Patients with metabolic syndrome (MetS) and diabetes mellitus (DM) are at increased risk for coronary artery disease (CAD) and heart failure (1). Impaired coronary flow reserve (CFR), the ratio of stress to rest myocardial blood flow, is a marker of coronary microvascular dysfunction (CMD) in the absence of obstructive epicardial CAD and associates with adverse outcomes (2). We sought to test the hypothesis that in subjects without overt obstructive epicardial CAD or left ventricular dysfunction, the presence of impaired CFR, reflecting CMD, would be associated with adverse events across the spectrum of metabolic impairment.
A total of 959 consecutive patients referred for positron emission tomography myocardial perfusion imaging (PET MPI) with global CFR quantitation from 2007–14 at Brigham and Women’s Hospital (Boston, MA) met inclusion criteria. All patients had normal regional myocardial perfusion by visual and semiquantitative analysis and left ventricular ejection fraction (LVEF) ≥40%. Patients with active malignancy, chronic renal impairment (glomerular filtration rate <45 mL/min/1.73 m2), cardiomyopathy, severe valvular disease, known obstructive epicardial CAD, end-stage solid organ disease or prior transplantation were excluded. PET MPI was performed using validated methods (2). A blinded physician assessed for major adverse cardiac events (MACE), a composite of non-fatal myocardial infarction, heart failure admission and death over a median follow up of 5.4 years. Using accepted clinical criteria, patients were grouped by metabolic health as follows: 1) no MetS or DM, 2) MetS without DM, and 3) DM (3,4). Cox proportional hazards (CPH) regression analyses were performed to evaluate the association between baseline variables and MACE. Event-free survival by metabolic group and CFR was plotted using Kaplan-Meier (unadjusted) and CPH survival (adjusted) analyses. The criteria used for metabolic classification were excluded from the analyses to avoid collinearity.
The population was 70% female, reflecting the sex distribution of subjects with normal MPI, with median age 62.1 years. Group 1 had 164 subjects (17.1% of total), group 2 had 439 (45.8%), and group 3 had 356 (37.1%). With worsening metabolic impairment, median body mass index increased (25.4 vs. 30.9 vs. 34.9 kg/m2, p<0.001) and the rate of Caucasian ethnicity decreased (71.3% vs. 62.2% vs. 41.3%, p<0.001) in addition to the expected increase in hypertension and hyperglycemia. There was a stepwise increase in the frequency of abnormal CFR (<2.0) with worsening metabolic impairment: 40%, 43%, and 59%. In univariable analysis, CFR (p<0.001), age (p<0.001), male sex (p=0.03), group 3 vs. 1 (p<0.04), LVEF (p<0.001), tobacco use (p=0.02), peripheral artery disease (p=0.001), chronic obstructive pulmonary disease (p<0.001), atrial fibrillation (p<0.001), and creatinine (p=0.02) were associated with MACE. In a multivariable model that included the above variables and ethnicity, CFR (adjusted hazard ratio per unit decrease: 2.03, p<0.001), age (p<0.001), LVEF (p=0.005), peripheral artery disease (p=0.03) and atrial fibrillation (p=0.003) were associated with MACE. Importantly, metabolic grouping was not informative to the survival model. Unadjusted and adjusted survival decreased with abnormal CFR across the three groups (Figure 1A and 1B, respectively).
Figure 1.
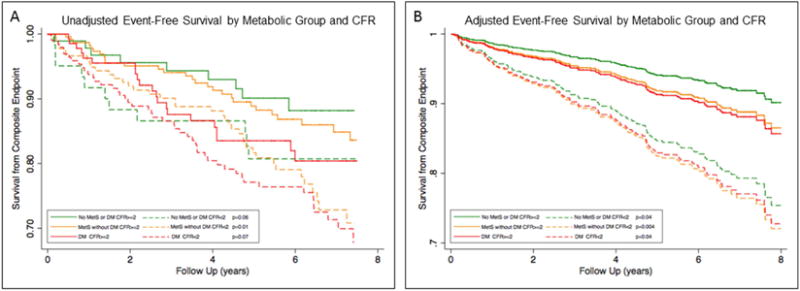
Event-Free Survival by Metabolic Group and CFR. A) Adjusted and B) Unadjusted
This single-center observational study was subject to the inherent limitations of such a design. Although some of the included patients may have had significant epicardial CAD, PET MPI is a sensitive technique for the detection of obstructive CAD (5). Despite including all available subjects meeting inclusion criteria, we lacked power to evaluate the association between CFR and individual endpoints or cause of death.
In patients without overt obstructive epicardial CAD or reduced LVEF, reduced CFR, reflecting CMD, associates with MACE across the spectrum of metabolic disease and provides incremental cardiovascular risk stratification beyond metabolic classification in this population.
Acknowledgments
We would like to thank Josh Klein, Victoria Morgan, Meagan Harrington and the other fellows, residents, faculty and staff of Brigham and Women’s Hospital Nuclear Medicine Department and Cardiovascular Imaging Program for their efforts and contributions to this data and research.
Funding: M.T.O. is supported by the National Institutes of Health (Bethesda, MD) grant T32 HL076136. N.S.B., A.G., and P.E.B. are supported by the National Institutes of Health (Bethesda, MD) grant T32 HL094301 and M.F.D. is supported by the National Institutes of Health (Bethesda, MD) grant R01 HL132021.
M.F.D. receives research grant support from Spectrum Dynamics, and S.D. receives research grant support from Astellas Global Pharma Development for unrelated research endeavors.
Abbreviations
- CAD
Coronary artery disease
- CFR
Coronary flow reserve
- DM
Diabetes mellitus
- LVEF
Left ventricular ejection fraction
- MACE
Major adverse cardiac events
- MetS
Metabolic syndrome
- MPI
Myocardial perfusion imaging
- PAD
Peripheral artery disease
- PET
Positron emission tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The other authors do not have any significant disclosures to report for this research.
References
- 1.Bozkurt B, Aguilar D, Deswal A, et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e535–e578. doi: 10.1161/CIR.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 2.Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–68. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adult. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39:S13–22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 5.Hsiao E, Ali B, Blankstein R, et al. Detection of obstructive coronary artery disease using regadenoson stress and 82Rb PET/CT myocardial perfusion imaging. J Nucl Med. 2013;54:1748–54. doi: 10.2967/jnumed.113.120063. [DOI] [PMC free article] [PubMed] [Google Scholar]


