal cancer (18% AIAN vs. 16% NHW; 43% AIAN vs. 40% NHW, respectively). A smaller proportion of AIAN women with breast cancer received hormone therapy treatment (40% AIANs vs. 47% NHWs, p < 0.001). A greater proportion of AIANs with colorectal cancer received radiation therapy treatment (22% AIANs vs. 11% NHWs, p < 0.001). Similarly, a greater proportion of AIAN males with prostate cancer received radiation therapy (34% AIANs vs. 26% NHWs, p < 0.05), although a smaller proportion received definitive surgery (21% AIANs vs. 27% NHWs, p < 0.05).
Table 2.
Cancer Characteristics of Study Sample by Cancer Site and Race/Ethnicity from Kaiser Permanente Northern California, 1997–2015 (N=83,278)
| Patient group | American Indian and Alaskan Natives (582) |
Non-Hispanic White (82,696) |
||
|---|---|---|---|---|
| n | % | n | % | |
| All Cancer patients: | ||||
| AJCC Stage | ||||
| Localized | 310 | 53.3 | 46,852 | 56.7 |
| Regional | 146 | 25.1 | 18,681 | 22.6 |
| Distant | 113 | 19.4 | 15,252 | 18.4 |
| Unknown | 13 | 2.2 | 1,911 | 2.3 |
| Treatment | ||||
| Definitive surgery | 345 | 59.3 | 46,712 | 56.5 |
| Chemotherapy | 194 | 33.3 | 21,623 | 26.2 |
| Radiation therapy | 154 | 26.5 | 21,926 | 26.5 |
| Hormone therapy | 111 | 19.1 | 18,674 | 22.6 |
| Vital Status | ||||
| Deceased | 268 | 46.1 | 38,231 | 46.2 |
| Alive | 314 | 54.0 | 44,465 | 53.8 |
|
| ||||
| Prostate Cancer patients: | ||||
| Follow-up time, years Median (IQR) | 4.8 | (2.4–9.1) | 6.5 | (3.4–10.4) |
| AJCC Stage | ||||
| Localized | 119 | 81.0 | 21,489 | 82.0 |
| Regional | 15 | 10.2 | 2,391 | 9.1 |
| Distant | 7 | 4.8 | 1,389 | 5.3 |
| Unknown | 6 | 4.1 | 936 | 3.6 |
| Treatment | ||||
| Definitive surgery | 31 | 21.1 | 7,005 | 26.7 |
| Chemotherapy | 0 | 0.0 | 100 | 0.4 |
| Radiation therapy | 50 | 34.0 | 6,860 | 26.2 |
| Hormone therapy | 40 | 27.2 | 6,738 | 25.7 |
| Vital Status | ||||
| Deceased | 43 | 29.3 | 8,118 | 31.0 |
| Alive | 104 | 70.8 | 18,087 | 69.0 |
|
| ||||
| Breast Cancer Patients: | ||||
| Follow-up time, years Median (IQR) | 4.5 | (2.4–8.0) | 5.8 | (2.9–10.2) |
| AJCC Stage | ||||
| Localized | 112 | 62.6 | 17,028 | 66.7 |
| Regional | 59 | 33.0 | 7,336 | 28.7 |
| Distant | 7 | 3.9 | 952 | 3.7 |
| Unknown | 1 | 0.6 | 232 | 0.9 |
| Treatment | ||||
| Definitive surgery | 170 | 95.0 | 24,066 | 94.2 |
| Chemotherapy | 75 | 41.9 | 9,749 | 38.2 |
| Radiation therapy | 48 | 26.8 | 9,018 | 35.3 |
| Hormone therapy | 71 | 39.7 | 11,872 | 46.5 |
| Vital Status | ||||
| Deceased | 55 | 30.7 | 7,462 | 29.2 |
| Alive | 124 | 69.3 | 18,086 | 70.8 |
|
| ||||
| Lung Cancer Patients: | ||||
| Follow-up time, years Median (IQR) | 0.8 | (0.3–2.3) | 0.7 | (0.2–1.9) |
| AJCC Stage | ||||
| Localized | 22 | 18.0 | 2,748 | 16.2 |
| Regional | 25 | 20.5 | 3,746 | 22.1 |
| Distant | 75 | 61.5 | 10,049 | 59.4 |
| Unknown | 0 | 0.0 | 390 | 2.3 |
| Treatment | ||||
| Definitive surgery | 33 | 27.1 | 3,595 | 21.2 |
| Chemotherapy | 64 | 52.5 | 6,548 | 38.7 |
| Radiation therapy | 27 | 22.1 | 4,532 | 26.8 |
| Hormone therapy | 0 | 0.0 | 53 | 0.3 |
| Vital Status | ||||
| Deceased | 100 | 82.0 | 14,997 | 88.6 |
| Alive | 22 | 18.0 | 1,936 | 11.4 |
|
| ||||
| Colorectal Cancer Patients: | ||||
| Follow-up time, years Median (IQR) | 3.2 | (1.1–7.4) | 3.3 | (1.2–7.4) |
| AJCC Stage | ||||
| Localized | 57 | 42.5 | 5,587 | 39.9 |
| Regional | 47 | 35.1 | 5,208 | 37.2 |
| Distant | 24 | 17.9 | 2,862 | 20.4 |
| Unknown | 6 | 4.5 | 353 | 2.5 |
| Treatment | ||||
| Definitive surgery | 111 | 82.8 | 12,046 | 86.0 |
| Chemotherapy | 55 | 41.0 | 5,226 | 37.3 |
| Radiation therapy | 29 | 21.6 | 1,516 | 10.8 |
| Hormone therapy | 0 | 0.0 | 11 | 0.1 |
| Vital Status | ||||
| Deceased | 70 | 52.2 | 7,654 | 54.6 |
| Alive | 64 | 47.8 | 6,356 | 45.4 |
Proportions may not equal 100 due to rounding
All-cause and Cancer-specific Mortality
Results of the Cox proportional hazards analyses for each cancer site are given in Table 3. Among patients with breast and prostate cancer, all-cause and cancer-specific mortality rates were higher for AIAN compared to NHW patients. HRs were not attenuated when further adjusting for comorbidity. AIAN men had nearly twice the prostate cancer-specific mortality risk compared to NHW men (HR = 1.87; 95% CI: 1.14, 3.06). Similarly, breast cancer all-cause mortality risk for AIAN women was 47% greater than for NHW women (95% CI: 1.13, 1.92). In contrast, AIAN patients with lung cancer or colorectal cancer were not at elevated risk of all-cause or cancer-specific mortality compared to NHW patients; the test of the adjusted HR representing differences in survival among AIAN as compared to NHW was not statistically significant. Additional adjustment for income had little to no effect on HR estimates. For example, after adjusting for patient factors, disease characteristics, and comorbidity the HR for breast-cancer specific mortality was 1.31 (95% CI: 0.88, 1.94); it was 1.29 (95% CI: 0.87, 1.91) after additional adjustment for income.
Table 3.
Hazard Ratio of all-cause mortality and cancer-specific mortality among AIAN patients compared to NHW patients with prostate, breast, lung or colorectal cancer during years 1997 – 2015 (N=83,278)
| Patient group | Adjusting for patient1 + disease characteristics2 | Adjusting for patient + disease characteristics + Charlson Score | ||
|---|---|---|---|---|
|
| ||||
| HR | (95% CI) | HR | (95% CI) | |
|
|
||||
| Prostate cancer patients3 | ||||
| All-cause mortality | 1.36 | (1.01, 1.84) | 1.30 | (0.96, 1.76) |
| Prostate cancer-specific mortality | 1.97 | (1.20, 3.22) | 1.87 | (1.14, 3.06) |
|
| ||||
| Breast cancer patients4 | ||||
| All-cause mortality | 1.52 | (1.17, 1.99) | 1.47 | (1.13, 1.92) |
| Breast cancer-specific mortality | 1.31 | (0.89, 1.95) | 1.31 | (0.88, 1.94) |
|
| ||||
| Lung cancer patients5 | ||||
| All-cause mortality | 0.91 | (0.75, 1.11) | 0.87 | (0.71, 1.06) |
| Lung cancer-specific mortality | 0.89 | (0.72, 1.11) | 0.85 | (0.69, 1.05) |
|
| ||||
| Colorectal cancer patients6 | ||||
| All-cause mortality | 1.08 | (0.85, 1.37) | 1.07 | (0.85, 1.36) |
| CRC cancer-specific mortality | 0.96 | (0.70, 1.31) | 0.96 | (0.70, 1.31) |
Abbreviations: AIAN, American Indian and Alaskan Natives; NHW, non-Hispanic White; HR, hazard ratio; CI, confidence interval; CRC, colorectal cancer.
Adjusting for age and sex.
Adjusting for stage, and treatment.
NHW prostate cancer patients (n=26,205), AIAN prostate cancer patients (n=147).
NHW breast cancer patients (n=25,548), AIAN breast cancer patients (n=179).
NHW lung cancer patients (n=16,933), AIAN lung cancer patients (n=122).
NHW colorectal cancer patients (n=14,010), AIAN colorectal cancer patients (n=134).
The adjusted survival curves for all-cause and cancer-specific mortality for AIANs and NHWs are shown in Figure 1. These adjusted survival curves were generated based on the Cox proportional hazards regression models, assuming covariate values age 65, female (except for prostate cancer), localized stage, surgery treatment, no chemotherapy, radiation or hormone treatment, no comorbidities, and high SES. Among breast cancer and prostate cancer patients, for whom we found statistically significant differences in survival for AIAN compared to NHW patients, we see a separation of the adjusted survival curves for AIAN compared to NHW patients (Fig. 1A and Fig. 1B, respectively). Among lung cancer and colorectal cancer patients, for whom we found no statistically significant differences in survival for AIAN compared to NHW patients, we see no clear separation of the adjusted survival curves for AIAN compared to NHW patients (Fig. 1C and Fig. 1D, respectively).
Figure 1.
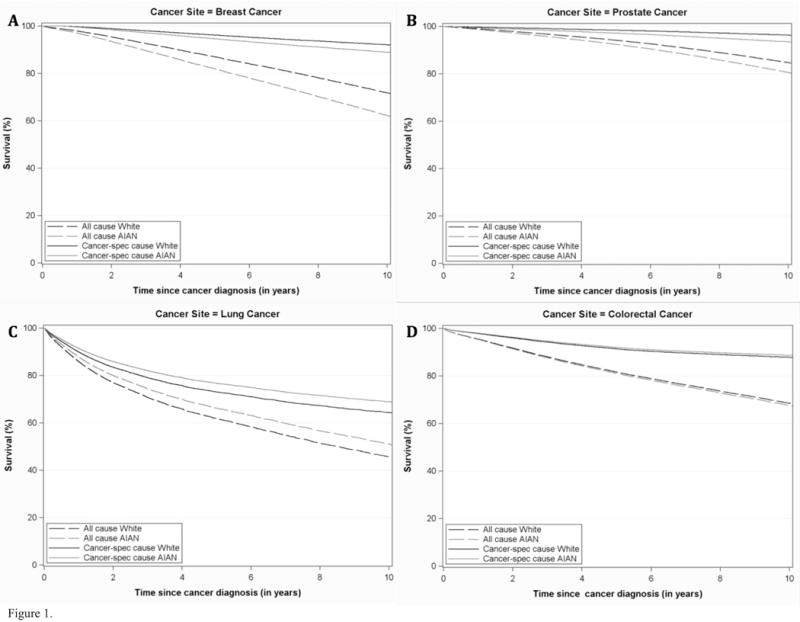
Adjusted plots of cancer-specific survival among AIAN patients compared to NHW patients with (A) breast cancer (n=25,548 for NHW, n=179 for AIAN), (B) prostate cancer (n=26,205 for NHW, n=145 for AIAN), (C) lung cancer (n=16,933 for NHW, n=122 for AIAN) and (D) colorectal cancer (n=14,010 for NHW, n=134 for AIAN) during years 1997 – 2015 (N=83,278). Differences between curves were tested by log-rank test with p-value (not shown), survival time.
DISCUSSION
In our study of 582 AIANs with cancer in an integrated health care system, where enrolled patients have approximately equal access to cancer care services, we found that prostate all-cause and cancer-specific survival and breast cancer all-cause survival among AIANs were significantly lower compared to NHWs. Specifically, after adjustment for disease characteristics and comorbidities, prostate cancer-specific mortality among AIAN men was 87% higher than among NHWs and the all-cause breast cancer mortality among AIAN women was 47% higher than among NHWs.
Our findings of higher mortality in AIANs compared to NHWs are consistent with previous studies conducted among AIANs using national population-based registries, including SEER, CHSDA and IHS (1,8,13,19–22). However, prostate and breast cancer-specific mortality, among AIANs compared to NHWs were higher than previous studies (19,22,23). Further, in our study, the lung and colorectal cancer mortality estimates were lower than 5-year adjusted risk of death estimates reported by Jemal et al. (22). Such differences between findings may be due to our study population all having insurance coverage or the fact that we are comparing our estimates to deaths rates of AIANs restricted to non-urban Contract Health Service Delivery Area counties, which include or are adjacent to tribal lands. Additionally, our results yield more stable estimates compared to 5-year estimates of other studies given our study has greater person-time to detect a difference. Yet, these data are considered to be the most reliable data to date on AIAN people, since it is based on linkages between IHS patient files and the National Death Index (which reduces AIAN racial misclassification), are not directly comparable. Importantly, then, our study of urban residing AIANs provides novel insight on cancer mortality outcomes and extends prior studies that are limited to defined geographic regions.
While there are several factors that may contribute to the cancer survival disparities observed in our study, poverty is three times higher among AIANs compared to NHWs and may lead to poorer access to healthcare, particularly among those who reside in rural settings (13). However, even urban-dwelling AIANs are two times as likely as the general population to be poor, unemployed and without a college degree (24). These factors have been shown to be associated with late-stage cancer diagnosis and lack of guideline-consistent screening behaviors among AIANs, which can contribute to poorer survival (25–27).
Our findings that AIANs with any cancer have a greater proportion of comorbid conditions compared to NHWs are consistent with previous reports using national data sources (28–30) and builds upon these previous reports by using data from an integrated delivery system. We expected that the differences in comorbid conditions between AIANs and NHWs might have explained some of the disparity in cancer survival between AIANs and NHWs. However, despite adjusting for key patient and clinical variables, the disparity between AIANs and NHWs was not attenuated. Similarly, cancer stage at diagnosis is considered an underlying factor in racial differences in cancer survival (31–33). However, despite greatly similar localized prostate and breast cancer stage distribution between AIANs and NHWs, disparity in all-cause and prostate and breast cancer-specific survival among AIANs persisted.
The findings reported in our study are subject to certain limitations. First, we did not control for racial differences in quality of care, treatment adherence or recommendations, or lifestyle factors that may vary across racial subgroups and influence differences in mortality risk. Second, our study population was comprised entirely of insured members of one integrated health plan in Northern California; this may limit the generalizability of the study. Third, these analyses could be further strengthened by data linkages with central cancer registries in California to further quantify racial misclassification. However, given data suggesting under-reporting of AIANs in mortality records, we believe AIANs are more likely to be misclassified as NHWs, than vice versa (5,6). If true, such misclassification would have a minimal impact on our survival and HR estimates, since only a very small percent of NHWs would actually be AIANs. Fourth, AIAN is presented as an aggregate group, which may limit tribe-specific estimates; knowing the proportion of Native Alaskan or the predominant tribes among the American Indians would be interesting and help give context when comparing to past results. Despite these limitations, our study has several strengths. We included a large number of AIANs using recent data that reflect currently available treatments. Previous analyses have been limited by misclassification of race due to underreporting and small sample size (8). Data were collected retrospectively, with long-term follow-up. We had comprehensive data, which enabled us to control for several key variables including insurance coverage, a common confounder in health outcomes research. All individuals in this study had health insurance, and equal access, in terms of ability to access the same services in this healthcare system, minimizing survival differences due to access to care issues that may be present in other studies that include information on AIAN cancer survival.
This study adds valuable and novel information and it is the first study, to our knowledge, identifying the most commonly diagnosed cancers and describing prostate, breast, lung and colorectal survival among AIANs outside of the Indian Health Service. Despite approximately equal access to health care in the study population, our results show a lower survival for AIANs than NHWs following invasive prostate and breast cancer. These differences persisted even after controlling for variation in income and the presence of comorbid conditions. This highlights the importance of further research into the factors responsible for higher mortality among AIAN populations with cancer beyond basic access to care and SES related-effects. While a focus on health equity as it relates to AIAN cancer mortality that considers historical and contemporary injustices among AIANs is critically important, the results presented here suggest that disparities in mortality may yet persist despite reduction of inequities in health care access and income. In addition to identifying other social-behavioral and cultural factors (e.g., diet, physical activity), cancer researchers should consider potential differences in tumor biology for AIANs, such as the higher prevalence of more aggressive breast cancer subtypes found in African American women (34).
Our findings suggest that in settings where AIAN have less access to health care, these cancer survival disparities may be even greater. Given that 71% of AIANs live in urban settings, research efforts to identify potentially unique drivers of cancer health disparities are warranted to improve outcomes for urban dwelling AIANs within non-IHS health care systems (9). Future studies should utilize big data systems to help address the needs of understudied and underserved populations for greatest public health impact.
Acknowledgments
Funding: While conducting this work, Marc Emerson was an Intramural Research Training Award Fellow in the Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health. This work also was supported by the National Cancer Institute at the National Institutes of Health (R01 CA098838 PI, Habel).
Footnotes
Conflict of interest disclosure statement: The authors declare no potential conflicts of interest.
References
- 1.National Cancer Institute. Cancer Trends Progress Report 2011–2012. NIH, HHS; Bethesda, MD: Mar, 2015. https://progressreport.cancer.gov/after/survival. [Google Scholar]
- 2.Espey DK, Jim MA, Cobb N, Bartholomew M, Becker T, Haverkamp D, et al. Leading causes of death and all-cause mortality in American Indians and Alaska Natives. Am J Public Health. 2014;104(Suppl 3):S303–11. doi: 10.2105/AJPH.2013.301798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiggins CL, Espey DK, Wingo PA, Kaur JS, Wilson RT, Swan J, et al. Cancer among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008;113:1142–52. doi: 10.1002/cncr.23734. [DOI] [PubMed] [Google Scholar]
- 4.Frost F, Taylor V, Fries E. Racial misclassification of Native Americans in a surveillance, epidemiology, and end results cancer registry. J Natl Cancer Inst. 1992;84:957–62. doi: 10.1093/jnci/84.12.957. [DOI] [PubMed] [Google Scholar]
- 5.Jim MA, Arias E, Seneca DS, Hoopes MJ, Jim CC, Johnson NJ, et al. Racial misclassification of American Indians and Alaska Natives by Indian Health Service Contract Health Service Delivery Area. Am J Public Health. 2014;104(Suppl 3):S295–302. doi: 10.2105/AJPH.2014.301933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espey DK, Jim MA, Richards TB, Begay C, Haverkamp D, Roberts D. Methods for improving the quality and completeness of mortality data for American Indians and Alaska Natives. Am J Public Health. 2014;104(Suppl 3):S286–94. doi: 10.2105/AJPH.2013.301716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espey D, Paisano R, Cobb N. Regional patterns and trends in cancer mortality among American Indians and Alaska Natives, 1990–2001. Cancer. 2005;103:1045–53. doi: 10.1002/cncr.20876. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society. Cancer facts and figures 2014. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf Accessed Novemeber 20, 2016.
- 9.U.S. Census Bureau. Census 2010 American Indian and Alaska Native Summary File; Table: PCT2; Urban and rural; Universe Total Population; Population group name: American Indian and Alaska Native alone or in combination with one or more races [Google Scholar]
- 10.Kaiser Family Foundation. Race, ethnicity, and health care issue brief: A profile of American Indian and Alaska Natives and their health coverage 2009. https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7977.pdf Accessed November 20, 2016.
- 11.Kaiser Family Foundation. Health Insurance Coverage and Access to Care Among American Indians and Alaska Natives. 2011 https://kaiserfamilyfoundation.files.wordpress.com/2013/01/health-insurance-coverage-and-access-to-care-among-american-indians-and-alaska-natives.pdf Accessed November 20, 2016.
- 12.Zuckerman S, Haley J, Roubideaux Y, Lillie-Blanton M. Health service access, use, and insurance coverage among American Indians/Alaska Natives and Whites: what role does the Indian Health Service play? Am J Public Health. 2004;94:53–9. doi: 10.2105/ajph.94.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 14.Ogunwole SU. We the People: American Indians and Alaska Natives in the United States Census 2000 Special Reports (CENSR-28) US Census Bureau; 2006. [Google Scholar]
- 15.Norris T, Vines PL, Hoeffel EM. The American Indian and Alaska Native Population: 2010 Census Briefs. US Census Bureau; 2012. [Google Scholar]
- 16.Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH. Kaiser Permanente Medical Care Program. In: Strom BL, editor. Pharmacoepidemiology. Fourth. John Wiley & Sons; 2005. pp. 241–59. [Google Scholar]
- 17.U.S. Census Bureau. American Community Survey 1-Year Estimates; Table: B02014; American Indian and Alaska Native alone for selected tribal groupings; Universe: People who are American Indian and Alaska Native alone and people with no tribe reported 2015 [Google Scholar]
- 18.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102:1584–98. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS. Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. Am J Public Health. 2014;104(Suppl 3):S377–87. doi: 10.2105/AJPH.2013.301673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 21.Plescia M, Henley SJ, Pate A, Underwood JM, Rhodes K. Lung cancer deaths among American Indians and Alaska Natives, 1990–2009. Am J Public Health. 2014;104(Suppl 3):S388–95. doi: 10.2105/AJPH.2013.301609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White A, Richardson LC, Li C, Ekwueme DU, Kaur JS. Breast cancer mortality among American Indian and Alaska Native women, 1990–2009. Am J Public Health. 2014;104(Suppl 3):S432–8. doi: 10.2105/AJPH.2013.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castor ML, Smyser MS, Taualii MM, Park AN, Lawson SA, Forquera RA. A nationwide population-based study identifying health disparities between American Indians/Alaska Natives and the general populations living in select urban counties. Am J Public Health. 2006;96:1478–84. doi: 10.2105/AJPH.2004.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162:1985–93. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 26.Bleed DM, Risser DR, Sperry S, Hellhake D, Helgerson SD. Cancer incidence and survival among American Indians registered for Indian health service care in Montana, 1982–1987. J Natl Cancer Inst. 1992;84:1500–5. doi: 10.1093/jnci/84.19.1500. [DOI] [PubMed] [Google Scholar]
- 27.Frost F, Tollestrup K, Hunt WC, Gilliland F, Key CR, Urbina CE. Breast cancer survival among New Mexico Hispanic, American Indian, and non-Hispanic white women (1973–1992) Cancer Epidemiol Biomarkers Prev. 1996;5:861–6. [PubMed] [Google Scholar]
- 28.Guraya SY. Association of type 2 diabetes mellitus and the risk of colorectal cancer: A meta-analysis and systematic review. World J Gastroenterol. 2015;21:6026–31. doi: 10.3748/wjg.v21.i19.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) Prevalence of diagnosed diabetes among American Indians/Alaskan Natives United States, 1996. MMWR Morbidity and mortality weekly report. 1998;47:901–4. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. US Department of Health and Human Services; 2011. [Google Scholar]
- 31.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. Jama. 2015;313:165–73. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 32.Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012;30:401–5. doi: 10.1200/JCO.2011.37.5527. [DOI] [PubMed] [Google Scholar]
- 33.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 34.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]


