Abstract
The practice of offering payment to individuals in exchange for their participation in clinical research is widespread and longstanding. Nevertheless, such payment remains the source of substantial debate, in particular about whether or the extent to which offers of payment coerce and/or unduly induce individuals to participate. Yet, the various laws, regulations, and ethical guidelines that govern the conduct of human subjects research offer relatively little in the way of specific guidance regarding what makes a payment offer ethically acceptable—or not. Moreover, there is a lack of definitional agreement regarding what the terms coercion and undue inducement mean in the human subjects research context. It is, therefore, unsurprising that investigators and Institutional Review Boards (IRBs) experience confusion about how to evaluate offers of payment, and lean toward conservative approaches. These trends are exemplified by our pilot data regarding the ways in which some IRB members and investigators (mis)understand the concepts of coercion and undue inducement, as well as the ways in which certain research institutions oversee offers of payment at a local level.
This article systematically examines the legal and ethical dimensions of offering payment to research participants. It argues that many concerns about offers of payment to research participants can be attributed to the misguided view that such offers ought to be treated differently than offers of payment in other contexts, a form of “research exceptionalism.” We show that rejection of research exceptionalism with respect to payment helps settle open debates about both how best to define coercion and undue influence, and how to understand the relation between these concepts and offers of payment. We argue for adoption of our preferred definitions, ideally by regulatory authorities, and against the conventional conservatism toward payment of research participants. Instead, we draw attention to the rarely asked, even radical, question: are research participants paid enough? We conclude by arguing that we ought to change the default to favor, rather than encourage suspicion of, offers of payment to research participants.
Introduction
In the early days of 2016, news broke that six men had been hospitalized—one of whom was pronounced brain-dead—after a “serious accident” in the course of a drug trial conducted in France.1 The men were all participants in a Phase I, or first-in-human, trial of BIA 10-2474,2 a novel compound designed to treat “anxiety and motor disorders associated with Parkinson’s disease, and chronic pain in people with cancer and other conditions.”3 Each participant had been paid €1,900 (about $2,060), “including travel expenses; in return, they agreed to stay at [the testing] facility in Rennes [France] for 2 weeks, swallow a drug on 10 consecutive days, undergo extensive medical tests, and provide at least 40 blood samples.”4 The amount of payment was widely reported in the wake of the tragedy, with the implication that the offer of payment, or the amount of payment, signaled that the trial itself was ethically questionable.
Clearly, something went terribly wrong in France.5 Yet, if we focus on what was known at the time the offer of payment was made, rather than allowing retrospective judgments and suspicions about pecuniary incentives to cloud our ethical evaluations, was it acceptable to offer the research participants €1,900? And if it was not, why not?
Offers of payment made to research participants6 have been described as “one of the more contentious ethical problems” facing institutional review boards (IRBs).7 The U.S. federal regulations and the leading international codes of research ethics require that consent to participation in research be obtained in a manner that minimizes the possibility of coercion and undue influence (a term used interchangeably with undue inducement). Offers of payment made to research participants have been linked to both concepts, and yet the various laws, regulations, and ethical guidelines that govern the conduct of human subjects research offer relatively little in the way of specific guidance about what factors or features render offers of payment ethically acceptable, or not—or even how to define coercion and undue inducement. Therefore, IRBs—the administrative bodies “established to protect the rights and welfare of human research subjects recruited to participate in research activities conducted under the auspices of the institution with which [the IRB is] affiliated”8—and investigators are left largely without a compass to determine whether any particular offer of payment is appropriate.
Given the lack of clear regulatory guidance, one would fully expect the space inhabited by IRBs and investigators to be characterized by confusion and a general trend toward conservative approaches to offers of payment—better to be safe than sorry in the midst of uncertainty. To the extent that IRBs and investigators are identifying legitimate ethical concerns about payment, such conservatism is appropriately protective of research participants. On the other hand, if ethical concerns about payment are overestimated (or simply wrong), the limits that follow from a conservative approach are not only unnecessary to protect research participants, but could actually be ethically inappropriate to the extent that they prevent research participants from receiving offers of payment that would fairly compensate them for the risks and burdens of their participation. Unnecessarily conservative approaches to payment might also hinder trial recruitment,9 thereby delaying scientific and medical progress and/or unethically exposing research participants to risks and burdens that cannot be justified by their scientific value if studies fail to complete.10 Moreover, such conservative approaches might result in an unfair distribution of the burdens and/or benefits11 of research participation over the broader population, by failing to attract a more diverse group of participants. All of this is to say that there are potential practical and ethical costs to the confusion experienced by IRBs and investigators, and the “better safe than sorry” approach is not necessarily safer at all.
This article systematically examines the legal and ethical dimensions of offering payment to research participants. It argues that many concerns about offers of payment in this context are attributable to misguided “research exceptionalism”—simply put, the idea that research is meaningfully different from other contexts in which individuals assume risk. As we show, the rejection of research exceptionalism with respect to payment helps settle open debates within the research ethics community about both how best to define coercion and undue inducement and how to understand their relation to offers of payment. Recognition that research exceptionalism is problematic, coupled with the adoption of our preferred definitions of coercion and undue inducement, should help resolve the confusion exhibited by IRBs and investigators with regard to offers of payment for research participation. Moreover, it should allow IRBs and investigators—two groups that have traditionally focused on whether offers of payment are too high—to focus on the more ethically salient question: are research participants being paid enough? We think the answer to that question is often “No.”
The article proceeds as follows: Part I provides background on why payment is sometimes considered ethically problematic, and reviews the existing literature on offers of payment made to research participants. Such offers are a pervasive feature of research involving both “healthy volunteers” and “patient volunteers,” individuals who have the disease or condition under study. Moreover, offers of payment span the spectrum of studies from those that pose minimal risk to participants to those that are far riskier and more burdensome. The relative frequency with which payment is offered means that investigators who design payment schedules and the IRBs that review those payment schedules routinely confront questions about the ethical acceptability of payment.
Part II surveys regulations and guidelines on the ethics of biomedical research at two levels: national and international. First, we briefly describe the U.S. federal regulations and relevant guidance documents governing human subjects research from both the Office of Human Research Protections (OHRP) within the Department of Health and Human Services (HHS) and the Food and Drug Administration (FDA). Next, we examine international guidelines, which are highly influential and may be formally (or even legally) applicable, depending on where research is conducted. Treatment of payment within these regulations and guidelines is highly uneven: some fail altogether to address offers of payment, while others address the purpose, amount, mechanism, and timing of offers of payment, albeit in a fairly high-level way. As a result, IRBs and investigators bear significant responsibility both for determining what the terms coercion and undue influence mean in the context of offers of payment and for correctly identifying and addressing those ethical concerns when they see them. While we concede that discretion will always be needed to determine whether coercion and undue inducement are present in particular circumstances, the lack of clear definitions and guidance can lead to unnecessary confusion and conservative approaches.
In Part III, we consider a potential explanation for the debate surrounding offers of payment to research participants: research exceptionalism. Research exceptionalism is the view that biomedical research is meaningfully different from other contexts in which individuals assume risk. Although many individuals implicitly endorse the idea that research is different, we suggest that nine common justifications for research exceptionalism ultimately fail, at least when it comes to offers of payment. Though we favor robust regulatory protections for participants in human subjects research, we maintain that common arguments for research exceptionalism do not identify characteristics of research that can justify regulating offers to payment to research participants more heavily than offers of payment made in other areas.
Part IV explores the considerable academic discussion related to coercion and undue inducement in the context of research ethics generally and in relation to payment specifically. No clear consensus has materialized regarding what these concepts mean, but we review the dominant themes and arguments that have emerged. We argue for our preferred definitions of coercion and undue inducement and show that some definitions necessarily fail with the rejection of research exceptionalism.
To demonstrate how the regulatory underdevelopment and conceptual confusion play out in practice, Part V reviews selected institutional policies related to payment of research participants. Such policies, typically promulgated by IRBs in conjunction with administrators, guide both investigators’ design of and IRBs’ deliberations regarding offers of payment to research participants. The want of substantive direction from either regulatory authorities or international bodies has unsurprisingly resulted in correspondingly wide variation in institutional policy.
In Part V, we also present the results of two small pilot surveys we conducted with a sample of IRB members, administrators, investigators, and study coordinators. Our aim was to examine how individuals who are actively engaged in human subjects research and protection think about offers of payment generally, and about the concepts of coercion and undue inducement specifically. While these are preliminary findings, and we call for more research, our data contribute to the growing empirical literature showing that confusion exists among IRB members regarding how to define the terms coercion and undue inducement.12 Our pilot survey is the first to examine how investigators define those terms; it is unsurprising but valuable to see that investigators are confused in much the same way that IRB members are. Moreover, both groups subscribe to definitions that are consistent with research exceptionalism, and inconsistent with our preferred approaches.
Finally, Part VI builds on our analysis, definitions, and findings to make recommendations for policy and practice. We recognize that it may be impossible for IRBs and investigators to reach consensus amongst themselves on what the terms coercion and undue inducement mean, given the relative ambiguity of U.S. federal regulations and international guidelines and the persistent lack of agreement among bioethicists about the features of ethically acceptable offers of payment. In the short-term, it is desirable that IRB members and investigators stop assuming that labels—that is, calling an offer “coercive” or “unduly influential”—alone do sufficient explanatory work when deciding whether a payment is ethically acceptable. In the long-term, we believe that official regulatory guidance and educational efforts by enforcement agencies are needed to clarify these concepts.
Helping the research community speak with greater precision about their concerns regarding offers of payment by adoption of common definitions will enable a more concrete separation of ethically acceptable and unacceptable payment structures, which may have the effect of improving trial recruitment and promoting fair compensation of research participants, with new attention paid to the problem of underpayment.
I. Background: Offers of Payment in Biomedical Research
Human subjects research is research in which human beings (“as opposed to animals, atoms, or asteroids”13) are the subjects of study. A “human subject” is defined by the regulations governing most federally-funded human subjects research as “a living individual about whom an investigator … conducting research obtains (1) data through intervention or interaction with the individual, or (2) identifiable private information.”14
Clinical research is that “subset of human subjects research which focuses on improving human health and well-being.”15 Clinical research is “designed to test an hypothesis, permit conclusions to be drawn, and thereby develop or contribute to generalizable knowledge …Research is usually described in a formal protocol that sets forth an objective and a set of procedures designed to reach that objective.”16
Central to the distinction between research and care is “the idea that the purpose of clinical research is fundamentally different from that of clinical medicine: whereas medical care focuses on providing optimal care to individual patients, clinical research is primarily concerned with producing generalizable knowledge for the benefit of future patients,” even when individual research participants may fortuitously accrue benefits themselves.17 Other characteristics of research include the use of distinctive methodologies—such as randomization, placebo controls, and blinding—that “sacrifice personalization of care” in favor of scientific validity and the inclusion of some “procedures that hold no prospect of medical benefit for the research participant, but which are justified in light of their scientific value.”18 Research also presents a distinctive relationship between the research participant and the investigator, which is best understood in opposition to the relationship between a patient and her doctor. Franklin Miller and Howard Brody explain:
[W]hen physicians of integrity practice medicine, physicians’ and patients’ interests converge. The patient desires to regain or maintain health to relieve suffering; the physician is dedicated to providing the medical help that the patient needs. In clinical research, by contrast, the interests of investigators and patient volunteers are likely to diverge, even when the investigator acts with complete integrity.19
Again, this is because the purpose of research is to advance science and medicine, not necessarily to benefit individual participants. Given these key differences between research and care, it is unsurprising that the two activities are governed by distinctive normative commitments.20
The phrase “offer of payment” is an umbrella term used to capture all instances in which money—either cash or cash equivalent—is provided to research participants. Although controversy persists surrounding offers of payment to research participants, the practice is widespread and growing.21
A. Why Might Offers of Payment Be Ethically Concerning?
The practice of offering payment to individuals in exchange for their participation in certain types of clinical studies is generally recognized as an important—and often essential—tool to reach enrollment targets.22 Despite the longstanding nature of the practice, whether payment is a “necessary evil” or legitimate compensation for services rendered is the source of substantial debate. A minority of commentators contends that altruism should be an individual’s sole motivation for research participation, such that payment beyond reimbursement of a participant’s out-of-pocket costs is ethically inappropriate.23 The majority of academic literature on this topic, however, has focused on establishing those circumstances under which offers of payment may be ethically acceptable, addressing concerns related to the amount, mechanism, timing, and context of payment.24
As mentioned above, and as will be discussed at greater length in Part II, the U.S. federal regulations, as well as the leading international codes of research ethics, explicitly stipulate that consent to participation in research should be obtained in a manner that minimizes the possibility of both coercion and undue inducement.25 Informed consent, central to ethical clinical research, serves to “ensure not only that individuals control whether or not they enroll in clinical research,” but also that “they participate only when doing so is consistent with their values and interests.”26 In order to provide adequate informed consent, prospective research participants must be: (1) informed of the purpose, methods, risks, benefits, and alternatives to research participation; (2) comprehend this information and understand its particular relevance to them; and (3) make a voluntary decision to participate.27
Unfortunately, there is no broad consensus in the research ethics literature as to what constitutes coercion or undue inducement—a matter we delve into at length in Parts II and IV. Therefore, we will not define the terms here, instead reserving that discussion for later. There is, however, general consensus that coercion and undue inducement render consent invalid, though the mechanism by which they do so remains open to debate. Many understand both coercion and undue inducement to compromise voluntariness,28 whereas others argue that coercion compromises voluntariness while undue inducement chiefly compromises comprehension.29
The potential effect of offers of payment on research participants has been described as either coercive, unduly influential, or both, and therefore potentially problematic in terms of satisfying the ethical (and legal) requirement for valid informed consent. Simply put, many think that the offer of money can hold an overwhelming allure for research participants, the result of which is to render invalid their consent to research participation. To pick but one example, a writer discussing the adverse events in the BIA 10-2474 trial described at the outset of this article stated that “[w]ith many in poverty, there is an inherent coercion in this type of trial” and concluded that it is “imperative … that we … minimize the coercion of financial incentives” in clinical research.30
Because people have highly disparate views on the necessary and sufficient conditions for coercion and undue inducement, there is great heterogeneity regarding when offers of payment are thought to be acceptable. To fully appreciate the controversy engendered by offers of payment, it is necessary to consider them at a more granular level. Various characteristics of both the payment itself and the study for which payment is being offered are thought to have normative importance when determining the ethical acceptability of an offer of payment. That is what we turn to next.
B. Which Research Participants Receive Offers of Payment?
From an investigator’s perspective, research participants are selected through the development of inclusion and exclusion criteria, as well as through recruitment strategies.31 Inclusion and exclusion criteria are standards prospectively set forth in a study protocol that are used to determine whether an individual is or is not eligible to participate in a particular study.32 For example, inclusion and exclusion criteria may account for age, pregnancy-status, comorbidities, or an individual’s treatment history.
Although inclusion and exclusion vary widely by study, a basic and fundamental distinction can be drawn between research participants who are healthy volunteers—individuals with no known health problems—and those who are patient volunteers—individuals at risk for or with the condition under study. Presently, demand for research participants often outstrips the number of individuals willing to take part.33
From a potential research participant’s perspective, diverse factors may prompt agreement to participate in clinical research.34 For instance, healthy volunteers may be motivated by a wish to help others, to move science forward, or to receive financial compensation.35 Patient volunteers may be motivated by these factors as well, but they may also wish to receive innovative therapies only available in the research context in hopes that they will receive direct medical benefit. A direct benefit to research participants is a benefit that arises from receiving the intervention being studied, as opposed to other types of so-called collateral benefits that may be associated with trial participation, such as access to specialists and more attentive care.36
There is a common perception “that money is offered only to healthy subjects in research, and rarely to patient-subjects with the disease or condition under study.”37 Relatedly, commentators sometimes assume (or argue) that while it is legitimate to offer payment to healthy volunteers for their participation in research, one should not offer to pay patient volunteers, at least when they stand to accrue other benefits from research participation.38 Others, however, have persuasively argued that there is no inherent reason to treat healthy volunteers and patient volunteers differently with respect to payment.39 Data suggest that, in practice, researchers do in fact nearly always offer payment to healthy research participants, and also increasingly offer payment to patients who participate in clinical research, even when the study holds the prospect of direct medical benefit.40
C. Why Are Offers of Payment Made to Research Participants?
Investigators may be motivated to offer payment to research participants for a number of reasons, and the perceived ethical acceptability of these reasons varies greatly.41 Figure 1 shows possible reasons for offering payment that have been identified by IRBs and regulators, arrayed from least to most controversial.42 It is important to appreciate that it is not just the dollar value of payment that is subject to ethical critique, but also the function that the payment is understood to serve by the investigator and the IRB.43
Figure 1.

Reasons for Offering Payment to Research Participants, arrayed from least to most controversial.
First, money might be offered to reimburse participants for research-related expenses, for example, travel to the study site. Such offers may enable individuals who could not otherwise afford to participate or who would not be willing to make a financial sacrifice to participate to do so.44 The practice of offering money as reimbursement is uncontroversial and widely accepted.45
Additionally, money may compensate individuals for time and effort expended or inconvenience experienced in the course of participating in research, beyond true out-of-pocket costs. Payment may be used as a recruitment incentive, too, particularly if the amount offered is high enough to overcome lack of interest, or—for certain subgroups within the population—lack of awareness or distrust.46 Money also can serve as a token of appreciation; in contrast to an incentive, which is offered prospectively, and in contrast to compensation, which aims to match the value of what has been given, a token of appreciation is generally small and offered only after the decision to participate has already been made.47 While offers of compensation and tokens of appreciation are generally not controversial, because they aim to make a participant whole, are quite minimal or are offered in a way that would not influence decisions to participate, use of money as an incentive garners mixed reactions.48
Finally, money could be viewed as a benefit to research participants in assessing whether the risks of participation are reasonable in comparison to the benefits.49 This approach, however, is extremely controversial since it could allow even very risky research to proceed so long as the “price” was right.50 Indeed, IRBs are warned not to consider remuneration as a way of offsetting risks when it comes to approving research.51 Nonetheless, this does not preclude consideration of risks when setting appropriate remuneration amounts, and there are no restrictions on how prospective research participants might view or perceive the offer of payment when deciding whether or not to participate.52
D. How Much Payment is Offered to Research Participants?
Published journal articles rarely mention whether payment was offered to research participants, and almost never mention the amount.53 Additionally, most research studies do not specify a dollar value for any given procedure in either the protocol or consent document.54 Yet, some efforts have been made to quantify what research participants are paid. In 2012, ethicists at the National Institutes of Health (NIH) Clinical Center reviewed four years of data to estimate payment amounts for common research procedures.55 They estimated $20 for a blood sample, $10 for a urine sample, and $30 for a 1-hour questionnaire.56 This is generally consistent with data from a national survey conducted by Elizabeth Ripley and colleagues,57 as well as with suggested monetary compensation for routine research procedures outlined by the Boston-based Partners Healthcare Human Research Protection Program.58 Others have found that the procedure-related dollar value for MRIs can range from $25 to $120 (mean $58) and that variation can occur even within the same institution.59
While these are valuable benchmarks, they hardly exhaust the spectrum of offers of payment—particularly as studies vary with respect to complexity, number of procedures, length, et cetera.60 One study of consent documents for thirteen HIV cure studies found a range from “no payment to nearly $2,000,” though neither the median nor mean payment was identified.61 In 2005, a review of IRB-approved protocols and consent forms from 467 studies offering payment to research subjects approved by eleven IRBs across the United States found that the total amount of compensation offered for a complete study varied from $5 to $2,000.62 The authors found that nearly two-thirds of studies offered less than $250, and the median total across all studies was $155.63 Studies with some prospect of direct medical benefit, studies having at least one invasive procedure, and studies with a greater number of clinic visits were associated with higher dollar amounts offered.64
It is not possible to offer a straightforward explanation for the observed variation in offers of payment. The methods by which investigators determine how much payment to offer have proven difficult to discern, as there is no clear-cut correlation between the amount offered and explicit factors, such as procedures or visits.65 This has led some to speculate that these decisions are simply “guesstimates.”66 That is, investigators pick a lump sum that feels appropriate to them and/or that is likely to pass muster with their IRB. Variation, then, may be the result, among other factors, of vague guidance regarding the appropriateness of payment or different understandings of how to value research participation or of the functions that payment serves. More concretely, variation can be explained by the constraints established by study budgets and desires to avoid certain paperwork, tax reporting, or other requirements that are triggered when payments exceed a certain threshold.67
Considered together, these figures suggest that the offer of payment made to participants in the French experiment discussed at the beginning of this paper is on the higher end of the spectrum, but certainly not off the charts.68
II. Regulations and Guidelines Related to Payment of Research Participants
With this background in mind, we now turn to regulations and guidelines governing human subjects research to describe what they say about coercion and undue inducement generally and what, if anything, they say about offers of payment specifically. In short, the answer is not much. The want of meaningful guidance at both the U.S. and international levels may help to explain the heterogeneity of offers of payment described in the preceding section, as well as the conservative approaches to payment we see both anecdotally69 and in many institutional policies, as described in Part V. In what follows, we outline the various definitions of coercion and undue inducement offered in these regulations and guidelines, but we refrain from normative evaluation until Part IV because the shortcomings of these definitions are most evident when facilitated by the discussion of research exceptionalism provided in Part III.
A. American Regulations and Guidelines
Federal laws governing human subjects research demonstrate “a societal commitment to the advancement of scientific knowledge provided that the advances occur in accord with ethically sound principles and practices.”70 Although federal regulations and guidelines call attention to some of the ethical issues that payment raises, they offer little substantive guidance regarding how ethically to offer payments to research participants.71
1. The Belmont Report
The Belmont Report,72 promulgated by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, is one of the foundational documents of bioethics, setting forth ethical principles and guidelines to govern the conduct of human subjects research. The report itself is not legally binding, but we begin with it here because its principles underlie the current U.S. federal regulations.73
The Belmont Report explains that “[r]espect for persons requires that subjects, to the degree that they are capable, be given the opportunity to choose what shall or shall not happen to them. This opportunity is provided when adequate standards for informed consent are satisfied.”74 As described above, informed consent is understood to ensure that individuals control whether they participate in research and that they participate only when participation is consistent with their values, preferences, and interests. The Belmont Report states that
[a]n agreement to participate in research constitutes a valid consent only if voluntarily given. This element of informed consent requires conditions free of coercion and undue influence. Coercion occurs when an overt threat of harm is intentionally presented by one person to another in order to obtain compliance. Undue influence, by contrast, occurs through an offer of an excessive, unwarranted, inappropriate or improper reward or other overture in order to obtain compliance. Also, inducements that would ordinarily be acceptable may become undue influence if the subject is especially vulnerable.75
The authors of the Belmont Report clearly understood coercion and undue inducement as distinct concepts, but it is implied that both affect the voluntariness of consent. It is worth noting that the authors resisted drawing a bright line between that which is a mere inducement (i.e., ethically acceptable) and that which is undue (i.e., ethically unacceptable), instead emphasizing the contextual nature of undue inducements. The Belmont Report does not directly address payment.
2. The Common Rule
The Federal Policy for the Protection of Human Subjects is codified in the separate, but identical, regulations of eighteen Federal departments and agencies, and accordingly referred to as the “Common Rule.”76 The Common Rule is “a uniform regulatory floor for human subjects research … which generally requires informed consent, independent ethical review, and the minimization of avoidable risks.”77 Common Rule standards apply to all research funded by these eighteen departments and agencies, regardless of where that research occurs. The FDA has not adopted the Common Rule, but applies essentially the same standards to all clinical investigations of products regulated by FDA involving human subjects, regardless of funding source.78
The Common Rule requires IRBs to ensure that investigators will secure research participants’ informed consent.79 It states that “[a]n investigator shall seek [informed] consent only under circumstances that provide the prospective subject … sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence.”80 The Common Rule does not define either term, nor does it directly address offers of payment. However, to the extent such offers trigger concerns about either coercion or undue influence, they fall within the IRB’s regulatory purview to address and responsibility to resolve.
The fact that the Common Rule (and its FDA equivalent) cover almost all clinical research conducted in the U.S., and a broad swath of research conducted abroad,81 underscores the important role of IRBs in reviewing offers of payment to research participants and the importance of understanding the many open questions IRB members—and investigators—face when assessing the acceptability of said offers.
3. OHRP Frequently Asked Questions About Human Research
Created in 2000,82 OHRP is the office within HHS that “provides clarification and guidance, develops educational programs and materials, maintains regulatory oversight, and provides advice on ethical and regulatory issues in biomedical and behavioral research”83 funded or conducted by the Department. OHRP’s website addresses a number of Frequently Asked Questions (FAQs) about human subjects research, including questions regarding offers of payment. Because the FAQs “provide guidance that represents OHRP’s current thinking on these topics”,84 they offer helpful insight, though they “should [merely] be viewed as recommendations, unless specific regulatory requirements are cited.”85
On the one hand, OHRP acknowledges that “[p]aying research subjects in exchange for their participation is a common and, in general, acceptable practice.”86 On the other, it cautions that despite, or perhaps because of, the “lack of clear-cut standards on the boundaries of inappropriate and appropriate forms of influence, investigators and IRBs must be vigilant about minimizing the possibility of coercion and undue influence.”87 Although more research is needed, one might infer that a call to be “vigilant” from an important oversight body—one with a variety of enforcement mechanisms available to it, including institution-wide suspension of research—coupled with limited substantive guidance on how best to offer payment to research participants could lead to extreme caution and support expansive understandings of coercion and undue inducement. A review of OHRP enforcement letters in complaint-initiated investigations uncovered only a handful of instances in which the agency found “unethical inducement through large offers of money,”88 but the mere threat of regulatory action in this space is often enough to shape behavior.89 This is supported by our pilot data, described below, as well as anecdotal experience with IRB administrative staff and members.
4. Definitions
In one FAQ, the following question is posed: “What does it mean to minimize the possibility of coercion or undue influence?”90 In response, OHRP provides definitions of coercion and undue inducement that largely—though incompletely—align with those found in the Belmont Report, as well as examples.
Coercion occurs when an overt or implicit threat of harm is intentionally presented by one person to another in order to obtain compliance. For example, an investigator might tell a prospective subject that he or she will lose access to needed health services if he or she does not participate in the research.91
Undue influence, by contrast, often occurs through an offer of an excessive or inappropriate reward or other overture in order to obtain compliance. For example, an investigator might promise psychology students extra credit if they participate in the research. If that is the only way a student can earn extra credit, then the investigator is unduly influencing possible subjects. If, however, she offers comparable non-research alternatives for earning extra credit, the possibility of undue influence is minimized.92
With respect to undue inducement, the FAQ observes that “it is often difficult for IRBs to draw a bright line delimiting undue influence” because it is highly contextual.93
5. Substantive Recommendations Regarding Payment
OHRP acknowledges that “difficult questions must be addressed by the IRB.”94 The FAQ “When does compensating subjects undermine informed consent or parental permission?” advises that:
-
□
“Remuneration for participation in research should be just and fair. However, the specifics of each protocol will influence how those determinations are made. Both researchers and IRBs need to be familiar with the study population and the context of the research in order to make reasonable judgments about how compensation might affect participation.”95
-
□
“IRBs should be cautious that payments are not so high that they create an ‘undue influence’ or offer undue inducement that could compromise a prospective subject’s examination and evaluation of the risks or affect the voluntariness of his or her choices.”96
-
□
“IRBs and investigators should ensure that the consent process includes a detailed account of the terms of payment, including a description of the conditions under which a subject would receive partial or no payment (e.g., what will happen if he or she withdraws part way through the research or the investigator removes a subject from the study for medical or noncompliance reasons).”97
-
□
“[I]n studies of considerable duration or that involve multiple interactions or interventions, OHRP recommends that payment be prorated for the time of participation in the study rather than delayed until study completion, because the latter could unduly influence a subject’s decision to exercise his or her right to withdraw at any time.”98
It noteworthy that this FAQ links offers of payment only to undue inducement and not to coercion, suggesting that offers of payment cannot be coercive. We take precisely this position below, although it is one that is disputed in the research ethics community. The FAQ does not, however, explicitly say that offers of payment cannot be coercive, which would be an even clearer — and we suggest more desirable — statement on the matter. Additionally, the FAQ suggests that undue inducement affects the voluntariness element of consent.
6. FDA Information Sheet
FDA also offers an Information Sheet on Payment to Research Subjects,99 which like the OHRP FAQs is a non-binding guidance document, but also the most extensive guidance IRBs have when seeking to implement and adhere to FDA regulations. The Information Sheet acknowledges that “[i]t is not uncommon for subjects to be paid for their participation in research, especially in the early phases of investigational drug, biologic or device development.”100
Among other things, the Information Sheet advises IRBs to “review both the amount of payment and the proposed method and timing of disbursement to assure that neither are coercive or present undue influence.”101 Specific guidelines for evaluating offers of payment include:
-
□
“All information concerning payment, including the amount and schedule of payment(s), should be set forth in the informed consent document.”102
-
□
“Any credit for payment should accrue as the study progresses and not be contingent upon the subject completing the entire study. Unless it creates undue inconvenience or a coercive practice, payment to subjects who withdraw from the study may be made at the time they would have completed the study (or completed a phase of the study) had they not withdrawn.”103
-
□
“While the entire payment should not be contingent upon completion of the entire study, payment of a small proportion as an incentive for completion of the study is acceptable to FDA, providing that such incentive is not coercive.”104
-
□
“The IRB should determine that the amount paid as a bonus for completion is reasonable and not so large as to unduly induce subjects to stay in the study when they would otherwise have withdrawn.”105
Unlike the OHRP FAQ, the FDA guidance clearly links offers of payment to both coercion and undue inducement. As noted above and discussed further below, we disagree with this approach. Therefore, it is useful to note that OHRP and FDA could be seen as coming out on different sides of this debate.
B. International Guidelines
While the Common Rule and its FDA equivalent cover most clinical research conducted in the United States,106 investigators’ and IRBs’ deliberations regarding what constitutes an acceptable offer of payment may also be influenced by a number of prominent ethical guidelines relating to the conduct of biomedical research. Some countries have adopted these as regulatory requirements, while in other places, they are merely advisory. Investigators may voluntarily import them into protocols or be mandated to do so under certain conditions.
Many of these international guidelines were written in the aftermath of ethics scandals or in response to the perceived shortcomings of prior documents.107 As a result, there is a tendency to emphasize some ethical requirements while overlooking others.108 This context may help explain why the guidelines provide little specific guidance regarding offers of payment.
1. Nuremberg Code
The Nuremberg Code was formulated by American judges “sitting in judgment of Nazi doctors accused of conducting murderous and torturous human experiments in the concentration camps.”109 Although the Code says nothing about payment specifically, it does address coercion. The first principle is: “The voluntary consent of the human subject is absolutely essential.” The Code goes on to specify that “[t]his means that the person involved should … be able to exercise free power of choice, without the intervention of any element of … coercion; and should have sufficient knowledge and comprehension of the elements of the subject matter involved, as to enable him to make an understanding and enlightened decision.”110 Coercion is not defined, however.
2. Declaration of Helsinki
The World Medical Association’s Declaration of Helsinki is “a statement of ethical principles for medical research involving human subjects … . addressed primarily to physicians.”111 Like other guidelines and regulations discussed in this article, the Declaration places an emphasis on the importance of voluntary consent to participation in research. Additionally, the 2013 revision of Declaration states that “[t]he protocol should include information regarding … incentives for subjects” and be submitted for consideration and approval to an IRB.112 The Declaration does not define coercion or undue inducement, nor does it raise these concerns in relation to offers of payment.113
3. Good Clinical Practice Guidelines
The International Conference on Harmonization (ICH) Good Clinical Practice (GCP) guidelines are “an international ethical and scientific quality standard for designing, conducting, recording, and reporting trials that involve the participation of human subjects.”114 They provide “a unified standard for the European Union, Japan, and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in those jurisdictions.”115
According to the ICH GCP E6 guidelines, the IRB should “review both the amount and method of payment to subjects to assure that neither presents problems of coercion or undue influence on the trial subjects. Payments to a subject should be prorated and not wholly contingent on completion of the trial by the subject.”116 Additionally, the IRB “should ensure that information regarding payment to subjects, including the methods, amounts, and schedule of payment to trial subjects, is set forth in the written informed consent form and any other written information to be provided to subjects. The way payment will be prorated should be specified.”117 Unlike the OHRP FAQs but like the FDA information sheet on payment, the GCP guidelines suggest that payments can be both coercive and unduly influential. Neither term is defined.
4. CIOMS International Ethical Guidelines for Biomedical Research
Compared with the preceding guidelines, the recently revised 2016 International Ethical Guidelines for Health-related Research Involving Humans, prepared by the Council for International Organizations of Medical Sciences (CIOMS) in collaboration with the World Health Organization (WHO), offer a more definitive answer to questions about offers of payment to research participants.118 Guideline 13 (Reimbursement and compensation for research participants) states:
Research participants should be reasonably reimbursed for costs directly incurred during the research, such as travel costs, and compensated reasonably for their inconvenience and time spent. Compensation can be monetary or non-monetary. The latter might include free health services unrelated to the research, medical insurance, educational materials, or other benefits.
Compensation must not be so large as to induce potential participants to consent to participate in the research against their better judgment (“undue inducement”). A local research ethics committee must approve reimbursement and compensation for research participants.119
Helpfully distinguishing between reimbursement and other types of payment, the Commentary on Guideline 13 explains further that participants should not have to pay to participate in research in the form of bearing direct expenses like transportation costs themselves, and calls for participants to be reasonably reimbursed for such expenses. In addition, “participants must be appropriately compensated for the time spent and other inconveniences resulting from study participation” – although explicitly not for risk that participants agree to undertake – and payment amounts “should be calculated using the minimum hourly wage” in the trial location. The commentary goes on to clarify that the “obligation to reasonably reimburse and compensate” participants arises even when participants otherwise stand to benefit from their participation.120
Recognizing the relevance of a study’s risk level, the commentary notes that “[e]specially when the research poses low risks, providing compensation should not raise concerns about undue inducement.” This is notable among all the guidance discussed so far, as it is the only statement of a reason not to worry about payment in some contexts. However, the commentary does state that “as the risks of research procedures having no potential individual benefit for participants increase, so does the concern that compensation may constitute an undue inducement. Monetary or in-kind compensation for research participants must not be so large as to persuade them to volunteer against their better judgment or deeply held beliefs (‘undue inducement’).”121
The commentary acknowledges the contextual nature of undue inducement in the sense that individuals may view compensation differently depending on their personal situation. Thus, the responsibilities laid on research ethics committees are substantial:
Research ethics committees must evaluate monetary and other forms of compensation in light of the traditions and socio-economic context of the particular culture and population in order to determine whether the average participant expected to enrol [sic] in the study is likely to participate in the research against his or her better judgment because of the compensation offered. The appropriateness of compensation is likely better judged by local research ethics committees than by international ones. Consultation with the local community may help to ascertain this even in the case of research conducted in the researcher’s own community.122
In total, CIOMS offers the most explicit guidance regarding offers of payment to research participants – providing additional guidance regarding persons who are incapable of giving informed consent themselves, the timing of payment in relationship to early withdrawal, and the need for empirical study of financial incentives themselves. Nonetheless, it still leaves a considerable amount of discretion to the IRB to determine what constitutes an acceptable offer of payment. Emphasis is placed on the possibility that offers of payment will be unduly influential, rather than coercive.
In this section, we have reviewed payment-related guidance at both the U.S. and international levels. This is important because discussions of payment-related regulations are often focused on the Common Rule, and it serves as a useful reference to assemble these documents together.
As we have indicated throughout, these documents may or may not be legally applicable depending on where research is conducted, but they are nevertheless highly influential. They consistently emphasize the importance of research participants’ informed consent and point out that coercion and undue influence can vitiate consent. Yet, treatment of payment within these regulations and guidelines is highly uneven and at times contradictory. For example, whereas one might reasonably infer that OHRP does not worry about offers of payment being coercive, FDA clearly links payment to coercion, as does the ICH GCP E6 guideline.
As a result, IRB members and investigators bear significant responsibility both for determining what the terms coercion and undue influence mean, how (if at all) they apply to offers of payment, and for correctly identifying and addressing those ethical concerns when they arise.
III. An Argument Against Research Exceptionalism With Regard to Payment
As Part II established, regulations and guidelines regarding offers of payment to research participants generally establish as the default that such offers are to be subjected to scrutiny because they may be unduly influential, coercive, or both, and so might undermine the validity of research participants’ informed consent. Given this default, it is perhaps unsurprising that in the context of human subjects research, offers of payment are often viewed with a high index of suspicion, despite being quite common. We attribute much of the concern about offers of payment to research participants to the problem of research exceptionalism.
Many people have been taught—or intuitively believe—that research is meaningfully different than other areas of life in which we accept burdens, discomforts, and risks. They are, therefore, much more concerned about threats to the validity of consent posed by payment in the research context than they are in other contexts, such as employment.123 As a result, research in general, and offers of payment made to research participants in particular, are more stringently regulated and scrutinized than many other activities that involve both payment and the imposition of seemingly similar—or even greater—levels of risk.124 While people often worry that offers of payment made to research participants may be too high, we do not hear comparable concerns voiced about payment to individuals engaged in risky work, such as police offers, firefighters, pilots, and even commercial truck drivers.125 Indeed, many would argue that these individuals are not paid enough. Why the discrepancy?
Of course, the fact of this divergent thinking is not in and of itself proof that the current level of oversight and scrutiny applied to clinical research payments is, as a normative matter, too great. Instead, one might argue that (1) offers of payment made elsewhere are insufficiently scrutinized, and that we should not level-down in the research context, or (2) there are sound ethical reasons why offers of payment made to research participants, in particular, should be treated differently.126 Position (2) is consistent with a view of justified research exceptionalism.
Here, we will identify nine arguments made in favor of research exceptionalism, some with more force and frequency than others, and show that they all ultimately fail to justify the more stringent regulation of offers of payment made to research participants. There may, we concede, be reasons to think that research is meaningfully different from other contexts and that some enhanced protections are appropriate for research participants in general. However, in our view, these reasons do not relate to payment.
A. History of Ethical Abuses
Probably the foremost reason given in favor of special regulation of human subjects research is the history of egregious ethical abuses.127 Many of the ethical guidelines and regulations governing human subjects research have grown out of particular scandals.128 The scandal-and-reform dynamic has led to a progressive ratcheting up of research participant protections.129
We don’t dispute the seamy history. Yet, we agree with James Wilson and David Hunter that
[t]hese cases do provide prima facie evidence that unregulated research can be abused. However, they fall short of demonstrating the case for research exceptionalism… . First, they do not show that these risks are specific to research: Abuses can and have occurred in many other areas of human existence. Second, they do not show that regulation will prevent these abuses. To justify research exceptionalism, we need to demonstrate that there are risks that are either specific to research or are more likely in research.130
Additionally, and most importantly for our purposes, these foundational and transformational abuses have nothing directly to do with offers of payment. Instead, they were related to concerns with outright torture (e.g., Nazi experimentation131), deception (e.g., the Tuskegee syphilis studies132), researcher conflicts of interest (e.g., the Jesse Gelsinger gene therapy case133), and the like.
Even in high-profile cases where the offer of payment was subsequently subject to scrutiny, ethical fault laid with the way the trials were conducted, rather than with the offer of payment itself (e.g., the TeGenero TGN1412 trial134). Critically, the tragic outcomes attributable to ethical violations in these cases would have been no more acceptable if payment had not been offered to research participants.135 The mere fact that money was offered to research participants should not, therefore, bias our evaluation of whether the research was conducted ethically. Scandal does not make payment in the research context exceptional.
B. Risk of Harm to Research Participants
Another common argument given in support of research exceptionalism is that research exposes participants to the risk of harm. Research-related risks can be analyzed as a function of two distinct components: (1) the likelihood that harm will occur, and (2) should it occur, the magnitude of the harm.136
Admittedly, participation in research can be associated with significant risks: individuals have been seriously injured and even died as a result of their participation.137 Yet, “research participation … is not usually as risky as the general public perceives it to be.”138 Additionally, many quotidian activities expose individuals to at least some risk of harm. The pervasive nature of risk is acknowledged in the Common Rule, which defines minimal risk research in terms of risks “ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests.”139 Even granting that some research studies are riskier than the risks we ordinarily assume in daily life, “[i]t is not clear that research per se is specifically risky.”140 Therefore, the risk of harm does not itself justify research exceptionalism.
The argument from risk of harm also clearly fails when applied more narrowly to offers of payment to research participants. As explained in detail in our other scholarship, we think that participation in research is most appropriately analogized to labor; relevant comparators include police work and military service, jobs that are important to the community but also offer personal benefit.141 There is little normative debate about whether it is acceptable to offer payment, or higher payment, to people who accept risky jobs. To the contrary, outside the research context, the main concern seems to be that people will be unfairly compensated—that is, exploited—if they are paid too little. For example, “[t]he life-and-death nature of the job [policing] is used to push for extremely generous … pay packages.”142
[I]n theory, the market should dictate (and some laws do) that risky work be better compensated, a phenomenon called the compensating wage differential. Further, even when risky jobs are held by those with few other options for less risky work that is comparably compensated, the law does not require that their payment be restricted on that basis.143
Thus, the fact that research participation exposes people to risk of harm cannot stand alone as an argument against offering payment—even generous payment—research participants.
C. Uncertainty of Risk in Research
The next possibility we consider is that it is not the risk of harm per se but some characteristic of that risk that justifies research exceptionalism. For example, it might be that the risk in research is uniquely amorphous. Research is, after all, intended to answer open questions regarding interventions about which knowledge is limited; therefore, “[u]ncertainty is a fundamental characteristic of research.”144 At the outset, it may be impossible to know with certainty the scope of potential or likely harms—as well as the potential benefits—faced by research participants.145
Yet, there is less uncertainty about research risks than it may appear, particularly as investigational products proceed through their development. Before a study of a new FDA-regulated product can proceed to human trials, for example, FDA must be convinced that there is adequate data from laboratory and animal testing to support the claim that the drug is safe enough to give to research participants;146 IRB approval will be required as well, as a further check on whether the risks are appropriately minimized and reasonable. Moreover, as clinical research progresses through the different phases, there will be a substantial accretion of data; therefore, uncertainty should dissipate over time.
While granting that there is some degree of uncertainty in clinical research, it is necessary to point out that there is uncertainty about risks in many contexts—consider, for example, exposure to environmental pollutants, or even approved drug products. When risk is uncertain, regulation can be an appropriate response, but the key observation to our present analysis is that it is not clear why research should be regulated more stringently than other areas similarly characterized by uncertainty.
Looking to offers of payment specifically, even if uncertainty about research risks was somehow unique, it is unclear why that uncertainty would be a reason to pay research participants less. Above, we discussed the compensating wage differential for risky work, and here, we would reiterate that it may be appropriate to pay research participants more when risks are uncertain, precisely as compensation for that uncertainty. The argument from uncertainty of risks does not necessarily or even obviously lead to the conclusion that offers of payment to research participants should be constrained, and so further justificatory work is needed to defend research exceptionalism with respect to payment.
D. Risk Assumed for the Benefit of Others
A fourth possible argument in favor of research exceptionalism is that the purpose of research is to generate socially valuable knowledge. As discussed above, research-related risks and burdens are justified not in light of the potential to benefit the individual research participant but in light of their potential to benefit future patients. In research, unlike in other activities, the argument goes, there is tension between the individual good and the public good because risk is assumed for the benefit of others, and so additional scrutiny is needed.
This apparent distinction also proves illusory, however. First, at least some individuals may, in fact, benefit from participation in research, for example from a successful experimental intervention or from free medical care that is delivered in the course of the study.147 Even when individuals are motivated to participate in clinical research solely by altruism, they may benefit by contributing to research when they share the ends for which the research is undertaken.148
Second, assumption of risk in other areas of life cannot accurately be characterized as entirely self-interested; it is often also for the benefit of society. Again, consider police officers. While it is clearly in their personal interests to work in order to collect a paycheck, their jobs only exist because others experience a clear benefit and, therefore, create demand for such jobs. Additionally, consider the job of commercial fishing – a risky occupation that exists to satisfy consumer demand for fish; the social benefit is mere satisfaction of consumers’ taste for fish.
If society is willing to pay people to engage in risky but socially beneficial activities – even when the benefits are arguably frivolous, as in the fishing example – “then consistency seems to require that they also be allowed to receive payments for participating in socially beneficial research involving serious risk.”149 Thus, the argument that risk is assumed for the benefit of others in clinical research also fails to support the exceptional scrutiny given to research payments.
E. The Optional Nature of Medical Progress
A fifth possible argument—a variant of that just considered—is that medical progress is optional, whereas other risky but socially beneficial endeavors are not. Hans Jonas has, for instance, admonished us “not [to] forget that progress [in the conquest of disease] is an optional goal.”150
Relatedly, and arguing specifically against payment of research participants, Paul McNeil concedes that some dangerous work, such as fire fighting, is necessary, but he denies that “experiments are … necessary to society in the way in which some dangerous work may be.”151 He argues that the risks of research cannot be justified in the same way as the risks of necessary work. McNeil’s distinction, fails, however. As we have explained elsewhere:
If dangerous work such as fire fighting is necessary … why is dangerous work such as research participation — which may also save lives and meet basic human needs — any less so? There seems to be no reason to distinguish between different types of potentially preventable deaths when people have voluntarily put themselves at risk in the service of a greater good.152
On our view, medical progress is not optional. Some kinds of research are morally obligatory to conduct, assuming they can be conducted ethically. One might respond that a fire fighter who rushes into a burning building to save someone offers an immediate benefit, whereas participation in research saves lives over a much longer time-scale. Admittedly, that will often be the case. Yet, as a matter of intergenerational equity, it is unclear why we should favor lives currently in existence (or presently in jeopardy) over lives not yet in existence (or not presently in jeopardy). Our moral impulse to save identifiable lives should not blind us to the imperative to save statistical lives when possible.153
Yet, even if we were to assume arguendo that medical progress is optional, one must allow that some risky jobs that yield social benefits but are indisputably optional, like commercial fishing, exist without controversy. If we allow payment for those jobs—and we do—then the optional nature of social benefit, if true, could not justify research exceptionalism with respect to payment.
F. Difficulty Securing Research Participants’ Informed Consent
Another argument for research exceptionalism stems from the now substantial evidence that many who participate in research suffer from the therapeutic misconception—that is, they confuse the goals of clinical research (social benefit) with the goals of clinical care (individual benefit)—and, at least some individuals may be unaware that they are participating in research at all.154 More generally, some people may assume the risks of research participation despite a failure to fully comprehend them. Some commentators use this fact to argue that “we should not allow people to make significant life choices without fully understanding the potential consequences for their lives.”155
Yet, as Wilson and Hunter astutely point out, “[W]hile research protocols may be difficult to understand, they are no more difficult and often considerably less difficult to understand than many official documents such as the fine print on mortgage documentation.”156 Of course, the risks are not clearly analogous (e.g., physical v. financial), but as the housing crisis made clear, signing a mortgage without full comprehension can have devastating repercussions. Moreover, the conduct of research—like mortgages—is heavily regulated, and there are calls to make informational documents easier to understand in both contexts.157 Nevertheless, the fact that it is difficult to secure truly informed consent from research participants does not, on its own, justify research exceptionalism. True understanding is a challenge in many contexts.
In fact, difficulty in securing research participants’ genuinely informed consent may be a stronger argument in favor of payment than against it. Offers of payment may help research participants distinguish clinical research from clinical care, since offering payment to research participants “might send the message that they were participating in these trials for the sake of science and should be compensated for it, which would not occur if they were … expected to benefit from it.”158 Certainly, our doctors do not pay us in the course of clinical care; instead, we pay them. Accordingly, any offer of payment might help flag for research participants the distinct risks and burdens of research, presumably with higher payments offering even stronger signals. This is an empirical claim that deserves further examination.
G. Commodification
One potential justification for research exceptionalism with respect to payment, in particular, is that offering to pay people who participate is wrongful commodification. It has been said, for example, that “[p]ayment to patients to serve as research subjects is an ethically unacceptable commodification of research practice.”159 Individuals concerned with commodification feel that it is improper to offer money for certain goods or services, even if the validity of the consent is not in doubt. This may be a threshold concern as to whether payment can be offered at all—and not just the amount of payment.
Commodification concerns do animate certain laws and policies outside the research context. For example, a central provision of the National Organ Transplant Act (NOTA), § 301(a), bans the buying and selling of human organs.160 The legislative history of NOTA clearly shows that Congress felt that buying and selling of organs was contrary to society’s moral values.161 One might question—as many have—whether prohibitions against organ sales are appropriate on these grounds.162 Yet, even if one accepts that commodification concerns are relevant in some contexts, services offered by research participants are not the same as selling the constituent parts of one’s body. As we have suggested throughout this section, participation in research is most appropriately analogized to essential (albeit unskilled) labor.163 In the context of unskilled labor—and skilled labor as well—we generally permit people to sell their bodily services,164 even when sale of those services exposes them to risk of bodily harm. It should be “no more worrisome to commodify a person’s labor as a research subject than to commodify a person’s labor in other contexts, which happens all the time.”165
H. Crowding Out Altruism
As mentioned above, a minority of commentators believes that altruism should be the sole motivation for research participation.166 For them, this may be a threshold concern as to whether payment can be offered at all for research participation. Most commentators, however, have focused on the conditions under which offers of payment can be ethical, suggesting that research participation does not have to be exclusively or even primarily altruistically motivated.
Yet, even some who accept a role for offers of payment continue to emphasize the importance of preserving altruistic motivation. Lynn Jansen observes, “Those who seek to justify clinical research often point to the possibility that participants … have altruistic motives for participating.”167 The argument goes that if research participants have genuinely altruistic motives, “then it is easier to justify imposing costs and sacrifices on them in the course of a trial” than if they do not.168 That is, altruism plays an ethically significant role in justifying the imposition of risk on research participants. Another argument for research exceptionalism regarding payment, then, is that offers of payment must be closely scrutinized to avoid the perverse consequence of diluting prospective participants’ intrinsic motivation to enroll in research.169
In practice, and as mentioned above, research participants—even those who are paid—report experiencing a variety of motivations, including altruism.170 This is comparable to studies of police officers that have found individuals enter policing for both altruistic and practical reasons; they value the opportunity to help others but also the attractive job benefits.171 These findings are both unsurprising and untroubling; if individuals are capable of satisfying a role’s requirements, why should their motivations matter? Moreover, given that a variety of motivations can simultaneously coexist within a single individual, there is no clear argument for why altruistic motivation should be valued more highly than financial motivation in research, or than it is (or should be) in other contexts.
Two possible practical implications of crowding out altruistic motivations among research participants in favor of financial motivations are more troubling, and could potentially justify greater scrutiny of offers of payment in the research context than elsewhere. If offering payment dilutes altruistic motivation, this might (1) reduce the overall pool of prospective research participants, i.e., some altruists may not participate at all if payment is offered because they find the offer repugnant, and/or (2) selectively appeal to individuals who are somehow less desirable as research participants due to their motivation by payment.172 While a number of experimental studies have examined the effects of financial incentives on altruistic motivations in other contexts, particularly blood donation, and generally found results consistent with the crowing out hypothesis,173 data is needed about research participation in particular. We grant that these concerns may be valid in some research contexts; however, they cannot justify restrictive approaches to payment in all instances. Rather, a more tailored approach is appropriate, focused on those situations in which payment might have damaging instrumental effects, and also considering whether those effects might be avoided through mechanisms other than limiting payment.
I. Importance of Public Trust
The final argument we consider in favor of research exceptionalism has nothing to do with protecting research participants themselves, but rather with protecting the research enterprise of which they are a part. Public trust is “essential to secure funding and institutional support for research and to recruit human subjects.”174 Therefore, the argument goes, research exceptionalism is justified if it promotes and preserves the public trust. Wertheimer observed,
Whereas society accepts with a relative yawn the fact that people incur job related injuries or deaths as coal miners, fishermen, and off-shore oil service workers, society seems to react with great intensity to research related injuries and deaths, as evidenced by the public concern with the Jesse Gelsinger case.175
As our replies to prior arguments suggest, we believe the public is mistaken to react more intensely to harms attributable to research participation than to harms attributable to traditional work. Yet, even if that more intense response is mistaken, “the public trust argument maintains that public beliefs are a fact that must be accommodated.”176
In response, we first note that there is little evidence that “members of the public are both generally aware of the existence of [IRBs] and find the notion reassuring.”177 In other words, they may simply be unaware of the ways in which they are protected from research risks, such that these protections cannot possibly contribute to trust building. More specifically, it is only speculative that research exceptionalism with respect to payment specifically promotes public trust. To the contrary, rigorously restricting offers of payment to research participants—indeed, “protecting” them from offers of payment—could erode public trust by suggesting that research is more dangerous than it really is, and that participation is something to be avoided. If individuals nonetheless choose to participate, restricting payment could also cause research participants to feel they have been treated unfairly as a result of inadequate compensation.
Beyond these considerations, we believe it would be a mistake to accommodate erroneous beliefs that research is dramatically different from other potentially risky/uncertain endeavors, and instead favor attempts at education that build the right kinds of trust. Therefore, public trust—while doubtlessly important to the research enterprise—is not an acceptable argument for research exceptionalism, particularly with regard to payment.
We have considered nine arguments sometimes made in favor of research exceptionalism with respect to payment—that is, in favor of the view that offers of payment to research participants need to be regulated more stringently than offers of payment made to individuals in other contexts where they also assume risks for the benefit of others. For the reasons outlined above, we maintain that each of these arguments fails. Significantly, we do not claim that these arguments have failed to identify characteristics of research that might merit regulatory attention; indeed, we favor robust regulatory protections for human subjects research, including IRB review. Rather, we claim that these nine arguments fail to identify factors that justify regulating offers of payment to research participants more heavily than offers of payment made in other areas.
IV. From Confusion to Clarity: Defining Coercion and Undue Inducement
As we have discussed in the preceding sections, despite a general consensus that coercion and undue inducement are to be avoided, there is a lack of clear regulatory guidance about what constitutes an acceptable offer of payment and disagreement about when offers of payment to research participants violate ethical norms. In this section, we will look at the considerable debate within the research ethics community about how best to define coercion and undue inducement. For both terms, we will highlight areas of consensus, briefly review the range of definitions offered within the literature, and offer our preferred definitions.
A. Coercion
As discussed above, there is a general ethical requirement that prospective participants give their voluntary consent to participate in research.178 The main worry about coercion is that it affects the voluntariness of consent, and the most prominent definitions from the bioethics literature relate to voluntariness. Here, we will consider three commonly used definitions and also address a divisive question: can offers ever be coercive?
1. Threatening to Make One Worse Off
Recall that the influential Belmont Report states that coercion “occurs when an overt threat of harm is intentionally presented by one person to another in order to obtain compliance.”179 It is perhaps unsurprising, then, that broad consensus exists that coercion includes the use of a threat of harm to compel another to do something against his or her will.180 Christine Grady, for example, has stated, “By definition, coercion is understood to involve a threat of physical, psychological, or social harm in order to compel an individual to do something, such as participate in research.”181 Given the consistent references to harm, it is generally understood that the person coercing is threatening to make the person coerced worse off than he would be at his status quo baseline.
2. Threatening to Violate Rights
Alan Wertheimer182 and Franklin Miller offer a view of coercion that is similar—but not identical—to that of the Belmont Report.183 On their rights-violating view of coercion,
A coerces B to do X in a way that invalidates B’s consent only if (1) A proposes or threatens to violate B’s rights or not fulfill an obligation to B if B chooses not do X and (2) B has no reasonable alternative but to accept A’s proposal. Both conditions are necessary.184
Wertheimer and Miller state that “the main point is that A’s proposal is coercive only if A’s ‘declared unilateral plan’—[that is,] what A proposes to do if B does not do X—would violate B’s rights.”185 A classic example would be when a mugger pulls a knife on someone and says: “Your money or your life.” The mugger is threatening to kill his victim, which would violate the victim’s right not to be wantonly harmed by others, if the victim does not acquiesce to surrender his property. Thus, the victim is coerced to hand over his wallet.
Wertheimer and Miller concede that “[t]here is often little difference between the worse-off and the rights-violating accounts.”186 After all, both views of coercion will reach the same conclusion in the case of the mugger—what the mugger has done is coercive.
However, when the two differ, the rights-violating approach is more accurate, because it allows us to handle (1) cases in which A has a right to make B worse off than B’s status quo, and also (2) cases in which A has an obligation to render B better off than B’s status quo.187
To illustrate (1), a prosecutor does not coerce defendants into pleading guilty to a crime in exchange for a relatively lenient sentence when he proposes to take them to trial if they do not plead guilty, even though both options—pleading guilty and going to trial—are worse than B’s status quo. Why? Because the prosecutor’s declared unilateral plan to take the defendants to trial does not violate their rights relative to that option, the prosecutor is actually making an offer of leniency rather than a threat of severity… . The defendants’ guilty pleas are voluntary.
To illustrate (2), if a physician (A) has an obligation to provide a patient (B) with medical services free of charge, say, because A is employed by the national health service, then A actually does coerce B into paying a fee if A proposes not to provide such services unless B pays. And this is so even though A does not propose to make B worse off than at present if B declines.188
We emphasize that in the example for (2), Wertheimer and Miller say A does not propose to make B worse off than B is at present. In other words, B is presently untreated and would continue to be untreated if B refuses to capitulate to A’s demand, so B’s status quo is unchanged and B is, at least in a sense, not made any worse off. However, A has an obligation to help B achieve something superior to the status quo at present, which is why we find coercion under the rights-violating view when we may not under the worse-off view. Note that there may be disputes about how to identify the appropriate status quo, however, because under an alternative approach, one might suggest that A is indeed threatening to make B worse off by failing to achieve the status quo to which B is entitled, which is to be treated by A.
Resolving this question about which status quo baseline is the proper one to focus on under the rights-violating view can be the source of reasonable debate. However, it is unnecessary to resolve the matter here because we argue momentarily that offers of payment cannot be coercive. Thus, in the payment context, it is unnecessary to strictly distinguish between the worse-off and rights-violation definitions of coercion, since neither will be present.
That said, we favor the rights-violating account because of its broader explanatory power. In other words, simply asking if the threat would cause harm inappropriately identifies coercion in scenarios in which harm is justifiable (e.g., when an investigator threatens to remove a subject from a potentially beneficial clinical trial for failure to comply with the study procedures), and might fail to identify coercion when harm is arguably not present, but there is an obligation to make one better off. Importantly, neither the worse-off view nor the rights-violating view of coercion falls prey to research exceptionalism, since they both reflect common views of coercion applied outside of the context of research as well.
3. No Reasonable Alternative
The notion of coercion as existing only when threats of adverse consequences (harm or rights violation) override the exercise of genuinely free choice has been characterized as “cramped” by some commentators.189 Thus, another proposed definition of coercion is that an individual is coerced when she has no reasonable alternative but to accept another’s proposal.190
In contrast to the two prior definitions, this definition does not require a threat at all. Proponents of this view classify having no reasonable alternative as a sufficient condition of coercion, not merely a necessary one.191 Importantly, due to its expansive scope, this approach might result in a substantial portion of research being deemed coercive, since research participation may be a patient volunteer’s best available alternative for therapeutic improvement or a healthy volunteer’s best available alternative to make a comparable amount of money in a given period of time. Both types of participants may feel that they have no reasonable alternative, even though individuals always have the option not to participate in research as a regulatory matter.
Importantly, if one rejects research exceptionalism, the no-reasonable-alternative view is clearly wrong. Consider these familiar examples from outside the research context: first, a woman is diagnosed with breast cancer, and her oncologist tells her that she is unlikely to survive more than a year without surgery. We would not say that the oncologist has coerced the woman by offering surgery, and it would be nonsensical to claim that the woman cannot give valid consent to the surgical intervention because she has “no choice” but to have it. Second, turning to an instance in which payment changes hands, it is unlikely anyone would say an individual had been coerced to take an unpleasant, risky (but perfectly legal) job if that was his best or even only option to earn sufficient funds to cover his bills. In common parlance, we may suggest that both of these individuals were “forced” in some way to make an unpleasant decision, but we would not maintain that there had been any ethical violation. If we do not think that morally problematic coercion occurs in these circumstances, it would be unjustifiable research exceptionalism to argue that it occurs when research participants believe—in the absence of any threat—that they have no reasonable alternative but to participate in research due to an offer of payment.
4. Coercive Offers?
A notable fissure in the literature relates to whether genuine offers, rather than threats, can ever be coercive.192 One of the most visible advocates of the view that offers can be coercive is Ruth Macklin.193 In a 1989 article, she noted that the “reason for holding that it is ethically inappropriate to pay patients to be research subjects is that [offers of payment are] likely to be coercive.”194 Joan McGregor more explicitly links the concept of coercive offers to the no-reasonable-alternative view just discussed. She suggests that coercive offers are “offers because they propose to make the person ‘better off’ relative to his or her baseline … but they are coercive since, because of the recipient’s lack of options, the proposal is likely to present the only eligible choice.”195 Others have accepted that offers may be coercive on the condition that the offerer is responsible for the offeree’s bad circumstances.196
Many, however, have reached a contrary conclusion and assert that genuine offers (as opposed to veiled threats) cannot be coercive.197 While threats reduce the choices available to an individual, genuine offers expand the individual’s choice set and, therefore, by definition, do not coerce.198 Wertheimer and Miller are emphatic that the “claim that the offer of financial payments can actually constitute a coercive offer in a manner that undermines informed consent is both false and incoherent, because genuine offers cannot coerce.”199 If one thinks that coercion requires a threat (whether of harm or of rights violations), as we do, offers of payment to research participants cannot be coercive.
For emphasis, our view is that coercion is not a valid or relevant concern when evaluating offers of payment, although that is not to say that subjects may not be coerced to participate in other ways. This conclusion does not definitively resolve the question of whether offers of payment in the research context are ethically permissible, however, since they may, in some circumstances, cause undue inducement.
B. Undue Inducement
Although there is also a lack of consensus about how to define undue inducement, there are several points of general agreement. First, if an inducement is undue, it could “prompt subjects to lie, deceive, or conceal information that, if known, would disqualify them as participants in a research project.”200 This not only threatens to harm research participants—for example, by exposing them to risks that the exclusion criteria were designed to shield them from—but also jeopardizes the scientific integrity of the research.
A second area of agreement is that determining the existence of an undue inducement is highly contextual. For example, Emanuel, Wendler, and Grady state, “[L]ocal traditions and economic conditions will influence when financial payments may constitute undue inducements.”201 Wertheimer and Miller suggest that an individual’s situation determines whether there is undue inducement; they emphasize that the “distinction between an unproblematic … inducement and an undue inducement is not a feature of the inducement itself. It is a function of the relation between the inducement and the subject’s response to it.”202 Ruth Grant and Jeremy Sugarman have written that “[u]nder certain conditions, incentives are implicated in problems of manipulation in the form of undue influence.”203 Finally, Ruth Macklin explored the question of how large a payment constitutes undue inducement and found it “impossible to arrive at a single, objective criterion serving to mark off due from undue monetary inducements to participate in research.”204
Taking these areas of consensus as our starting point, we will consider three commonly used definitions of undue inducement and also review the empirical evidence regarding the actual existence of undue inducement in research.
1. Excessive Reward
According to the Belmont Report, “undue influence… occurs through an offer of an excessive, unwarranted, inappropriate or improper reward or other overture in order to obtain compliance.”205 On this view, the defining feature of an undue inducement is an offer so disproportionate to what the person is asked to do that it alone appears as evidence of nefarious intent. Of course, what constitutes a disproportionate offer may be subjective.
2. Excessive Reward Producing Bad Judgment Entailing Risk of Harm
Ezekiel Emanuel offers a four-part definition of undue inducements, of which a reward’s excessiveness is only one feature:
First, they entail an offer of a welcomed good, a positive incentive. The induced person is getting something he or she deems desirable. Second, the incentive, by some metric, appears excessive or irresistible. While there is no physical force or external psychological pressure, there is considerable internal attraction because of the quantity or type of the incentive. Third, the incentive does not just make the person do something they are not otherwise induced to do. The incentive must produce bad judgments. Finally, the bad judgments must in turn engender ethically, legally, or prudentially undesirable activities. The activities are undesirable because they contravene the person’s interests and thereby harm them. While bad judgment is necessary, alone it is insufficient to constitute undue inducement. Undue inducement requires the action entail a substantial risk of serious harm … That is, there must be a risk of a serious adverse effect for the person. Absent potentially serious adverse consequences of the bad judgment there is no undue inducement.206
Emanuel stresses that all four elements are necessary for an undue inducement to exist.207 The first condition, that the thing offered be a positive incentive, immediately distinguishes undue inducement from our preferred view of coercion, which requires a threat. The second condition requires—like the excessive-reward view—that the incentive is relatively large in light of what is being asked. Condition three distinguishes undue inducements from mere inducements, which is a critical distinction since mere inducements are not morally problematic (e.g., paying employees a salary so they show up to work, which they would not be inclined to do for free). By contrast, an undue inducement is a genuine offer that “distorts people’s reasoning abilities to such a degree that they undertake something that exposes them to unreasonable risks, the kind of risks they would not do were they more sober and reasoning clearly, or to forsake deeply held value.”208 The fourth condition requires that engaging in the activity be unreasonably against a person’s interests. The irresistible nature of the inducement coupled with the cognitive distortion results in acceptance of unreasonable risks.
Unlike the excessive-reward view, which speaks solely to the size and nature of the offer, the Emanuel account of undue inducement has the advantage of speaking to how the offer affects the target (i.e., the potential research participant). Emanuel writes that “[i]nducements prompt ethical concern when they distort people’s judgment, encouraging them to engage in activities that contravene their interests because they are harmful.”209 Thus, his account is superior to the excessive-reward view because it clearly articulates the widely held concern that an undue inducement creates a cognitive distortion that impacts the validity of consent to enroll.210 It also provides additional criteria that more comprehensively articulate what is wrong about undue inducement.
On our view, as on Emanuel’s, if an offer of payment, even an extremely large one, simply motivates people to enroll in research when they otherwise would not—and does not distort their perception of the risks or lead them to lie—then it is a mere inducement and not an undue one.
Given that inducement is a common element of human life, it seems difficult to see what would be uniquely worrisome about inducement in research. Working life often involves inducements and in particular sometimes involves inducements for engaging in risky working behavior (so-called “danger money”) … . If we are to complain about inducement in research, it seems apt to consider it elsewhere as well.211
Thus, without research exceptionalism, it is difficult to show that anything is wrong with the use of offers of payment merely to induce participation in research. In contrast, it is consistent with views of offers of payment outside of research to be concerned when amounts are so high as to cause people to behave irrationally in ways that could result in unreasonable harm.
What are the practical implications of this definition? According to Emanuel, “[u]ndue inducement cannot occur in otherwise ethical clinical research because there is no possibility of excessive risks, of assuming risks a reasonable person would not assume.”212 This is because IRB approval is conditioned on a determination that a study has a favorable risk-benefit ratio, completely independent of any offer of payment, and a person could reasonably decide to participate.213 IRBs “are required to determine that any risks of serious harm are offset or outweighed by either the prospect of individual benefit or by the value of the knowledge that the trial is designed to generate.”214 Even when the social value of a proposed study is very high, IRBs must ensure that risks to individual participants have been minimized. Thus, according to Emanuel, once a protocol has been approved by an IRB, it is essentially by definition a reasonable proposal to put before potential participants.
Nonetheless, because an IRB is approving a protocol for a general population, and not evaluating the circumstances of individual participants, we suggest that it remains possible that in some cases, an individual’s particular circumstances might make his or her participation in an approved study unreasonable, i.e., the result of bad judgment. In other words, it is possible that participation is against the individual interest of any particular research participant.215 One might, for example, think of a devout Jehovah’s Witness who is considering participating in an IRB-approved study that requires receiving a blood transfusion because it is high paying.216 For this reason, we do not ascribe to Emanuel’s view that undue inducements cannot occur in otherwise ethical research.217
However, we do think they are relatively unlikely to occur. This is because situations in which an individual’s interests may be so unique as to fall completely outside of the risks and benefits evaluated by the IRB are likely to be rare. A default position of encouraging highly restrictive approaches to offers of payment in research—intended to forestall undue inducements—is, therefore, inappropriate if IRB review functions as intended, i.e., as a bulwark against unethical research.
3. Coercive Offers
Professor Joan McGregor flatly rejects Emanuel’s four-part definition of undue inducement as “wrong.”218 She counters, “Only the first condition from his list, that a good is offered in exchange for something, is necessary for undue inducement. The other conditions are too vague to be useful or are clearly not necessary conditions.”219
McGregor instead favors defining undue inducements as “coercive offers.”220 Notably, this seemingly eliminates undue inducement as a distinct concept and places McGregor back in the discussion of coercion above. From McGregor’s perspective, the prohibition against undue inducements is intended to guard against taking advantage of vulnerable populations, including impoverished persons with few, if any, alternatives.221 Note the similarity of this position to the view of coercion as simply having no reasonable alternative. For reasons discussed above, we find this definition untenable.
4. Empirical Evidence of Undue Inducement
Once undue inducement is defined to include distortion of a person’s rational risk assessment as a necessary condition, we have an empirical question: does such distortion actually occur in practice? Importantly, available empirical research suggests that it may not. To the contrary, some studies indicate that offers of payment draw prospective research participants’ attention to risks (rather than causing risks to be ignored), while other studies have found no association between offers of payment and perceived research risk.
Cynthia Cryder and colleagues found that while higher offers of payment increased willingness to participate, these offers also increased perceived risk and the time spent reviewing information about research-related risks.222 Jacquelyn Slomka and colleagues conducted in-depth interviews with individuals taking part in three HIV prevention studies.223 While the interviewees saw money as a necessary incentive to attract research participants, at least some expressed a belief that large financial incentives might raise concerns about risks.224 Scott Halpern and colleagues found that, although higher payment motivates research participation, there was no evidence that higher payments altered patient’s perceptions of the risks of research participation, that is, their comprehension.225 John Bentley and P.G. Thacker determined that higher levels of payment increase willingness to participate, but, perhaps counterintuitively, there was no association between monetary payment and perceived risk.226 Finally, Eleanor Singer and Mick Couper conducted an online vignette-based survey and concluded that while larger incentives induced greater overall participation, “respondents do not appear to exchange higher incentives for greater risks.”227 Although more data are needed, these studies do not indicate that higher payment necessarily or even frequently leads to cognitive distortion regarding the risks of research participation.
That said, however, empirical evidence does suggest that higher payments may prompt research participants to lie, deceive, or otherwise conceal information from investigators.228 Some individuals interviewed by Slomka and colleagues “believed that if a large amount of money was offered, individuals would be more likely to provide false information to investigators and ‘say anything’ to obtain the money.”229 Bentley and Thacker’s study “showed that higher levels of monetary payment may influence subjects’ behaviors regarding concealing information about restricted activities.”230 They expressed concern that “[I]f such activities were actually engaged in, the results of the hypothetical studies may have been distorted.”231 In our view, this act of deception may indicate a distorted understanding of risks or an unreasonable willingness to assume risks of participation, for example, by circumventing exclusion criteria or lying about adverse events that could lead to disqualification. Thus, some concern about undue inducement in practice remains.232
Nonetheless, we note that “[w]orkers may lie about their qualifications too, in ways that put both themselves and their employers’ output in jeopardy, and they may be enticed to do so by money.”233 Without research exceptionalism, the fact that highly-compensated research participants might be more likely to lie than unpaid or less-compensated research participants cannot justify a limit on compensation to research participants but not for other jobs. The immediate response to deceit by research participants should not be to reduce payment. Regulatory oversight bodies, sponsors, and investigators “could implement national subject registries to track participants [to avoid duplicative enrollment for financial gain], … utilize more extensive screening before enrollment [to better check against inclusion/exclusion criteria], and increase use of physical testing rather than relying on qualitative subject feedback whenever possible.”234 In some instances, it may be necessary to limit payment to avoid the problems entailed by deceitful research participants, but these cannot justify blanket limits on offers of payment in all clinical research.235
C. The Relationship Between Coercion and Undue Inducement
On one view, coercion and undue inducement are not distinct concepts, but rather fall on a sliding scale, with one being a more extreme version of the other. This view purports that the “quantity of payment is directly correlated with the ‘pressure’ on the decision-maker, and the threshold of pressure necessary to constitute undue influence is less than the threshold of pressure necessary to constitute coercion.”236 The sliding scale view is intuitively appealing and may be implied by some of the leading regulatory and ethical guidelines, like the U.S. Common Rule, which mention coercion and undue inducement together and do not draw a clear conceptual distinction between them.237
Nevertheless, we join others in forcefully arguing for distinguishing undue inducement and coercion as distinct concepts. Emanuel, for instance, contends that “[u]ndue inducement is the diametric opposite of coercion. While both make a person do what may be unethical, illegal, or imprudent, the former dangles a good, a positive offer to induce bad judgment that leads to harm, while the latter entails an overwhelming threat… . Coercion requires a threat of what the person considers a worse consequence, while undue inducement offers a positive good.”238 Additionally, whereas undue inducement may compromise the validity of consent by creating a cognitive distortion and impairing comprehension, coercion compromises the voluntariness of consent by the threat of harm.239
Additional support for the argument that these are distinct concepts may be found in the legal rules, or canons, of statutory interpretation. It is a “cardinal principle of statutory construction that a statute ought, upon the whole, to be so construed that, if it can be prevented, no clause, sentence, or word will be superfluous, void, nugatory, or insignificant.”240 In the case of the Common Rule, quoted above, this would favor understanding coercion and undue inducement as distinct concepts, rather than one as an extreme form of the other. Moreover, as discussed above, both the Belmont Report and OHRP’s FAQs distinguish conceptually coercion from undue influence.
In this section, we have illustrated the lack of definitional consensus within the bioethics community pertaining to coercion and undue inducement. The conceptual definitions are highly variable, and as a result, different individuals reviewing an offer of payment may reach different conclusions in practice about whether that offer is coercive or unduly influential, and in turn, whether it is ethically permissible or impermissible. Moreover, it is easy to see that, depending on how two individuals define the respective terms, they could talk past one another. They may be using the same term to refer to different ethical concerns; different terms to refer to the same concern; or different terms to refer to different concerns.
Clearly, it is desirable for the human subjects research community to come to consensus on what these terms mean. We have argued that once one rejects research exceptionalism, certain definitions come to the fore, as depicted in Figure 2. Yet, even if one continues to defend research exceptionalism with regard to payment, it is possible to endorse our preferred definitions on the grounds of their superior explanatory power and consistency with the canon of non-surplusage.
Figure 2.
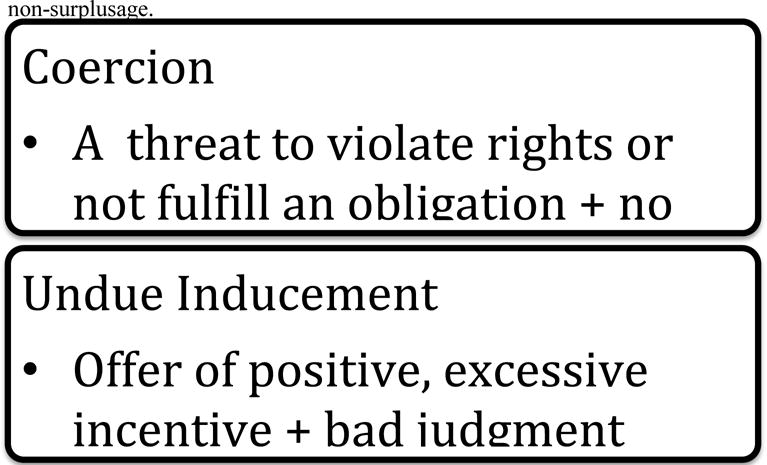
Best Definitions of Coercion and Undue Inducement
V. Case Study: Confusion in Practice
As the preceding sections have highlighted, it is reasonable to expect that the lack of substantive guidance regarding offers of payment from key regulatory agencies and other influential bodies in research ethics, the misguided tendency toward research exceptionalism, and the want of clarity about how to define coercion and undue influence will result in conceptual confusion among IRBs and investigators, as well as a general trend toward conservative approaches to payment. In this section, we present preliminary research that illustrates precisely such confusion and an emphasis on protecting subjects from payments that are deemed to be “too high.” The purpose of this case study is to show that the challenges identified herein are not just theoretical, but can have concrete effects in practice.
A. Institutional Guidelines
IRBs—and the institutions with which they are affiliated—have wide discretion when it comes to overseeing offers of payment made to research participants. As a result, one finds predictably wide variation in institutional policies. As part of this project, we reviewed payment-related policies for all of the IRBs affiliated with Harvard Catalyst. Harvard Catalyst, Harvard’s Clinical and Translational Science Center, is part of the National Clinical and Translational Science Award (CTSA) consortium241 and “works with Harvard schools and the academic healthcare centers (hospitals) to build and grow an environment where discoveries are rapidly and efficiently translated to improve human health.”242
In 2015, we reviewed official copies of policies and guidelines regarding payment of research participants for each of the Harvard Catalyst-affiliated institutions.243 Although we do not suggest that these institutions provide a representative sample of research institutions across the country, they do range from world-renowned academic medical centers to local community hospitals. Because the goal is simply to demonstrate variety, rather than to praise or criticize any institution’s policy, we refrain in this discussion from attributing particular policies to particular institutions.244
Several of the Harvard Catalyst-affiliated institutions share umbrella IRBs (and therefore were covered by a single policy). In all, six institutions had no policy governing offers of payment to research participants, whereas 13 IRBs (covering the remainder of the participating institutions) did have a payment-specific policy or policies.245 Of those with policies, there is a great deal of heterogeneity: whereas some largely parrot the regulations, others go into much more extensive detail. In Appendix 1, we have compiled information about each of these policies on a range of parameters.
When an institution has a policy regarding offers of payment to research participants, that policy can reasonably be expected to establish the default for how payment is viewed by both IRB members and investigators. Two policies were particularly striking in their contrast. The first of these stated: “It is sometimes desirable to provide payments to subjects and their families for their participation in research projects.”246 By contrast, the second stated:
It is not necessary, required, or desirable that all subjects involved in clinical research receive monetary compensation for their participation. Some subjects derive medical benefit as a result of their participation; some subjects volunteer out of sheer altruism … or for other personal reasons.247
The former sets a default that is much more favorable to offers of payment than the latter, and also seems to be more in line with approaches to payment that might be expected outside of the research context, whereas the latter appears to be influenced by research exceptionalism.
In reviewing these policies, we observed several trends relevant to our present discussion. First, and most notably, the vast majority of policies do not include definitions of either coercion or undue inducement, despite (or perhaps because of) the fact that these terms are not clearly defined in the U.S. federal regulations, nor are there broadly accepted definitions in the research ethics literature. There were two notable exceptions. The first defines coercion, roughly correctly, as “undue pressure.”248 The second, however, suggests coercion means “unduly inducing individuals to participate because compensation would be difficult to refuse.”249 Not only is this definition of coercion clearly incorrect on our preferred definitions, it mistakenly conflates coercion with undue influence, suggesting the terms are interchangeable when they are correctly understood as distinct.
Second, the policies reviewed also reflected the widespread—albeit mistaken on our view—belief that offers of payment can be coercive. One policy states, for instance: “Payment should not be coercive.”250 Another explains, “When subjects are being paid, the [IRB] will review both the amount of payment and the proposed method and timing of disbursement to assure that neither is coercive.”251 A third states, “The IRB reviews remuneration plans to assess whether the amount, schedule and type of any proposed compensation … could be considered coercive.”252 As we have stressed above, genuine offers of payment are never coercive because they do not threaten to violate an individual’s rights but instead expand an individual’s options.
Third, the policies generally allowed advertisements to indicate that payment would be offered, as long as undue emphasis was not placed on the offer of payment.253 A typical policy stated, “[A]dvertisements may state that Human Subjects will be paid, but should not emphasize the payment or the amount to be paid, by such means as larger or bold type.”254 None of the polices we reviewed expressly forbade inclusion of payment nor did they require that offers of payment be explicitly mentioned in the advertising materials. While the policies do not explicitly link limits on advertising to either coercion or undue inducement, presumably such limits are motivated by a fear that research participants could be inappropriately influenced to participate in research by an emphasis on payment in advertising materials. Given our view on the broad acceptability of offers of payment made to research participants, we believe policies that allow inclusion of reasonable information about payment at the investigators’ discretion are not only appropriate but ideal.
Of course, we understand the difficulty of drafting these policies in the absence of clear regulatory guidance and the presence of robust academic debate. The confusion they reflect is reasonable given the confused circumstances from which they emerge. Ideally, however, institutions would bridge the gap between policy and practice, defining crucial terms and providing substantive guidance on ethically acceptable offers of payment that could guide investigators and IRB members as they design and evaluate offers of payment made to research participants. There is, as we have shown, an unfortunate divergence between the ideal and reality. While this divergence is neither unexpected nor blameworthy, the lack of clear institutional guidance, layered upon a lack of clear regulatory guidance, likely reinforces a tendency toward conservative approaches to payment among IRB members and investigators.
B. Individual Survey Data
In addition to a review of institutional policies, we conducted pilot surveys of individuals at Harvard Catalyst-affiliated research institutions in order to develop preliminary data about attitudes of both IRB members and investigators regarding payment generally, and about their beliefs regarding coercion and undue inducement in particular. This is the first survey to assess how investigators, as opposed to IRB members alone, define these terms.
We included investigators in our sample because they are responsible for designing—and oftentimes justifying—the offer of payment that is submitted to the IRB for review. While factors extrinsic to ethical concerns about coercion and undue influence, most notably the study budget, will influence how much payment an investigator offers, their understanding of coercion and undue influence may be relevant, as well as their expectations regarding likely IRB response. Furthermore, it is useful to know how much daylight there is between the perspectives of IRB members and investigators on these issues to determine how best to address conservative approaches to payment moving forward.
1. Methods
Two online surveys were conducted. The first (hereafter, the “IRB Survey”) was sent to IRB members and administrators and was distributed via the Harvard Catalyst Regulatory Committee, which “is comprised of institutional officials, compliance officers, and directors of human research protections from Harvard Catalyst-participating institutions.”255 The second survey (hereafter, the “Investigator Survey”) was sent to investigators and study coordinators and distributed via the Harvard Catalyst Clinical Research Center (HCCRC) email list.256
Two draft survey instruments, one for IRB members and one for investigators, were developed using an iterative process that began with a comprehensive review of the literature on coercion and undue inducement and offers of payment to research participants and included several rounds of revision based on input from IRB members, administrators, and experts on the ethics of human subjects research. Because much of our work was exploratory in nature, we used a combination of open- and close-ended questions. The draft surveys were pretested with IRB members, administrators, and investigators who were asked to comment on the content and design of the survey. Feedback was incorporated to refine and clarify survey items. The Investigator Survey was finalized after we had the results from the IRB Survey, and several additional changes were made to further enhance clarity.257
Potential participants received an email embedded with an HTML link to the confidential, self-administered survey instrument, which was administered in Qualtrics, a web-based survey tool. Two subsequent reminder emails were sent. Responses received by June 1, 2015 were included in our analysis. This project was approved by the Committee on the Use of Human Subjects, the IRB for Harvard University’s Cambridge campus. No compensation was provided to participants.
Because this study was designed as an exploratory analysis, we summarized data using frequency distributions and descriptive statistics. We evaluated associations between responses using simple frequencies and evaluated the interrelationships between survey response items using cross-tabulations without adjustment for multiple comparisons. Statistical significance by chi-square test was defined as p < 0.05.
2. Results and Analysis
Of the 694 emailed invitations to participate in the IRB survey, 116 surveys were completed, for a response rate of 16.7%.258 Of the 1,596 emailed invitations to participate in the investigator survey, 115 surveys were completed, for a response rate of 7.2%.259
Respondents who provided demographic information were predominately non-Hispanic white (90%) and female (62%), with a mean age of 54 (±13) for IRB members and administrators and a median age of 41–50 for investigators.260 The majority of respondents (76%) held a masters, doctorate, or professional degree. Those with experience serving on an IRB had an average of 8 (±6) years of experience, and all but 7% said that their IRB reviewed biomedical research. Investigators reported submitting an average of 14 (±20) protocols to their current IRB. All respondents held a role or roles related to human subjects research (see Table 1).
Table 1.
Respondents’ Current Roles Related to Human Subjects Research
| Role1 | Frequency | Percent |
|---|---|---|
| Researcher | 85 | 36.8% |
| IRB Member | 91 | 39.4% |
| Study Coordinator | 54 | 23.4% |
| Research Nurse | 6 | 2.6% |
| Clinician, Non-Researcher | 14 | 6.1% |
| Professor | 39 | 16.9% |
| Ethicist | 8 | 3.5% |
| Sponsor | 3 | 1.3% |
| Regulator | 4 | 1.7% |
| Subject Recruiter | 11 | 4.8% |
| Evaluate Grants | 14 | 6.1% |
| Write Policy | 16 | 6.9% |
| Member of Human Research Protection Program | 14 | 6.1% |
| Other Study Staff | 10 | 4.3% |
| Other | 16 | 6.9% |
Respondents could choose more than one role
Beyond these demographics, however, we will generally present the results for investigators and IRB members together because there were few instances in which the differences in their answers reached statistical significance; where the difference was statistically significant, we have included a footnote indicating that to be the case. This is an interesting finding in itself because it shows that IRB members and investigators think about coercion and undue influence in similar ways.
Respondents were asked to select which of a given series of definitions properly defined coercion, and were permitted to select more than one option; we did not indicate which definition reflected our preferred view. See Table 2. Nearly all respondents agreed that a research participant is coerced if threatened with harm or loss of benefits to which he is otherwise entitled if he doesn’t participate in research (87.0%),261 a definition consistent with the rights-violating view of coercion we endorse. The vast majority also agreed that a research participant is coerced if he participates as the result of intimidation, or some other form of pressure or force (90.0%), consistent with the worse-off view. While we favor the rights-violating view, for reasons discussed above, there is often little difference between the two views in practice. These results are encouraging in the sense that they indicate that most respondents include the correct (by our analysis) definitions of coercion in their understanding of the term.
Table 2.
Definitions of Coercion and Undue Inducement
| % of respondents who agreed that if … | Then … it is coercion |
Then … it is undue inducement |
|---|---|---|
| The research participant is threatened with harm or loss of benefits to which he is otherwise entitled if he doesn’t participate in research | 87.0% | 55.8% |
| The research participant participates as the result of intimidation, or some other form of pressure or force | 90.0% | 60.6% |
| The offer of payment makes the research participant participate in research he would not otherwise participate in | 51.1% | 58.9% |
| The offer of payment distorts the research participant’s ability to perceive accurately the risks and benefits of research | 63.6% | 74.5% |
| The offer of payment causes the research participant to feel he has no reasonable alternative but to participate in research | 71.0% | 69.3% |
Less encouraging, however, is that respondents might also be including incorrect definitions. A majority agreed that a research participant is coerced if the offer of payment causes him to feel he has no reasonable alternative but to participate in research (71.0%), if the offer of payment distorts his ability to perceive accurately the risks and benefits of research (63.6%),262 or if the offer of payment makes him participate in research he would not otherwise participate in (51.1%). From our perspective, that a majority of respondents would endorse these definitions demonstrates a widespread and fundamental misunderstanding of what coercion is. With respect to the first option, although some ethicists defend the no-reasonable-alternative view of coercion, we indicated above why this approach is inconsistent with understandings of what counts as coercive outside of the research context, and why it must be rejected as an instance of inappropriate research exceptionalism. The second option, that offers of payment may distort comprehension of risks and benefits is the correct definition for undue inducement, not for coercion. This illustrates how the two terms are often conflated. Finally, the third option is consistent not with coercion but with an ethically unproblematic mere inducement. More than two-thirds (68.6%) of respondents agreed with the following statement, which we view to be false: “Offers of payment can be coercive.”
Next, respondents were given the same series of definitions and asked which defined undue influence. See Table 2. Three-quarters (74.5%) of respondents agreed that a research participant is unduly influenced if the offer of payment distorts his ability to perceive accurately the risks and benefits of research, which means that a full quarter of respondents failed to identify what we view to be the correct definition of undue inducement. It is perhaps most worrisome that more than half of the respondents (58.9%) agreed that research participants are unduly influenced if the offer of payment makes them participate in research they would not otherwise participate in. Again, this seems more accurately to describe a mere inducement (i.e., something that one would not otherwise have done), not one that is undue per se, and is an expansive view potentially at odds with the pervasive use of offers of payment as an incentive for participation in research.
In these numbers, we again see evidence that IRB members and investigators often conflate undue influence and coercion. The majority agreed that research participants are unduly influenced if they participate as the result of intimidation, or some other form of pressure or force (60.6%)263 or if they are threatened with harm or loss of benefits to which they are otherwise entitled if they do not participate in research (55.8%),264 both of which are definitions applicable instead to coercion.
Undue inducement and coercion are often said to be conflated,264 a claim consistent with our findings. Our data suggest that people use these terms somewhat interchangeably. Some individuals chose the same definitions for both coercion and undue inducement. Moreover, a majority of respondents (65.2%) agreed with the statement that “coercion is an extreme form of undue influence,” consistent with the “sliding scale view” and demonstrating a failure to appreciate that coercion and undue inducement are distinct concepts.266
Finally, two-thirds (67.4%) of respondents agreed with the statement “offering to pay subjects is different from offering to pay people in other contexts.” This finding is consistent with widespread research exceptionalism, which may, in addition to confusion about how to define the key terms, encourage conservative approaches to payment.
3. Limitations
This was an exploratory study without a nationally representative sample and with a low response rate, which imposes limits on the conclusions we can draw. While the respondents are professionally diverse and have considerable experience in human subjects research, they may have views that differ from others involved in the research enterprise, especially given that our results were generated exclusively from Harvard Catalyst-affiliated research institutions. Yet, as mentioned above, Harvard Catalyst encompasses institutions ranging from academic medical centers to community hospitals to schools of medicine and public health.
Another limitation to this exploratory data is that we asked about concepts only in the abstract, rather than including case studies. Thus, it is possible that even if IRB members and investigators adopt overly expansive definitions of coercion and undue inducement when asked about these terms in the abstract, these definitions have little impact on their decisions to approve or not approve offers of payment in specific instances. Yet, the federal Common Rule requires investigators to seek informed “consent only under circumstances…that minimize the possibility for coercion or undue influence,”267 and OHRP cautions investigators and IRBs to “be vigilant about minimizing the possibility of coercion or undue influence.”268 Therefore, although more research is needed, we hypothesize that these confused views do influence how IRBs interpret offers of payment as well as how investigators structure offers of payment.
In response to our pilot survey, some IRB members and investigators readily admitted to their confusion,269 and many others showed themselves to have a faulty conceptual understanding of coercion and undue inducement on our preferred definitions. Some respondents identified the best definitions while also endorsing incorrect views, suggesting that their understanding of these concepts is overly expansive. In some instances, respondents identified a legitimate ethical concern but called it by the wrong name. In other instances, they expressed concern about something that is not a legitimate ethical concern at all, but called it by an ethically charged name.
As a result, we fear that IRBs sometimes incorrectly reject offers of payment that really ought to be ethically acceptable, thereby eliminating a potentially important tool in clinical trial recruitment. The flip-side of this is that investigators share many of the misconceptions that IRB members have—not only do investigators have the same dearth of guidance on what these terms mean, they may also be reliant on the IRB to guide them in how to understand and apply these terms. As a result, they may not submit protocols with offers of payment that they expect will be met unfavorably by the IRB, or may fail to advocate for offers of payment once the IRB has questioned them, even when those payments really ought to be viewed as ethically acceptable.
While preliminary, our results suggest that guidance and educational efforts targeted at both IRB members and investigators are needed to clarify coercion and undue inducement and to address research exceptionalism if we are to advance the goals of research ethics to promote socially valuable research while providing appropriate protections for research participants.
VI. Implications for Policy and Practice: The Path Forward
Given the potential for confusion and conservative approaches to payment demonstrated above, it is clear that something must be done. Here, we will consider several possible solutions to the problems we have identified.
A. If Not Accuracy, Precision
In the field of science, accuracy tells us how close a measurement is to the true value. Precision, by contrast, refers to the closeness of two or more measurements to each other.270 Unfortunately, our data suggests that currently when IRB members and investigators define and use the terms coercion and undue inducement, they are often neither accurate nor precise. While we have argued above for the definitions that we think are best, we also recognize that reasonable disagreement is possible. In the face of disagreement among ethicists about what each of these concepts mean, it seems unrealistic—at least in the absence of a definitive statement from OHRP or FDA, which we discuss below—to ask that IRB members and investigators universally accept one meaning as factually correct. This may be particularly difficult, given an ingrained culture of payment conservatism. Therefore, accuracy might be too much to hope for, but precision is not.
How might we achieve precision? As a first step, we propose relying much less on these labels to do the heavy-lifting. It appears from our data and some strands of the bioethics literature that the terms coercion and undue inducement may be used as “catchalls” when something about research (e.g., an offer of payment) seems somehow not right. Because most everyone agrees that coercion and undue inducement in the context of human subjects research are wrong, use of these terms can be a conversation killer and result in not approving a protocol or an aspect of a protocol. Yet, to the extent that people understand these terms expansively or understand them in wildly different ways, people may well be talking past one another when these terms are used. Therefore, leveling the charge that an offer of payment is coercive or unduly influential should be the beginning, rather than the end, of the conversation. Individuals interested in protecting research participants should explain precisely why they think that a particular payment is problematic rather than assuming that the label alone does sufficient explanatory work, or that the label itself will carry the same meaning for the listener as it does for the speaker.
So, for example, instead of saying that a proposed offer of payment would create undue inducement, it would be vastly preferable to say that a proposed offer of payment appears so high that it might prevent prospective research participants from adequately evaluating the risks and burdens of enrolling in the associated trial, while also offering specific evidence for why that worry is present in this particular case. Employing that level of specificity will limit the extent to which individuals talk past each other and allow the conversation to be focused on the ethical concern at hand. To continue with the example, once the concern is expressed as money impinging on the evaluation of risks, it is possible to have a substantive discussion about whether the offer of payment is so high that it predictably creates a cognitive distortion, whether the research is otherwise ethical such that a reasonable person could agree to participate, or whether additional safeguards are needed for the informed consent process. Such questions would, for example, have been useful to assess prospectively the offer of payment made to research participants in France.
B. Changing the Default Rules to Favor Payment
As described above, we think that research exceptionalism is generally wrongheaded when it comes to offers of payment, and that offers of payment do not need to be subjected to greater scrutiny in the research context than elsewhere. If so, that is a strong argument in favor of changing the default to generally accept even high offers of payment to research participants unless there is compelling evidence that they are harmful. Even if one continues to accept some form of research exceptionalism, if coercion and undue inducement are not actually happening in practice when payment is offered to participants, then we are making mountains out of molehills when we set the default in favor of low (or no payment).
We have argued that coercion is incorrectly associated with genuine offers of payment. While undue inducement is a more credible concern when offers of payment are extended to research participants, we caution that there is little evidence that undue inducement is occurring in practice. As described above, empirical research has failed to substantiate the claim that offers of payment lead to irrational choices by research participants. In fact, some scholars have found that offers of payment heighten subjects’ attention to the risks and burdens of research participation. We suggest that many regulators, IRBs, investigators, and other stakeholders in human subjects research are, therefore, inappropriately concerned about offers of payment being too high in most cases. Offers of payment, even extremely high ones, should not generally be cause for ethical concern.
From our perspective, the larger concern is that subjects may be inadequately compensated for their contribution to socially beneficial research, which may slow recruitment, hinder retention, or exploit research participants who are not paid enough. According to Wertheimer, to exploit someone is to take unfair advantage of him or her.271 Exploitation occurs when, due to an asymmetry of bargaining power, one party to a transaction insufficiently benefits or assumes an unfair share of the burden relative to other parties to the transaction. The possibility of exploitation suggests that a default in favor of payment is preferable to a default against payment.
At a minimum, individuals “should not have to pay for making a contribution to the social good of research.”272 This entails providing reimbursement for any research-related expenses they incur and adequate compensation for their time and effort, as well as risks they willingly incur as a result of their participation in research. Such offers of payment demonstrate respect for research participants, and treat them in accordance to what would be expected outside the research context. In some studies, acceptable offers of payment may be de minimus (e.g., a study that consists of a one-time blood draw), but in other studies, the minimum acceptable payment may be substantially higher.
Additionally, offers of payment can unproblematically be used to incentivize research participation. We think it is fundamentally wrong to argue, as some have, that “the need for large incentives can be a rough indicator that there may be an ethical concern that requires attention.”273 People may simply wish to avoid the discomforts or burdens of research participation, and just as incentives are acceptable in other areas of life to override such reluctance, they are acceptable in the context of human subjects research—particularly if one accepts, as we do, the role of a well-functioning IRB in determining that the risks of a study are reasonable in relation to the benefits, either to the individual or to society.
We do note that some people worry “that poverty or otherwise compromised circumstances may force people to take an inducement that people in a better situation shun.”274 This concern is often raised when research is conducted in developing countries, but its application is not geographically limited. Yet, “tempting offers in desperate situations that have clear good results are not undue inducements”275 because accepting such an offer can be a reasoned judgment that does not necessarily contradict one’s interests. It is an unfortunate consequence of research exceptionalism to frame these offers as undue inducements, and it would be unacceptably paternalistic to protect competent research participants from their fully voluntary and rational undertakings. Moreover, it is backward to think that protecting them requires paying less in light of their poverty; ideally, the response should be to pay them more.
To demonstrate this point, consider that a person who is facing poverty might be willing to work as a day laborer, which may be risky and burdensome, whereas a more affluent person would not be willing to do so. Of course, this does not mean day laborers should be paid less. If we think paid day labor is acceptable, then it is an instance of research exceptionalism to suggest that paid research participation is unacceptable simply because more affluent individuals may not find participation a compelling offer, given other options they have available. The factors that lead some people to participate in research in order to earn a living or supplement their income might be circumstances we would all think of as unjust, and would prefer not to have occured, but those circumstances are not reasons to limit the options of competent adults given the realities—and other protections for research participants—that exist.
Additionally, although we do not think offers of payment are a panacea for recruitment problems, greater incentives may have the dual benefit of improving enrollment and drawing a more diverse pool of research participants. This could ensure that socially valuable research is completed and that the burdens and benefits of research participation are spread more broadly, more fairly over the population. While more empirical research is needed to determine the effect of offers of payment on participation,276 lack of completion due to low enrollment is known to be a problem. A 2015 study of 787 cancer trials, for example, found that 18% closed with low accrual or were accruing at less than 50% of target three years or more after initiation.277 A review of terminated trials in clinicaltrials.gov found that insufficient rate of accrual was a leading reason for trial termination.278 Additionally, and contrary to the logic that only the poor participate in trials, researchers have “found that patients with annual household incomes below $50,000 were 27% less likely to participate in [cancer] clinical trials.”279 These researchers speculated that “incentives or reimbursements may be appropriate” to promote fair access to cancer trials, but warned, mistakenly, that such payments “should not be coercive to patients.”280
In medicine, a false positive is an error where a result is improperly reported as positive when it actually is not. A false negative is an error where a result is improperly reported as negative when it actually is not.281 This is contrasted with a true result: a true positive or a true negative. The judgments of an IRB can be fallible just as medical tests can be fallible. We might equate disapproval of an offer of payment that is actually ethically acceptable with a false negative. Although our survey data do not allow us to determine conclusively how frequently this occurs, the attitudes reflected in the survey suggest that under the current scheme, there may be many false negatives.
Some false positives or false negatives may be unavoidable. One consequence of changing the default to generally accept offers of payment is that some offers of payment that are ethically concerning might get through—yet, we expect that this is only a slight possibility. We have argued that coercion and undue inducement are unlikely to occur in otherwise ethical clinical research. Given that the harms from overpayment are generally overstated, and the harms from underpayment are understated or even ignored, we advocate changing the default rules so that offers of payment will be deemed acceptable unless someone can articulate a clear (i.e., precise) and persuasive—as opposed to speculative—reason why it is not.
C. Policy Guidance and Rulemaking
Policy guidance and educational efforts are sorely needed to clarify the concepts of coercion and undue inducement as applied to payment in the research setting. Unfortunately, it is unlikely that the U.S. regulations will be amended to address this issue in the near future, but there are other avenues to improvement.
In November 2009, representatives from HHS and other departments convened to draft the first substantive reforms to the Common Rule since it was published in 1991; these representatives had the dual aims of enhancing research participant protections and increasing the efficiency of the research oversight process.282 Their meetings led to the release of an Advanced Notice of Proposed Rulemaking (ANPRM) entitled “Human Subjects Research Protections: Enhancing Protections for Research Subjects and Reducing Burden, Delay, and Ambiguity for Investigators” in July 2011.283 The ANPRM did not substantively address payment, coercion, or undue inducement.
In September 2015, the long awaited NPRM284 was published in the Federal Register.285 Coming in at 131 Federal Register pages, the NPRM proposed a number of significant changes to the Common Rule, as well as numerous minor ones.286 Again, however, payment was not substantively addressed.
Most recently, in January 2017, on the last day of President Obama’s administration, the final rule was published in the Federal Register, completing a long and drawn out regulatory process, the outcome of which remains unclear in light of its timing and the present political climate. Given the intense difficulty of getting to this point, it is extremely unlikely that new rulemaking will be forthcoming any time soon. The final rule modifies populations that are deemed likely to be vulnerable to coercion and undue influence, dropping reference to pregnant women and those with physical disabilities – but it does nothing to clarify the definition of the terms or their precise role in evaluating offers of payment.287
Unfortunately, this was likely a lost opportunity. If past experience is any guide, the research community will be working with the rule finalized in 2017 for some time (assuming it survives the political process and change in administrations), meaning that additional formal rulemaking specifically regarding payment is unlikely in the foreseeable future.
Therefore, we propose that OHRP update its FAQs and that the FDA update its Information Sheet on payment to research participants, at least as a first step. While this guidance would not be binding, as the embodiment of the agencies’ current thinking, it would likely be persuasive for many IRBs and investigators and could help to address the present payment-conservative IRB culture. Indeed, Jerry Menikoff, Director of OHRP, suggested at a recent public meeting that OHRP is not particularly worried about payment resulting in undue inducement, which he believes—as we do—to be rare.288 This perspective indicates that clarifying OHRP guidance on this topic would potentially be feasible, with the salutary effect of rendering IRBs less worried about enforcement actions should they approve higher payments.
Any such guidance should provide clear definitions of coercion and undue inducement, as well as of exploitation—a concern that is not currently addressed at all, but that we think is ethically salient, and increasingly so as more research is conducted in developing countries. We would strongly advocate for our preferred definitions. At a minimum, this guidance should clarify—by stating explicitly rather than leaving it for the reader to infer—that genuine offers of payment are never coercive and reflect the empirical evidence suggesting that undue inducement is rare. It should also emphasize the importance of offering reimbursement for research-related expenses and compensation for time, effort, and inconvenience. Ideally, the guidance would also state that use of offers of payment to incentivize research participation are generally acceptable and that payment can be used to address exploitation, or an unfair distribution of research benefits and burdens.
Additionally, we encourage efforts to reform international research guidelines pertaining to payment. The recently revised 2016 CIOMS guidelines, discussed above, are particularly welcome in this respect.289 While these documents are of variable legal effect, they can be very influential in how people think about the ethics of human subjects research.
Conclusion
The practice of offering payment to individuals in exchange for their participation in clinical research is widespread and longstanding. Nevertheless, offers of payment to research participants remain the source of substantial debate. Two ethical charges routinely arise in relation to these offers—that they are coercive or unduly influential. Because there is general agreement that coercion and undue inducement are wrong in human subjects research, such a charge can shut down conversation among IRB members and investigators, and result in rejection of an offer of payment, or failure to make an offer in the first place.
As we have recounted, the various laws, regulations, and ethical guidelines that govern the conduct of human subjects research offer relatively little in the way of specific guidance about what factors or features characterize ethically acceptable offers of payment. Additionally, there is a lack of agreement regarding what exactly the terms coercion and undue inducement mean in the human subjects research context. It is, therefore, unsurprising that the space inhabited by IRB members and investigators is characterized by confusion and conservatism. The results of our pilot survey suggest that IRB members and investigators are worried about things that they probably do not need to be worried about. That may lead to overprotection, and possibly distraction from things they should actually be worried about—particularly the possibility that offers of payment are too low. Ultimately, resolving misplaced concerns about offers of payment being too high will offer investigators a more powerful recruitment tool and, hopefully, speed the pace of innovation and discovery.
Acknowledgments
Thank you to Barbara E. Bierer, I. Glenn Cohen, Luke Gelinas, Mildred Solomon, Kathy Swartz, and Sabune Winkler for helpful comments on prior drafts, as well as the anonymous peer reviewers from the Yale Journal of Health Policy, Law, and Ethics. Thank you to Hila Bernstein for research assistance and to the individuals who participated in our surveys. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Appendix 1. Comparing Policies of Harvard Catalyst-Affiliated Research Institutions on Offers of Payment to Research Participants
| Institution A | Institution B | Institution C | |
|---|---|---|---|
| Policy Regarding Payment | Yes | Yes | Yes |
| Discussion of Coercion, Undue Influence, or Exploitation (either direct or indirect) | “Remuneration may not be sizeable enough to induce subjects to participate, regardless of how minimal the risk.” | “The goal of IRB oversight of research subject compensation is to ensure that stipends paid to research subjects provide fair compensation without undue pressure (coercion) to participate. Excessive monetary compensation may cause subjects to undertake risks or discomforts that they otherwise would not assume. This unfairly targets subjects of lower socioeconomic groups and places more of the “risk burden” of medical research on these groups. In the case of healthy volunteer studies, the IRB is often in the position of suggesting decreased compensation over that suggested by investigators, in an effort to decrease the element of financial coercion.” | “The [IRB] shall determine that Human Subjects are not subject to coercion or undue influence to participate in the Research. Factors such as, but not limited to, … payment for participation, and unfair inducements should be taken into consideration.” “The [IRB] is required to review payments to subjects to determine that: (1) The amount of payment and the proposed method and timing of disbursement is neither coercive [n]or presents undue influence… . (3) Any amount paid as a bonus for completion is reasonable and not so large as to unduly induce subjects to stay in the study when they would otherwise have withdrawn.” |
| Definitions of Key Terms | “undue pressure (coercion)” | ||
| Recognized Uses of Payment | Reimbursement Compensation Tokens of appreciation Incentives |
Reimbursement Compensation |
|
| Factors Influencing the Acceptability of Payment | “The [IRB] will consider the protocol, including the time commitment and the proposed procedures, when determining if the planned amount is appropriate… . The [IRB] recognizes that varying amounts and methods of remuneration may be appropriate depending on the particular circumstances of a protocol.” | ||
| Amounts | “There are no established policies as to the amount … of payments that may be offered.” “The [IRB] does not have a set list of recommended remuneration amounts for specific tests or length of visits, nor does it require that one method (gift cards, cash, etc.) must be used.” |
“[A] list of approximate monetary compensations for a variety of frequently performed clinical activities is listed below. This list is meant to guide investigators, and is based upon active protocols currently approved by the [IRB]. Although not every procedure is listed, these amounts may guide investigators by allowing comparison of new procedures in terms of time and discomfort.” | |
| Prorating | “Investigators may not require that a subject complete the research in order to receive compensation. If a subject withdraws from a study, he or she must be offered payment for the completed portion of the study.” | “It is a general policy that compensation for participation in research projects is pro-rated according to the amount of time devoted to the project.” | “The [IRB] is required to review payments to subjects to determine that … [c]redit for payment accrued as the study progresses is not contingent upon the subject completing the entire study.” |
| Completion Bonuses | “Completion bonuses” or additional payments above and beyond reimbursements … are generally discouraged in pediatric research however the [IRB] will consider whether an incentive unduly influences a child and/or family to participate when reviewing and approving this type of payment.” | “In many protocols where completion of all visits or procedures is paramount, there is some element of “incentive” provided by withholding some compensation until the end of the study, or providing a “bonus” for completion of all segments of the study. Such procedures should be explained and rationalized in detail in the research protocol, and clearly outlined in the informed consent documents.” | “The [IRB] is required to review payments to subjects to determine that … [a]ny amount paid as a bonus for completion is reasonable and not so large as to unduly induce subjects to stay in the study when they would otherwise have withdrawn.” |
| Informed Consent | Should include when participant will receive remuneration, what will be provided, and “other appropriate details” | Should include information on completion bonuses. | “Investigator should seek consent under circumstances that minimize coercion or undue influence” |
| Advertising | “If participants will receive compensation/reimbursements, it can be noted (e.g. reimbursement for parking and/or your time will be provided). However, do not overly stress the compensation. In general, the [IRB] does not allow dollar values to be specified.” | “All advertisements should be tastefully composed and not inappropriately emphasize monetary remuneration.” “Specify the amount of monetary compensation (if you wish).” “Don’t: Feature monetary compensation as a lead in before the description of study purpose and procedures; bold, italicize, underline or enlarge fonts on type describing monetary compensation.” |
“Advertisements may state that Human Subjects will be paid, but should not emphasize the payment or the amount to be paid, by such means as larger or bold type.” |
| General Attitudes Toward Payment | “It is sometimes desirable to provide payments to subjects and their families for their participation in research projects.” | “It is not necessary, required, or desirable that all subjects involved in clinical research receive monetary compensation for their participation.” |
| Institution D | Institution E | Institution F | |
|---|---|---|---|
| Policy Regarding Payment | Yes | Yes | Yes |
| Discussion of Coercion, Undue Influence, or Exploitation - direct or indirect | “The … IRB must determine that the following requirements are satisfied before it approves research: … There are appropriate additional safeguards included in the study to protect the rights and welfare of participants who are likely to be vulnerable to coercion or undue influence.” | “The IRB shall review both the amount of payment and the proposed method and timing of disbursement to determine that neither are coercive nor present undue influence.” | “The IRB reviews remuneration plans to assess whether the amount, schedule and type of any proposed compensation is fair for the participant, and to assess whether the payments could be considered coercive (i.e., by unduly inducing individuals to participate because compensation would difficult to refuse.” |
| Definitions of Key Terms | Remuneration | ||
| Recognized Uses of Payment | “Payment to research subjects for participation in studies is considered compensation for time and inconvenience rather than a benefit to subjects.” | Compensation | |
| Factors Influencing the Acceptability of Payment | “In general, remuneration … should be comparable to other projects involving similar time, effort, and inconvenience.” | ||
| Amounts | |||
| Prorating | “Payment(s) shall be made to the subject as the study progresses and shall not be contingent upon the subject completing the entire study. If, for example, payment is made for each appointment attended, the payment must be made after each appointment.” | “In general, remuneration … [s]hould be pro-rated based on the number of procedures and study visits and should not be conditioned on completing the entire study, although a bonus for completing the study may be acceptable.” | |
| Completion Bonuses | “Any amount paid as a bonus for completion must be reasonable and not so large as to unduly induce participants to stay in the study who otherwise would have withdrawn.” | ||
| Informed Consent | “[A] timetable for the payments themselves must be … presented to every subject as part of the Informed Consent process.” “The Informed Consent Form must clearly establish how the subject is to be paid, i.e. cash, check, etc. A subject must sign a receipt for any cash payment, and this procedure must also be described as part of the Informed Consent process.” |
“In general, remuneration … [s]pecifics (including the amount per visit and payment schedule) should be documented in the consent form under the “Compensation” section–but not under the “Benefits section.” | |
| Advertising | “Advertisements may state that subjects will be paid, but should not emphasize the payment or the amount of be paid, by such means as larger or bold type and compensation information should be added towards the bottom of the advertisement.” | “Advertising materials shall not include the following: … an emphasis on the payment or the amount to be paid, by such means as larger or bold type. The IRB has authority to approve whether compensation shall be included in the advertisement.” | “Recruitment materials should not emphasize remuneration for participation (e.g., larger or bold type).” |
| General Attitudes Toward Payment | “Remuneration … ordinarily offered as a form of appreciation for the individual’s time and effort in the research project” |
| Institution G | Institution H | Institution I | |
|---|---|---|---|
| Policy Regarding Payment | Yes | Yes | Yes |
| Discussion of Coercion, Undue Influence, or Exploitation - direct or indirect | “The [IRB] is required to review payments to subjects to determine that: … The amount of payment and the proposed method and timing of disbursement is neither coercive or presents undue influence.” See also ‘Completion Bonuses’ |
“Under Federal regulations, the [IRB] must review and approve methods used to recruit subjects to ensure that the methods are not coercive.” | “PIs are responsible to: … Ensure the informed consent process is free from coercion or undue influence.” “NOTE: Payment cannot be held until the end of the study as that is potentially coercive.” |
| Definitions of Key Terms | |||
| Recognized Uses of Payment | Reimbursement Compensation |
||
| Factors Influencing the Acceptability of Payment | |||
| Amounts | |||
| Prorating | “The [IRB] is required to review payments to subjects to determine that: … Credit for payment accrued as the study progresses is not contingent upon the subject completing the entire study.” | “Indicate how much subjects will receive for each portion of the study completed and the payment form (e.g., cash, check, gift card). Specify the payment schedule, including a prorated plan should a subject withdraw or be withdrawn from a study prior to his/her completion.” | |
| Completion Bonuses | “The [IRB] is required to review payments to subjects to determine that: … Any amount paid as a bonus for completion is reasonable and not so large as to unduly induce subjects to stay in the study when they would otherwise have withdrawn.” | ||
| Informed Consent | “The IRB, when appropriate, will … consider whether the following additional elements of informed consent are required and whether they are adequately included in the [informed consent document]: … An explanation of the payment plan or a statement that subjects will not be paid for participation.” | ||
| Advertising | “Advertisements may state that subjects will be paid, but should not emphasize the payment or the amount to be paid, by such means as larger or bold type.” | ||
| General Attitudes Toward Payment |
| Institution J | Institution K | Institution L | |
|---|---|---|---|
| Policy Regarding Payment | Yes | Yes | Yes |
| Discussion of Coercion, Undue Influence, or Exploitation - direct or indirect | In the consent process section, “Describe any steps that will be taken to minimize the possibility of coercion or undue influence. “ | “When subjects are being paid, the [IRB] will review both the amount of payment and the proposed method and timing of disbursement to assure that neither is coercive.” “The [IRB] must review both the amount of payment and the proposed method of disbursement to ensure that neither entails problems of coercion or undue influence.” “The [IRB] pays particular attention to remuneration and other inducements that might encourage people with limited resources to participate in research projects in which they might not otherwise participate. Compensation should not be the sole grounds for participation in a research project, and should not cause participants to assume risks that they would not ordinarily find acceptable. The [IRB] considers persons with limited resources to be vulnerable to the extent that inducements to participate in research may result in their acting against their own best interests. Where the population from which subjects will be recruited primarily consists of people with limited resources, … [t]he investigator will be asked to justify the compensation being offered. If the [IRB] finds it to be coercive, then the [IRB] will ask the investigator to provide alternative compensation so as not to impede the subjects’ decision about whether they should participate in the research project.” |
“Subjects may receive reasonable payment for the time and trouble associated with participating in a study. Payment should not be coercive.” |
| Definitions of Key Terms | |||
| Recognized Uses of Payment | Reimbursement Compensation | Reimbursement Incentive | |
| Factors Influencing the Acceptability of Payment | “In general payments should be proportional to the degree of risk, inconvenience, or discomfort associated with participation. “ | ||
| Amounts | “All subjects should be paid the same.” | ||
| Prorating | “If a subject withdraws before the conclusion of the experiment, payment must be pro-rated.” | ||
| Completion Bonuses | “Incentive or bonus payments may … be appropriate under certain circumstances to encourage completion of experiments. Such payments may not be given for assuming increased risk.” | ||
| Informed Consent | “Both the informed consent discussion and the written informed consent form and any other written information to be provided to participants should include explanations of the following … The anticipated prorated payment, if any, to the participant for participating in the trial.” “Include the following information … Money or other forms of compensation or reimbursement, e.g., gift certificate, meal voucher, parking voucher, and travel expenses. Include the method and timing of the compensation… . Include how the amount of compensation is calculated if the participant does not complete the entire study for any reason.” “If participants will not be paid or will not receive other forms of compensation for participation, please state so.” |
“The consent form must describe the terms of payment and the conditions under which subjects would receive partial payment or no payment (e.g., if they withdraw from the study before their participation is completed).” | “The informed consent document should mention the possibility of tax withholding, when appropriate.” “If some groups are either not paid or paid differently from other groups, the differences in payment must be explained in the informed consent form.” |
| Advertising | “The [IRB] will review advertisements to ensure that they do not … unduly emphasize the amount subjects receive in compensation.” | ||
| General Attitudes Toward Payment | “Payment to research subjects may be an incentive for participation or a way to reimburse a subject for travel and other expenses incurred due to participation. However, payment for participation is not considered a research benefit. Regardless of the form of remuneration, investigators must take care to avoid coercion of subjects. In general payments should be proportional to the degree of risk, inconvenience, or discomfort associated with participation.” |
| Institution M | |
|---|---|
| Policy Regarding Payment | Yes |
| Discussion of Coercion, Undue Influence, or Exploitation - direct or indirect | “Subjects must give consent without coercion or undue influence and the prospective subject or legally authorized representative must be provided with sufficient opportunity whether or not to participate in the research.” |
| Definitions of Key Terms | |
| Recognized Uses of Payment | |
| Factors Influencing the Acceptability of Payment | |
| Amounts | |
| Prorating | |
| Completion Bonuses | “If completion of research is not a condition of compensation, you must describe how compensation will be prorated and calculated for subjects who withdraw early.” |
| Informed Consent | “All information concerning payment to subjects, including the amount, type (cash, check, or in kind) and schedule of payments, must be included in the consent form.” |
| Advertising | |
| General Attitudes Toward Payment |
Contributor Information
Emily A Largent, Research Associate, Petrie-Flom Center for Health Law Policy, Biotechnology, and Bioethics, Harvard Law School Regulatory Foundations, Ethics, and Law Program, Harvard Catalyst | The Harvard Clinical and Translational Science Center.
Holly Fernandez Lynch, Executive Director, Petrie-Flom Center for Health Law Policy, Biotechnology, and Bioethics, Harvard Law School; Faculty, Center for Bioethics, Harvard Medical School; Regulatory Foundations, Ethics, and Law Program, Harvard Catalyst | The Harvard Clinical and Translational Science Center.
References
- 1.Chan Sewell. 6 Hospitalized, One of Them Brain-Dead, After Drug Trial in France. NY Times; Jan 15, 2016. http://www.nytimes.com/2016/01/16/world/europe/french-drug-trial-hospitalization.html [ https://perma.cc/H4LQ-BU73] [Google Scholar]
- 2.Brosky John, Sheridan Cormac. Six Hospitalized in Bial Clinical Trial in France. BioWorld; http://www.bioworld.com/content/six-hospitalized-bial-clinical-trial-france [ https://perma.cc/NM6D-KC2C] [Google Scholar]
- 3.Butler Declan, Callaway Ewen. Scientists in the Dark After French Clinical Trial Proves Fatal. Nature. 2016;529:263. doi: 10.1038/nature.2016.19189. 263. [DOI] [PubMed] [Google Scholar]
- 4.Enserink Martin. More Details Emerge on Fateful French Drug Trial. Science. 2016 Jan 16; http://www.sciencemag.org/news/2016/01/more-details-emerge-fateful-french-drug-trial [ https://perma.cc/6HFB-TTNL]
- 5.Butler Declan, Callaway Ewen. Researchers Question Design of Fatal French Clinical Trial. Nature. 2016 Jan 22; doi: 10.1038/nature.2016.19189. http://www.nature.com/news/researchers-question-design-of-fatal-french-clinical-trial-1.19221 [ https://perma.cc/J5JG-6JLJ] [DOI] [PubMed]
- 6.We prefer and will use the term “research participant” rather than “research subject.” While “subject” is the more traditional of the two terms, over the past several decades, there has been a shift to using “participant” because many see it as more respectful. There continues, however, to be debate. See; Hall Ali. What’s in a name? Research “participant” versus research “subject”. Prim&r. 2014 Jan 6; http://primr.blogspot.com/2014/01/whats-in-name-research-participant.html [ https://perma.cc/7KA7-865W]
- 7.Gordon Bruce G, Brown Joseph, Kratochvil Christopher, Prentice Ernest D. In: Paying Research Subjects. Amdur Robery J, Banker Elizabeth A., editors. 2002. (Institutional Review Board: Management and Function 154). [Google Scholar]
- 8.U.S. Dep’t of Health & Human Servs. Institutional Review Board Guidebook. 1993 ch 1. [Google Scholar]
- 9.See generally; Probstfield Jeffrey L, Frye Robert L. Strategies for Recruitment and Retention of Participants in Clinical Trials. JAMA. 2011;306:1798. doi: 10.1001/jama.2011.1544. [DOI] [PubMed] [Google Scholar]; Kitterman Darlene R, Cheng Steven K, Dilts David M, Orwoll Eric S. The Prevalence and Economic Impact of Low-Enrolling Clinical Studies at an Academic Medical Center. Acad Med. 2011;86:1360. doi: 10.1097/ACM.0b013e3182306440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halpern Scott D, Karlawish Jason HT, Berlin Jesse A. The Continuing Unethical Conduct of Underpowered Clinical Trials. JAMA. 2002;288:358. doi: 10.1001/jama.288.3.358. 358. [DOI] [PubMed] [Google Scholar]
- 11.Unger Joseph M, Gralow Julie R, Albain Kathy S, Ramsey Scott D, Hershman Dawn L. Patient Income Level and Cancer Clinical Trial Participation: A Prospective Survey Study. JAMA Oncology. 2016;2:137. doi: 10.1001/jamaoncol.2015.3924. 137. “[L]imiting income disparities is important for ensuring rapid enrollment and fair access to trials.”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Largent Emily A, Grady Christine, Miller Franklin G, Wertheimer Alan. Money, Coercion, and Undue Inducement: A Survey of Attitudes About Payments to Research Participants. IRB: Ethics & Hum Res. 2012;34:1. [PMC free article] [PubMed] [Google Scholar]; Klitzman Robert. How IRBs View and Make Decisions About Coercion and Undue Influence. J Med Ethics. 2013;39:224. doi: 10.1136/medethics-2011-100439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wendler David. (The Stanford Encyclopedia of Philosophy).Zalta Edward N., editor. The Ethics of Clinical Research. 2012 http://plato.stanford.edu/archives/fall2012/entries/clinical-research/ [ https://perma.cc/W7ME-A2H2]
- 14.45 C.F.R. § 46.102(f) (2015). The amended regulations, finalized in January 2017 and effective in 2018 (assuming no change before then), define a human subject as “a living individual about whom an investigator … conducting research: (i) Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or (ii) Obtains, uses, studies, analyzes, or generates identifiable private, information or identifiable biospecimens.”; Department of Homeland Security et al. Final rule: Federal policy for the protection of human subjects. 2017;(12):82. 7149. Fed Reg. 7260. See also 21 C.F.R. § 50.3(6) (“Human subject means an individual who is or becomes a participant in research, either as a recipient of the test article or as a control. A subject may be either a healthy human or a patient.”). [PubMed] [Google Scholar]
- 15.Wendler, supra note 13, at n.p. Social behavioral research studies individuals’ responses to internal and external stimuli. While social-behavioral research is not the focus of this paper, payment is often used in that research as well. Many of the concerns raised herein would also be relevant in that context. See also 21 C.F.R. § 50.3(c) (“Clinical investigation means any experiment that involves a test article and one or more human subjects and that either is subject to requirements for prior submission to the Food and Drug Administration under section 505(i) or 520(g) of the act, or is not subject to requirements for prior submission to the Food and Drug Administration under these sections of the act, but the results of which are intended to be submitted later to, or held for inspection by, the Food and Drug Administration as part of an application for a research or marketing permit.”).
- 16.National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (Nat’l Comm’n), Ethical Principles and Guidelines for the Protection of Human Subjects of Research (1979) [hereinafter The Belmont Report].
- 17.Largent Emily A, Joffe Steven, Miller Franklin G. Can Research and Care Be Ethically Integrated? Hastings Center Rep. 2011;41:37. doi: 10.1002/j.1552-146x.2011.tb00123.x. 37. [DOI] [PubMed] [Google Scholar]
- 18.Id. at 37–38.
- 19.Miller Franklin G, Brody Howard. A Critique of Clinical Equipoise: Therapeutic Misconception in the Ethics of Clinical Trials. Hastings Center Rep. 2003;33:19. 21. [PubMed] [Google Scholar]
- 20.Largent Emily A, Joffe Steven, Miller Franklin G. A Prescription for Ethical Learning. Hastings Center Rep. 2013;43:S28. doi: 10.1002/hast.135. S28. [DOI] [PubMed] [Google Scholar]
- 21.See, e.g.,; Dickert Neal, Grady Christine. What’s the Price of a Research Subject? Approaches to Payment for Research Participation. New Eng J Med. 1999;341:198. doi: 10.1056/NEJM199907153410312. 198. [DOI] [PubMed] [Google Scholar]; see also; Grady Christine, Dickert Neal, Jawetz Tom, Gensler Gary, Emanuel Ezekiel. An Analysis of U.S. Practices of Paying Research Participants. Contemp Clinical Trials. 2005;26:365. doi: 10.1016/j.cct.2005.02.003. 366. [DOI] [PubMed] [Google Scholar]; Grady Christine. Money for Research Participation: Does It Jeopardize Informed Consent? Am J Bioethics. 2001;1:40. doi: 10.1162/152651601300169031. 40. [DOI] [PubMed] [Google Scholar]
- 22.Hutt Leah E. Paying Research Subjects: Historical Considerations. Health L Rev. 2003;12:16. 16. Offers of payment to research participants are often defended on the pragmatic grounds that they facilitate timely recruitment of the right numbers and types of participants. [PubMed] [Google Scholar]; See, e.g.; Dunn Laura B, Gordon Nora E. Improving Informed Consent and Enhancing Recruitment for Research by Understanding Economic Behavior. JAMA. 2005;293:609. doi: 10.1001/jama.293.5.609. While there is a need for more empirical research to show how increasing incentives affects recruitment for clinical trials specifically, there is evidence from survey research that larger offers of payment improve recruitment. [DOI] [PubMed] [Google Scholar]; See, e.g.; Keating Nancy L, Zaslavsky Alan M, Goldstein Judy, West Dee W, Ayanian John Z. Randomized Trial of $20 Versus $50 Incentives to Increase Physician Survey Responses. Medical Care. 2008;46:878. doi: 10.1097/MLR.0b013e318178eb1d. [DOI] [PubMed] [Google Scholar]; Ulrich Connie M, Danis Marion, Koziol Deloris, Garrett-Mayer Elizabeth, Hubbard Ryan, Grady Christine. Does It Pay to Pay? A Randomized Trial of Prepaid Financial Incentives and Lottery Incentives in Surveys of Nonphysician Healthcare Professionals. Nursing Res. 2005;54:178. doi: 10.1097/00006199-200505000-00005. [DOI] [PubMed] [Google Scholar]; Halpern Scott D, Ubel Peter A, Berlin Jesse A, Asch David A. Randomized Trial of $5 Versus $10 Monetary Incentives, Envelope Size, and Candy to Increase Physician Response Rates to Mailed Questionnaires. Med Care. 2002;40:834. doi: 10.1097/00005650-200209000-00012. [DOI] [PubMed] [Google Scholar]; Asch David A, Christakis Nicholas A, Ubel Peter A. Conducting Physician Mail Surveys on a Limited Budget: A Randomized Trial Comparing $2 Bill versus $5 Bill Incentives. Med Care. 1998;36:95. doi: 10.1097/00005650-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 23.E.g.; Chambers Tod. Participation as Commodity, Participation as Gift. Am J Bioethics. 2001;1:48. doi: 10.1162/152651601300169077. [DOI] [PubMed] [Google Scholar]
- 24.See generally; Elliott Carl, Abadie Roberto. Exploiting a Research Underclass in Phase 1 Clinical Trials. New Eng J Med. 2008;358:2316. doi: 10.1056/NEJMp0801872. [DOI] [PubMed] [Google Scholar]; Emanuel Ezekiel J. Undue Inducement: Nonsense on Stilts? Am J Bioethics. 2006;5:9. doi: 10.1080/15265160500244959. [DOI] [PubMed] [Google Scholar]; Grant Ruth W, Sugarman Jeremy. Ethics in Human Subjects Research: Do Incentives Matter? J Med & Phil. 2004;29:717. doi: 10.1080/03605310490883046. [DOI] [PubMed] [Google Scholar]; Lemmens Trudo, Elliott Carl. Guinea Pigs on the Payroll: The Ethics of Paying Research Subjects. Accountability in Res. 1999;7:3. doi: 10.1080/08989629908573939. In addition to broad concerns about offers of payment to research participants, unique ethical concerns also arise with respect to particular sub-populations of participants, for example, drug users. [DOI] [PubMed] [Google Scholar]; See, e.g.; Fry Craig L, Hall Wayne, Ritter Alison, Jenkinson Rebecca. The Ethics of Paying Drug Users Who Participate in Research: A Review and Practical Recommendations. J Empirical Res on Hum Res Ethics. 2006;1:21. doi: 10.1525/jer.2006.1.4.21. [DOI] [PubMed] [Google Scholar]
- 25.E.g., 45 C.F.R. § 46 (2015); see also; Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines for Health-Related Research Involving Humans. 2016;44 http://www.cioms.ch/ethical-guidelines-2016/ [ https://perma.cc/AKC2-TXXC].(stating that the informed consent process requires “ensuring that the person has adequately understood the material facts and has decided or refused to participate without having been subjected to coercion, undue influence, or deception.”) [Google Scholar]
- 26.Emanuel Ezekiel J, Wendler David, Grady Christine. What Makes Clinical Research Ethical? JAMA. 2000;283:2701. doi: 10.1001/jama.283.20.2701. 2706. [DOI] [PubMed] [Google Scholar]
- 27.See generally; Berg Jessica W, Applebaum Paul S, Lidz Charles W, Parker Lisa S. Informed Consent: Legal Theory and Clinical Practice. 2001:249. [Google Scholar]
- 28.E.g.; Casarett David, Karlawish Jason, Asch David A. Paying Hypertension Research Subjects: Fair Compensation or Undue Inducement? J Gen Intern Med. 2002;17:651. doi: 10.1046/j.1525-1497.2002.11115.x. 651. “Undue inducements decrease voluntariness, an essential component of valid consent.”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.E.g.; Largent Emily, Grady Christine, Miller Franklin G, Wertheimer Alan. Misconceptions About Coercion and Undue Influence: Reflections on the Views of IRB Members. Bioethics. 2013;27:500. doi: 10.1111/j.1467-8519.2012.01972.x. 507. arguing that coercion compromises voluntariness, whereas undue influence compromises comprehension of risks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone Judy. Bial’s Clinical Trial in France Ends in Disaster. What Went Wrong? Forbes. 2016 Jan 16; http://www.forbes.com/sites/judystone/2016/01/16/bials-french-clinicial-trial-ends-in-disaster-what-went-wrong/#6a59c2f49b2c [ https://perma.cc/A72X-YYCF]
- 31.Emanuel, Wendler & Grady, supra note 26, at 2704 (discussing the ethical importance of fair subject selection)
- 32.Whereas inclusion criteria are characteristics that individuals must have in order to participate, exclusion criteria are characteristics the possession of which disqualifies an individual. See generally; Van Spall Harriette GC, Toren Andrew, Kiss Alex, Fowler Robert A. Eligibility Criteria of Randomized Controlled Trials Published in High-Impact General Medicine Journals: A Systematic Sampling Review. JAMA. 2007;297:1233. doi: 10.1001/jama.297.11.1233. 1233. [DOI] [PubMed] [Google Scholar]
- 33.Dominguez Dinora, Jawara Mandy, Martino Nicole, Sinaii Ninet, Grady Christine. Commonly Performed Procedures in Clinical Research: A Benchmark for Payment. Contemp Clinical Trials. 2012;33:860. doi: 10.1016/j.cct.2012.05.001. 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.See, e.g.,; Stunkel Leanne, Grady Christine. More Than Money: A Review of the Literature Examining Healthy Volunteer Motivations. Contemp Clinical Trials. 2011;32:342. doi: 10.1016/j.cct.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.E.g.; Almeida Luis, Azevedo Benedita, Nunes Teresa, Vaz-da-Silva Manuel, Soares-da-Silva Patrício. Why Healthy Subjects Volunteer for Phase I Studies and How They Perceive Their Participation? Eur J Pharmacol. 2007;63:1085. doi: 10.1007/s00228-007-0368-3. finding financial reward was the most important motivation. [DOI] [PubMed] [Google Scholar]
- 36.King Nancy MP. Defining and Describing Benefit Appropriately in Clinical Trials. JL Med & Ethics. 2000;28:332. doi: 10.1111/j.1748-720x.2000.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 37.Grady, Dickert, Jawetz, Gensler & Emanuel, supra note 21, at 366.
- 38.E.g.,; Lemmens Trudo, Elliott Carl. Justice for the Professional Guinea Pig. Am J Bioethics. 2001;1:51. doi: 10.1162/152651601300169095. 52. But see, Dickert & Grady, supra note 21, at 198. [DOI] [PubMed] [Google Scholar]
- 39.Dickert & Grady, supra note 21, at 198.
- 40.See; Grady Christine. Payment of Clinical Research Subjects. J Clinical Investigation. 2005;115:1681. doi: 10.1172/JCI25694. 1681. Grady, Dickert, awetz, Gensler & Emanuel, supra note 21, at 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Id.
- 42.This figure was developed, in part, using the empirical data presented in Largent, Grady, Miller & Wertheimer, supra note 12.
- 43.See, e.g., Office of Human Research Protections, U.S. Dep’t of Health & Human Servs., When does compensating subjects undermine informed consent or parental permission?, http://www.hhs.gov/ohrp/regulations-and-policy/guidance/faq/informed-consent/# [https://perma.cc/CUT4-BXXU].(“Information submitted to IRBs should indicate and justify proposed levels and purposes of remuneration, which also should be clearly stated in the accompanying consent forms.”).
- 44.Emanuel, Wendler & Grady, supra note 26, at 2701.
- 45.Largent, Grady, Miller & Wertheimer, supra note 12, at 5.
- 46.Grady, supra note 40, at 1682.
- 47.Grant & Sugarman, supra note 24, at 735 n.3 (2004) (“[I]n the research context, providing a benefit after the decision to participate has been made is a gift or a token of appreciation, not an incentive properly speaking because the benefit does not serve as a motivator.”).
- 48.Largent, Grady, Miller & Wertheimer, supra note 12, at 5.
- 49.Wertheimer Alan. Is Payment a Benefit? Bioethics. 2013;27:105. doi: 10.1111/j.1467-8519.2011.01892.x. 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Id. at 111 (discussing the “jacking-up” argument).
- 51.Office of Human Research Protections, supra note 43; see also; Lynch Holly Fernandez. Human Research Subjects as Human Research Workers. Yale J Health Pol’y L & Ethics. 2014;14:122. 156–157. “Although technically silent on the matter of whether payment to subjects may be based on risk, the [U.S. federal] regulations’ direction to avoid undue inducement is often taken to mean that risk-based payment is impermissible.”. [PubMed] [Google Scholar]
- 52.Office of Human Research Protections, supra note 43 (“remuneration to subjects may include compensation for risks associated with their participation in research and that compensation may be an acceptable motive for agreeing to participate in research.”).
- 53.Brown Brandon, et al. Transparency of Participant Incentives in HIV Research. Lancet. 2016;3:e456. doi: 10.1016/S2352-3018(16)30150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Klitzman Robert, Albala Ilene, Siragusa Joseph, Nelson Kristen N, Appelbaum Paul S. The Reporting of Monetary Compensation in Research Articles. J Empirical Res on Hum Res Ethics. 2007;2:61. doi: 10.1525/jer.2007.2.4.61. 64. [DOI] [PubMed] [Google Scholar]
- 54.Grady Christine. Payment of Clinical Research Subjects. J Clinical Investigation. 2005;115:1681. doi: 10.1172/JCI25694. 1681. Grady, Dickert, Jawetz, Gensler & Emanuel, supra note 21, at 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dominguez Dinora, Jawara Mandy, Martino Nicole, Sinaii Ninet, Grady Christine. Commonly Performed Procedures in Clinical Research: A Benchmark for Payment. Contemp Clinical Trials. 2012;33:860. doi: 10.1016/j.cct.2012.05.001. 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Id.
- 57.Ripley Elizabeth, Macrina Francis, Markowitz Monkia, Gennings Chris. Why Do We Pay? A National Survey of Investigators and IRB Chairpersons. J Empirical Res on Hum Res Ethics. 2010;5:43. doi: 10.1525/jer.2010.5.3.43. 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Partners HealthCare, Partners Human Research Committee, Remuneration for Research Subjects, http://navigator.partners.org/ClinicalResearch/Remuneration_for_Research_Subjects.pdf [https://perma.cc/2DEU-KBAJ].
- 59.Grady, Dickert, Jawetz, Gensler & Emanuel, supra note 21, at 369.
- 60.Our work focused on offers of payment to adults, but for data on offers of payment to adolescents see; Borzekowski Dina LG, Rickert Vaughn I, Ipp Lisa, Fortenberry J Dennis. At What Price? The Current State of Subject Payment in Adolescent Research. J Adolescent Health. 2003;33:378. doi: 10.1016/s1054-139x(03)00228-3. [DOI] [PubMed] [Google Scholar]
- 61.Henderson Gail E. The Ethics of HIV “Cure” Research: What Can We Learn from Consent Forms? AIDS Res & Hum Retroviruses. 2015;31:56. doi: 10.1089/aid.2014.0219. 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grady, Dickert, Jawetz, Gensler & Emanuel, supra note 21, at 370.
- 63.Id.
- 64.Id.; see also; Quorum Review IRB. The Ethics of Compensation for Healthy Trial Participants. 2015 Sep 10; http://www.quorumreview.com/ethics-compensation-healthy-trial-participants/ [ https://perma.cc/7KKM-PDJQ]
- 65.Grady, Dickert, Jawetz, Gensler & Emanuel, supra note 21, at 373.
- 66.Id.
- 67.For example, we know from talking with investigators that some institutions require that payments in excess of, e.g., $50 be paid by check. In order to satisfy participants’ preference for cash and to avoid the administrative burden and delays of having checks issued, offers of payment will be kept at or below $50, even if a higher level of payment could be justified.
- 68.The individuals who experienced severe adverse reactions in the 2006 TeGenero trial were paid approximately $3,500 to participate; Wadman Meredith. London’s Disastrous Drug Trial Has Serious Side Effects for Research. Nature. 2006;440:388. doi: 10.1038/440388a. 388. [DOI] [PubMed] [Google Scholar]
- 69.See, e.g.,; Singer Eleanor, Bossarte Robert. Incentives for Survey Participation: When Are They Coercive? Am J Prev Med. 2006;31:411. doi: 10.1016/j.amepre.2006.07.013. 413. relating how IRBs are “increasingly saying” that $40 to $100 incentives for survey response have been deemed “coercive”. [DOI] [PubMed] [Google Scholar]
- 70.Moreno Jonathan, Caplan Arthur L, Wolpe Paul Root. Updating Protections for Human Subjects Involved in Research. JAMA. 1998;280:1951. doi: 10.1001/jama.280.22.1951. 1951. [DOI] [PubMed] [Google Scholar]
- 71.Dickert & Grady, supra note 39, at 1983.
- 72.The Belmont Report, supra note 16. Congress established the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (“National Commission”) in 1974 amidst “public outrage and congressional uncertainty over the Tuskegee syphilis experiments and other questionable uses of humans in research.”; Beauchamp Tom L. In: The Belmont Report. Emanuel Ezekiel J, Grady Christine C, Crouch Robert A, Lie Reider K, Miller Franklin G, Wendler David D., editors. 2008. (The Oxford Textbook of Clinical Research Ethics 149). [Google Scholar]
- 73.Hyman David A. Institutional Review Boards: Is this the Least Worse We Can Do? Nw U L Rev. 2007;101:749. 750 n.3. “Although there were classified regulations governing human experimentation issued by the Atomic Energy Commission and Department of Energy in the 1940s and 1950s, and the National Institutes of Health issued regulations on research involving human subjects in 1966, most scholars date the beginning of comprehensive feral regulation of human subjects research to 1974, when the regulation that ultimately gave rise to the Common Rule was issued.”. [Google Scholar]
- 74.The Belmont Report, supra note 16, at n.p.
- 75.Id. (emphasis added).
- 76.Presidential Commission for the Study of Bioethical Issues (PCSBI) Moral Science: Protecting Participants in Human Subjects Research. 2011;2 http://bioethics.gov/sites/default/files/Moral%20Science%20June%202012.pdf [ https://perma.cc/SLX9-K4TN]. All participating departments and agencies include language identical to that of the HHS codification at 45 C.F.R. § 46, subpart A in their chapters of the Code of Federal Regulations. We will, therefore, refer to the HHS regulations. [Google Scholar]
- 77.Id.
- 78.21 C.F.R. § 50, 56 (2015).
- 79.45 C.F.R. § 46.116 (2015).
- 80.45 C.F.R. § 46.116 (2015) (emphasis added).
- 81.PCSBI, supra note 76, at 39–40.
- 82.Before OHRP was formed, the Office for Protection from Research Risks (OPRR) was housed at the NIH. OPRR was dissolved in 2000 and responsibility was transferred to the office of the Secretary of Health and Human Services.
- 83.Office of Human Research Protections, U.S. Dep’t of Health & Human Servs., About OHRP, http://www.hhs.gov/ohrp/about/ [https://perma.cc/4BQU-TZ3Q]. see also; Burris Scott, Welsh Jen. Regulatory Paradox: A Review of Enforcement Letters Issued by the Office for Human Research Protection. Nw U L Rev. 2007;101:643. 647. [Google Scholar]
- 84.Office of Human Research Protections, U.S. Dep’t of Health & Human Servs., Frequently Asked Questions about Human Research, http://www.hhs.gov/ohrp/policy/faq/ [https://perma.cc/Q45Y-DYPW].
- 85.Id.
- 86.Office of Human Research Protections, supra note 43.
- 87.Id. (emphasis added).
- 88.Burris & Welsh, supra note 83, at 664.
- 89.Consider, for example, that FDA inspection activity has a deterrent effect on industry non-compliance, though only a small portion of clinical trial sites are inspected.; Olson Mary K. Agency Rulemaking, Political Influence, Regulation, and Industry Compliance. JL Econ & Org. 1999;15:573. 599. [Google Scholar]; U.S. Dep’t of Health & Human Servs. Office of Inspector General, Challenges to FDA’s Ability to Monitor and Inspect Foreign Clinical Trials. 2010 Jun; http://oig.hhs.gov/oei/reports/oei-01-08-00510.pdf [ https://perma.cc/T6PJ-UCD7].FDA inspected only 1.9% of domestic clinical trial sites.
- 90.Office of Human Research Protections, U.S. Dep’t of Health & Human Servs., What does it mean to minimize the possibility of coercion or undue influence?, https://www.hhs.gov/ohrp/regulations-and-policy/guidance/faq/Informed-Consent/index.html [https://perma.cc/TJ8P-HXQU].
- 91.Elsewhere within the FAQs, “overt coercion” is defined as “e.g., threatening loss of services or access to programs to which the potential subjects are otherwise entitled.” Office of Human Research Protections, U.S. Dep’t of Health & Human Servs., Can non-financial enrollment incentives constitute undue influence?, https://www.hhs.gov/ohrp/regulations-and-policy/guidance/faq/Informed-Consent/index.html [https://perma.cc/TJ8P-HXQU].(emphasis added).
- 92.Office of Human Research Protections, supra note 90.
- 93.Id.
- 94.Id.
- 95.Office of Human Research Protections, supra note 43.
- 96.Id. (emphasis added).
- 97.Id.
- 98.Id. (emphasis added).
- 99.U.S. Food & Drug Admin. Payment to Research Subjects—Information Sheet. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126429.htm [ https://perma.cc/JG27-Z5RC]
- 100.Id.
- 101.Id. (emphasis added).
- 102.Id.
- 103.Id. (emphasis added).
- 104.Id. (emphasis added).
- 105.Id. (emphasis added).
- 106.PCSBI, supra note 76, at 31, 39–40.
- 107.Emanuel, Wendler & Grady, supra note 26, at 2701.
- 108.Id. at 2701–2702 (offering examples of selective emphases and oversights).
- 109.Shuster Evelyne. Fifty Years Later: The Significance of the Nuremberg Code. New Eng J Med. 1997;337:1436. doi: 10.1056/NEJM199711133372006. 1436. [DOI] [PubMed] [Google Scholar]
- 110.Nuernberg Military Tribunals. Trials of War Criminals before the Nuernberg Military Tribunals under Control Council of Law No 10. 1949;181–182 https://www.loc.gov/rr/frd/Military_Law/pdf/NT_war-criminals_Vol-X.pdf [ https://perma.cc/4GDC-Z7P4].(emphasis added) [Google Scholar]
- 111.World Medical Association (WMA) Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. 2013 doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 112.Id.
- 113.Id. (stating only that the research ethics committee must be free of “any other undue influence”).
- 114.U.S. Dep’t of Health & Human Servs. Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance. 1996 Apr; http://www.fda.gov/downloads/Drugs/…/Guidances/ucm073122.pdf [ https://perma.cc/2B9A-9VTY]
- 115.U.S. Food & Drug Admin. ICH Guidance Documents. http://www.fda.gov/ScienceResearch/SpecialTopics/RunningClinicalTrials/GuidancesInformationSheetsandNotices/ucm219488.htm [ https://perma.cc/2TYA-WR96]
- 116.Guidance for Industry E6, supra note 114, at 11 (emphasis added).
- 117.Id.
- 118.CIOMS, supra note 25, at 45 (emphasis added).
- 119.Id.
- 120.Id.
- 121.Id.
- 122.Id.
- 123.Largent, Grady, Miller & Wertheimer, supra note 12.
- 124.Wilson James, Hunter David. Research Exceptionalism. Am J Bioethics. 2010;10:45. doi: 10.1080/15265161.2010.482630. 45. offering a “qualified defense” of research exceptionalism. [DOI] [PubMed] [Google Scholar]
- 125.It may be that people in these jobs deserve higher payments for a variety of reasons—such as shift-work and specialized training or skill—but risk is among them.
- 126.Cf. Wilson & Hunter, supra note 124, at 45.
- 127.See generally; Beecher Henry K. Ethics and Clinical Research. New Eng J Med. 1966;274:1354. doi: 10.1056/NEJM196606162742405. detailing examples of unethical and questionably ethical studies. [DOI] [PubMed] [Google Scholar]
- 128.Emanuel, Wendler & Grady, supra note 26, at 2701.
- 129.Other ethics regulations also follow this scandal-reform dynamic. See, e.g.,; MacKenzie G Calvin, Hafken Michael. Scandal Proof: Do Ethics Laws Make Government Ethical? 2002:55–86. discussing cumulative efforts to regulate the ethical behavior of executive branch officials. [Google Scholar]
- 130.Wilson & Hunter, supra note 124, at 49 (emphasis added).
- 131.Annas George J, Grodin Michael A. In: The Nuremberg Code. Emanuel Ezekiel J, Grady Christine C, Crouch Robert A, Lie Reider K, Miller Franklin G, Wendler David D., editors. 2008. (The Oxford Textbook of Research Ethics 136–137). “The victims who did not die in the course of such experiments surely wished that they had.”. [Google Scholar]
- 132.See generally; Jones James H. Bad Blood: The Tuskegee Syphilis Experiment. 1992 [Google Scholar]
- 133.Gelsinger, who was 18 years old, participated in a gene therapy trial at the University of Pennsylvania. He experienced a severe immune reaction to the vector (i.e., the gene’s delivery vehicle) and became the first person to die because of participation in gene-therapy research. The major questions after his death involved informed consent and conflict of interest disclosure.; Stolberg Sheryl Gay. The Biotech Death of Jesse Gelsinger. N.Y. Times; Nov 28, 1999. (Magazine) [PubMed] [Google Scholar]
- 134.See generally; Emanuel Ezekiel J, Miller Franklin G. Money and Distorted Ethical Judgments about Research: Ethical Assessment of the TeGenero TGN1412 Trial. Am J Bioethics. 2007;7:76. doi: 10.1080/15265160601111800. Wadman, supra note 68. [DOI] [PubMed] [Google Scholar]
- 135.Emanuel & Miller, supra note 134, at 78.
- 136.Rid Annette, Emanuel Ezekiel J, Wendler David. Evaluating the Risks of Clinical Research. JAMA. 2010;304:1472. doi: 10.1001/jama.2010.1414. 1473. [DOI] [PubMed] [Google Scholar]
- 137.See, e.g.,; Savulescu Julian. Harm, Ethics Committees and the Gene Therapy Death. J Med Ethics. 2001;27:148. doi: 10.1136/jme.27.3.148. discussing the death of Jesse Gelsinger. [DOI] [PMC free article] [PubMed] [Google Scholar]; Steinbrook Robert. Protecting Research Subjects—The Crisis at Johns Hopkins. New Eng J Med. 2002;346:716. doi: 10.1056/NEJM200202283460924. discussing the death of 24-year-old Ellen Roche in an asthma study. [DOI] [PubMed] [Google Scholar]
- 138.Lynch, supra note 51, at 133; see generally; Zarafonetis Chris JD, et al. Clinically Significant Adverse Effects in a Phase 1 Testing Program. Clinical Pharmacology & Therapeutics. 1978;24:127. doi: 10.1002/cpt1978242127. [DOI] [PubMed] [Google Scholar]
- 139.45 C.F.R. § 46.102(i) (2015).
- 140.Wilson & Hunter, supra note 124, at 49.
- 141.See Lynch, supra note 51, at 141.
- 142.Feige David. The Myth of the Hero Cop. Slate. 2015 May 25; http://www.slate.com/articles/news_and_politics/politics/2015/05/the_myth_of_the_hero_cop_police_unions_have_spread_a_dangerous_message_about.html [ https://perma.cc/ZD2B-YZGA]
- 143.Lynch, supra note 51, at 157 (internal citations omitted).
- 144.Wilson & Hunter, supra note 124, at 51.
- 145.Id.
- 146.U.S. Food & Drug Admin. The FDA’s Drug Review Process: Ensuring Drugs are Safe and Effective. http://www.fda.gov/drugs/resourcesforyou/consumers/ucm143534.htm [ https://perma.cc/WCP7-U6W9] see also U.S. Food & Drug Admin., IND Application Procedures: Clinical Hold, http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/ucm362971.htm [ https://perma.cc/GF4A-LBJM]
- 147.King Nancy MP. Defining and Describing Benefit Appropriately in Clinical Trials. JL Med & Ethics. 2000;28:332. doi: 10.1111/j.1748-720x.2000.tb00685.x. 333. While payments made to research participants are, technically, a collateral benefit, they are treated separately in research ethics and policy. Id. [DOI] [PubMed] [Google Scholar]
- 148.Cf.; Jansen Lynn A. The Problem with Optimism in Clinical Trials. IRB: Ethics & Hum Res. 2006;28:13. 18. [PubMed] [Google Scholar]
- 149.Ackerman Terrence F. An Ethical Framework for the Practice of Paying Research Subjects. IRB: Ethics & Hum Res. 1989;11:1. 1. [PubMed] [Google Scholar]
- 150.Jonas Hans. Philosophical Reflections on Experimenting with Human Subjects. Daedalus. 1969:219. 245. Jonas goes on to say, “Let us also remember that a slower progress in the conquest of disease would not threaten society, grievous as it is to those who have to deplore that their particular be not yet conquered, but that society would indeed be threatened by the erosion of those moral values whose loss, possibly caused by too ruthless a pursuit of scientific progress, would make its most dazzling triumphs not worth having.” Id. [Google Scholar]
- 151.McNeill Paul. Paying People to Participate in Research: Why Not? Bioethics. 1997;11:390. doi: 10.1111/1467-8519.00079. 392. [DOI] [PubMed] [Google Scholar]
- 152.Lynch, supra note 51, at 157.
- 153.In our personal morality, we believe that we do have greater obligations to identified individuals than to individuals unknown to us. Personal morality cannot, however, be neatly transposed on the public sphere. Cf.; Largent Emily A, Pearson Steven D. Which Orphans Will Find a Home? The Rule of Rescue in Resource Allocation for Rare Diseases. Hastings Center Rep. 2012;42:27. doi: 10.1002/hast.12. 30. [DOI] [PubMed] [Google Scholar]
- 154.See, e.g.,; Appelbaum Paul S, Lidz Charles W, Grisso Thomas. Therapeutic Misconception in Clinical Research: Frequency and Risk Factors. IRB: Ethics & Hum Res. 2004;26:1. 4–5. “A total of 61.8% n=139 of participants were judged to have a TM.”. [PubMed] [Google Scholar]; Lidz Charles W, Appelbaum Paul S, Grisso Thomas, Renaud Michelle. Therapeutic Misconception and the Appreciation of Risks in Clinical Trials. Soc Sci & Med. 2004;58:1689. doi: 10.1016/S0277-9536(03)00338-1. 1693. “23.9% n = 37 of subjects reported no risks or disadvantages of any sort from participating in these trials.”. [DOI] [PubMed] [Google Scholar]; Joffe Steven, Cook E Francis, Cleary Paul D, Clark Jeffrey W, Weeks Jane C. Quality of Informed Consent in Cancer Clinical Trials: A Cross-Sectional Survey. Lancet. 2001;358:1772. doi: 10.1016/S0140-6736(01)06805-2. 1774. “A quarter of respondents did not agree that the main purpose of clinical trials is to benefit future patients. Many did not realise that the treatment being research was not proven to be the best for their cancer.”. [DOI] [PubMed] [Google Scholar]
- 155.Wilson & Hunter, supra note 124, at 50.
- 156.Id.
- 157.The Consumer Financial Protection Bureau’s (CFPB) Know Before You Owe mortgage disclosure rule is “designed to help consumers … avoid costly surprises at the closing table.” Know Before you Owe – Mortgages, Consumer Financial Protection Bureau, http://www.consumerfinance.gov/know-before-you-owe/ [https://perma.cc/AH2J-97KG]. Similarly, the NPRM aims to address concerns that “[i]nformed-consent documents grow ever longer and consistently exceed the eighth-grade reading level, with wide variation in participants’ comprehension.”; Emanuel Ezekiel J. Reform of Clinical Research Regulations, Finally. New Eng J Med. 2015;373:2296. doi: 10.1056/NEJMp1512463. 2297. [DOI] [PubMed] [Google Scholar]
- 158.Glannon William. Phase I Oncology Trials: Why the Therapeutic Misconception Will Not Go Away. J Med Ethics. 2008;32:252. doi: 10.1136/jme.2005.015685. 254. “[T]his option at best would ameliorate but not resolve the problem of misperception about research.”; see also Dickert & Grady, supra note 39, at 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Macklin Ruth. The Paradoxical Case of Payment as Benefit to Research Subjects. IRB: Ethics & Hum Res. 1989;11:1. 3. [PubMed] [Google Scholar]
- 160.42 U.S.C. § 274e(a) (2012).
- 161.Largent Emily A. NOTA: Not A Good Act for Tissues to Follow. Quinnipiac Health LJ. 2016;19:179. analyzing prohibitions against the sale of human organs and tissues. [Google Scholar]
- 162.See id.
- 163.Lynch, supra note 51, at 137.
- 164.Obvious exceptions would be surrogacy and sex work. While it is beyond the scope of the present article to defend this proposition, we are of the opinion that it should generally be permissible to sell the bodily services of surrogacy and sex. See, e.g.,; Nussbaum Martha C. “Whether From Reason or Prejudice”: Taking Money for Bodily Services. J Legal Studies. 1998;27:693. [Google Scholar]
- 165.Lynch, supra note 51, at 159.
- 166.Chambers Tod. Participation as Commodity, Participation as Gift. Am J Bioethics. 2001;1:48. doi: 10.1162/152651601300169077. 48. [DOI] [PubMed] [Google Scholar]
- 167.Jansen Lynn A. The Ethics of Altruism in Clinical Research. Hastings Center Rep. 2009;39:26. doi: 10.1353/hcr.0.0164. 26. [DOI] [PubMed] [Google Scholar]
- 168.Id. at 30.
- 169.Cf.; Titmuss Richard M. The Gift Relationship: From Human Blood to Social Policy. 1971 [PubMed] [Google Scholar]
- 170.See generally; Stunkel Leanne, Grady Christine. More Than Money: A Review of the Literature Examining Healthy Volunteer Motivations. Contemp Clinical Trials. 2011;32:342. doi: 10.1016/j.cct.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Raganella Anthony J, White Michael D. Race, Gender, and Motivation for Becoming a Police Officer: Implications for Building a Representative Police Department. J Crim Just. 2004;21:501. 509. [Google Scholar]
- 172.Cf.; Glynn Simone A, Williams Alan E, Nass Catherine C, Bethel James, Kessler Debra, Scott Edward P, Fridey Joy, Kleinman Steven H, Schreiber George B. Attitudes Toward Blood Donation Incentives in the United States: Implications for Donor Recruitment. Transfusion. 2003;43:7. doi: 10.1046/j.1537-2995.2003.00252.x. [DOI] [PubMed] [Google Scholar]
- 173.Lacetera Nicola, Macis Mario. Do All Material Incentives for Pro-Social Activities Backfire? The Response to Cash and Non-cash Incentives for Blood Donations. J Econ Psychol. 2010;31:738. 738. [Google Scholar]
- 174.Resnik David B. Public Trust as a Policy Goal for Research with Human Subjects. Am J Bioethics. 2010;10:15. doi: 10.1080/15265161003686506. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]; see also; Largent Emily A. What’s Trust Got to Do with It? Trust and the Importance of the Research-Care Distinction. Am J Bioethics. 2015;15:22. doi: 10.1080/15265161.2015.1062182. [DOI] [PubMed] [Google Scholar]
- 175.Wertheimer, supra note 49, at 116.
- 176.Id.
- 177.Wilson & Hunter, supra note 124, at 51.
- 178.Of course, there may be exceptions, such as in emergency research. See, e.g.,; Largent Emily A, Wendler David, Emanuel Ezekiel J, Miller Franklin G. Is Emergency Research without Informed Consent Justified? The Consent Substitute Model. Archives Internal Med. 2010;170:668. doi: 10.1001/archinternmed.2010.80. [DOI] [PubMed] [Google Scholar]
- 179.The Belmont Report, supra note 16, at n.p.
- 180.E.g.; Faden Ruth, Beauchamp Tom L. A History and Theory of Informed Consent. 1986:235–73. [Google Scholar]; Pearson Steven D, Miller Franklin G, Emanuel Ezekiel J. Medicare’s Requirement for Research Participation as a Condition of Coverage: Is it Ethical? JAMA. 2006;296:988. doi: 10.1001/jama.296.8.988. 989. “Coercion occurs when a threat of some harm compels a person to act in a manner that he or she would not otherwise choose. An example is that of a kidnapper demanding ransom. The kidnapped victim’s family may be coerced into giving up money to avoid the threatened harm to their loved one.” internal citations omitted. [DOI] [PubMed] [Google Scholar]
- 181.Grady Christine. Payment of Clinical Research Subjects. J Clinical Investigation. 2005;115:1681. doi: 10.1172/JCI25694. 1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wertheimer’s book Coercion “sets the current standard and starting point for continued scholarship” regarding coercion.; Anderson Scott. Zalta Edward N., editor. Coercion, The Stanford Encyclopedia of Philosophy. 2015 http://plato.stanford.edu/archives/sum2015/entries/coercion/ [ https://perma.cc/W8VK-UZLJ]
- 183.Largent, Grady, Miller & Wertheimer, supra note 12, at 505.
- 184.Wertheimer Alan, Miller Franklin G. Payment for Research Participation: a Coercive Offer? J Med Ethics. 2008;34:389. doi: 10.1136/jme.2007.021857. 390. [DOI] [PubMed] [Google Scholar]
- 185.Id.
- 186.Id.
- 187.Id.
- 188.Id.
- 189.Noah Lars. Coerced Participation in Clinical Trials: Conscripting Human Research Subjects. Admin L Rev. 2010;62:329. 350. [Google Scholar]
- 190.McGregor Joan. “Undue Inducement” as Coercive Offers. Am J Bioethics. 2005;5:24. doi: 10.1080/15265160500245048. 25. emphasis added. [DOI] [PubMed] [Google Scholar]
- 191.Wertheimer & Miller, supra note 184, at 391.
- 192.Obviously, a threat may be veiled such that it appears to be an offer (e.g., “I will refrain from shooting you if you give me your money.”). This would not be a genuine offer.
- 193.Macklin Ruth. ‘Due’ and ‘Undue’ Inducements: On Paying Money to Research Subjects. IRB: Ethics & Hum Res. 1981;3:1. Macklin demurred from saying more about this writing, “Space does not permit a discussion here of the distinction between undue inducement and coercive offers.” Id. at 3 n.7. [PubMed] [Google Scholar]
- 194.Macklin Ruth. The Paradoxical Case of Payment as Benefit to Research Subjects. IRB: Ethics & Hum Res. 1989;11:1. 3. [PubMed] [Google Scholar]
- 195.McGregor Joan. “Undue Inducement” as Coercive Offers. Am J Bioethics. 2005;5:24. doi: 10.1080/15265160500245048. 25. arguing that “undue inducements might be referred to as ‘coercive offers’”; [DOI] [PubMed] [Google Scholar]; see also; McGregor Joan. Bargaining Advantages and Coercion in the Market. Phil Res Archives. 1988;14:23. [Google Scholar]; McGregor Joan L. Free Markets, Bargaining Power, and the Rules of Exchange. Pub Aff Quarterly. 1991;5:353. [Google Scholar]
- 196.Wilkinson Martin, Moore Andrew. Inducement in Research. Bioethics. 1997;11:373. doi: 10.1111/1467-8519.00078. 378. [DOI] [PubMed] [Google Scholar]
- 197.Wertheimer & Miller, supra note 184, at 390; see also Faden & Beauchamp, supra note 180, at 235–73;; Wertheimer Alan, Miller Franklin G. There are (STILL) no Coercive Offers. J Med Ethics. 2014;40:592. doi: 10.1136/medethics-2013-101510. [DOI] [PubMed] [Google Scholar]
- 198.Wertheimer & Miller, supra note 184, at 390.
- 199.Id. at 389.
- 200.Macklin, supra note 194, at 2; see also U.S. Dep’t of Health & Human Servs., Institutional Review Board Guidebook ch. 3 (1993) (warning that undue inducements “may prompt subjects to lie or conceal information that, if known, would disqualify them from enrolling—or continuing—as participants in a research project”). But see; Emanuel Ezekiel. Ending Concerns About Undue Inducement. JL Med & Ethics. 2004;32:100. doi: 10.1111/j.1748-720x.2004.tb00453.x. 103–104. stating that it is unclear whether lying is a general problem. [DOI] [PubMed] [Google Scholar]
- 201.Emanuel, Wendler & Grady, supra note 26, at 2708.
- 202.Wertheimer & Miller, supra note 184, at 391.
- 203.Grant & Sugarman, supra note 24, at 732 (emphasis added). For Grant and Sugarman, incentives become problematic when conjoined with “the following factors, singly or in combination with one another. Where the subject is in a dependency relationship with the researcher, where the risks are particularly high, where the research is degrading, where the participant will only consent if the incentive is relatively large because the participant’s aversion to the study is strong, and where the aversion is a principled one—when these conditions are present, the use of incentives is highly questionable.” Id.
- 204.Macklin, supra note 194, at 2.
- 205.The Belmont Report, supra note 16, at n.p.
- 206.Emanuel, supra note 200, at 101.
- 207.Emanuel, supra note 24, at 9.
- 208.Id.
- 209.Emanuel, supra note 200, at 100.
- 210.See, e.g.,; U.S. Dep’t of Health & Human Servs. Institutional Review Board Guidebook. 1993 ch. 3. (warning that offers that are “too attractive may blind prospective subjects to the risks or impair their ability to exercise proper judgment” about the risks of participation in research). Wertheimer & Miller, supra note 184, at 391. [Google Scholar]
- 211.Wilson & Hunter, supra note 124, at 50.
- 212.Emanuel, supra note 24, at 11.
- 213.Id.
- 214.London Alex John. Undue Inducements and Reasonable Risks: Will the Dismal Science Lead to Dismal Research Ethics. Am J Bioethics. 2005;5:29. doi: 10.1080/15265160500245105. 30. [DOI] [PubMed] [Google Scholar]
- 215.Participants might also be motivated to lie in order to participate in research, thereby skirting IRB protections.
- 216.Jehovah’s Witnesses, Why Don’t Jehovah’s Witnesses Accept Blood Transfusions?, https://www.jw.org/en/jehovahs-witnesses/faq/jehovahs-witnesses-why-no-blood-transfusions/ [https://perma.cc/E7GU-HWKJ].
- 217.Largent Emily A, Lynch Holly Fernandez. Paying Research Participants: The Outsized Influence of “Undue Influence”. IRB: Ethics & Hum Res ___. 2017;39 forthcoming. [PMC free article] [PubMed] [Google Scholar]
- 218.McGregor Joan. “Undue Inducement” as Coercive Offers. Am J Bioethics. 2005;5:24. doi: 10.1080/15265160500245048. 25. suggesting that Emanuel’s account fails to capture our intuitions about Joel Feinberg’s “lecherous millionaire” example, in which a millionaire offers to pay for a sick boy’s medical care if his impoverished mother will be the millionaire’s mistress. [DOI] [PubMed] [Google Scholar]
- 219.Id. at 24.
- 220.Id.
- 221.Id.
- 222.Cryder Cynthia E, London Alex John, Volpp Kevin G, Lowenstein George. Informative Inducement: Study Payment as a Signal of Risk. Soc Sci & Med. 2010;70:455. doi: 10.1016/j.socscimed.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 223.Slomka Jacquelyn, McCurdy Sheryl, Ratliff Eric A, Timpson Sandra, Williams Mark L. Perceptions of Financial Payment for Research Participation among African-American Drug Users in HIV Studies. J Gen Internal Med. 2007;22:1403. doi: 10.1007/s11606-007-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Id. at 1405 (“In response to questions about monetary influences on risk assessment, some respondents said they would participate in a study if the price was right in spite of the risks, whereas others said they would decline certain risky studies no matter what amount of money was offered.”).
- 225.Halpern Scott D, Karlawish Jason HT, Casarett David, Berlin Jesse A, Asch David A. Empirical Assessment of Whether Moderate Payments are Undue or Unjust Inducements for Participation in Clinical Trials. Archives Internal Med. 2004;164:801. doi: 10.1001/archinte.164.7.801. 803. [DOI] [PubMed] [Google Scholar]
- 226.Bentley John P, Thacker PG. The Influence of Risk and Monetary Payment on the Research Participation Decision Making Process. J Med Ethics. 2004;30:293. doi: 10.1136/jme.2002.001594. 296–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Singer Eleanor, Couper Mick P. Do Incentives Exert Undue Influence on Survey Participation? Experimental Evidence. J Empirical Research on Human Research Ethics. 2008;3:49. doi: 10.1525/jer.2008.3.3.49. 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Investigators who responded to our pilot survey, described in Part V, raised this as a concern. For example, one respondent explained: “Recruiting through Craigslist or other online methods seems to draw a lot of people who are unduly influenced by the compensation, to the point that they will lie about their medical history.” Another stated, “‘Professional subjects’ are very problematic for us. They lie during the screening process in order to get into the study, they have poor compliance, and their data messes up our findings. For this reason, we compensate as little as possible, to decrease the number of these subjects that we enroll.”
- 229.Slomka, McCurdy, Ratliff, Timpson & Williams, supra note 223, at 1406.
- 230.Bentley & Thacker, supra note 226, at 297.
- 231.Id.
- 232.Largent & Lynch, supra note 217.
- 233.Lynch, supra note 51, at 162.
- 234.Id. (internal citations omitted).
- 235.Largent & Lynch, supra note 217.
- 236.Largent, Grady, Miller & Wertheimer, supra note 29, at 506.
- 237.Id.
- 238.Emanuel, supra note 200, at 101 (“The ‘your money or your life’ threat of coercion is clearly different from the $1 million offer of undue inducement.”).
- 239.Largent, Grady, Miller & Wertheimer, supra note 29, at 506; see also; Wilkinson Martin, Moore Andrew. Inducement in Research. Bioethics. 1997;11:373. doi: 10.1111/1467-8519.00078. 378. “Coercion is paradigmatically a case of the denial of autonomy, since it consists in the deliberate imposition of one person’s will on another. However, coercion usually takes the form of threats, which restrict people’s options. Inducements are offers, not threats, and they expand people’s options.”. [DOI] [PubMed] [Google Scholar]
- 240.Disregarding surplusage, 82 C.J.S. Statutes § 433.
- 241.Sixty medical research institutions are members of the CTSA Consortium, which is funded by the National Center for Advancing Translational Sciences (NCATS), a part of the National Institutes of Health (NIH). Harvard Catalyst, National CTSA Consortium, http://catalyst.harvard.edu/about/consortium.html [https://perma.cc/CA5T-83TH].
- 242.Harvard Catalyst, About Harvard Catalyst, http://catalyst.harvard.edu/about/ [https://perma.cc/J6WF-W5YQ].
- 243.There are thirty-one participating institutions. Id.
- 244.Policies are on file with the authors.
- 245.This is consistent with the findings presented in; Dickert Neal, Emanuel Ezekiel, Grady Christine. Paying Research Subjects: An Analysis of Current Policies. Annals Internal Med. 2002;136:368. doi: 10.7326/0003-4819-136-5-200203050-00009. 369. [DOI] [PubMed] [Google Scholar]
- 246.See infra app. at [page] (Institution A).
- 247.See infra app. at [page] (Institution B).
- 248.Id.
- 249.See infra app. at [page] (Institution F). Undue influence was never defined by this policy.
- 250.See infra app. at [page] (Institution L).
- 251.See infra app. at [page] (Institution K).
- 252.See infra app. at [page] (Institution F).
- 253.See generally; Wright Megan S, Robertson Christopher T. Heterogeneity in IRB Policies with Regard to Disclosures About Payment for Participation in Recruitment Materials. JL Med & Ethics. 2014;42:275. doi: 10.1111/jlme.12153. 375–376. [DOI] [PubMed] [Google Scholar]
- 254.See infra app. at [page] (Institution C) (emphasis added).
- 255.Harvard Catalyst, Regulatory Foundations, Ethics, and Law Program, https://catalyst.harvard.edu/programs/regulatory/howwework.html [https://perma.cc/YL4H-XM3T].
- 256.Harvard Catalyst, Harvard Catalyst Clinical Research Center (HCCRC), https://catalyst.harvard.edu/programs/hccrc/ [https://perma.cc/DA6U-M4G4].
- 257.Survey instruments on file with the author.
- 258.Some of the IRBs made the members’ emails publicly available or shared them upon request; in other cases, the IRB chair agreed to forward our emails. As we did not send all of the email invitations directly, we are unsure how many emails were returned as undeliverable and how many emails were forwarded without notifying us of that fact. Therefore, the adjusted response rate may differ.
- 259.As we did not send any of these email invitations directly, we are unsure how many emails were returned as undeliverable. Therefore, the adjusted response rate may be higher.
- 260.The CUHS asked us to change how we asked questions about age between the two studies, which is why the results are reported differently.
- 261.Investigators were significantly more likely than IRB members (p < 0.05) to say that a research participant was coerced if threatened with harm or loss of benefits to which he is otherwise entitled (92.2% vs. 81.7%). Thus, investigators were more likely to get it right in our view.
- 262.Investigators were significantly more likely than IRB members (p < 0.05) to say that a research participant was coerced if an offer of payment distorts the research participant’s ability to perceive accurately the risks and benefits of research, which is part of our definition of undue inducement (75.9% vs. 51.3%).
- 263.Investigators were significantly more likely than IRB members (p < 0.05) to say that a research participant was unduly induced if she participates as the result of intimidation, or some other form of pressure or force (69.8% vs. 51.3%), which is instead one of our definitions of coercion.
- 264.Investigators were significantly more likely than IRB members (p < 0.05) to say that a research participant was unduly induced if threatened with harm or loss of benefits to which they are otherwise entitled (64.7% vs. 47.0%), which is instead one of our definitions of coercion.
- 265.E.g.,; Emanuel Ezekiel J, Currie Xolani E, Herman Allen. Undue Inducement In Clinical Research in Developing Countries. Lancet. 2005;366:336. doi: 10.1016/S0140-6736(05)66992-9. 337. (describing how it is not unusual for undue inducement to be “conflated with coercion, exploitation, injustice, deception, misunderstanding, and other ethical transgressions as if they were equivalent or interchangeable”) [DOI] [PubMed] [Google Scholar]
- 266.Largent, Grady, Miller & Wertheimer, supra note 29, at 506.
- 267.45 C.F.R. § 46.116 (2015).
- 268.Office of Human Research Protections, supra note 90.
- 269.For instance, a handful (5%) of respondents to the IRB survey explicitly stated that they were not certain how to define undue influence in answer to a free response question.
- 270.Imagine you have a box that you know weighs exactly 10 pounds. You take it home and weigh it five times on your bathroom scale. Each time, the scale says that the box weighs 7.5 pounds. Your scale is precise because it said that the box weighed 7.5 pounds each time, but your scale is not accurate because 7.5 pounds is not close to the known value of 10 pounds.
- 271.Alan Wertheimer, Exploitation 22–23 (1996).
- 272.CIOMS, supra note 25, at 65.
- 273.Grant & Sugarman, supra note 24, at 734.
- 274.Emanuel, Currie & Herman, supra note 265, at 338.
- 275.Id.
- 276.See, e.g.,; Jennings Claudine G, MacDonald Thomas M, Wei Li, Brown Morris J, McConnachie Lewis, Mackenzie Isla S. Does Offering an Incentive Payment Improve Recruitment to Clinical Trials and Increase the Proportion of Socially Deprived Elderly Participants? Trials. 2015;16:1. doi: 10.1186/s13063-015-0582-8. finding a £100 incentive payment led to “small but significant improvements” in the number of patients who consent to be screened for a clinical trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bennette Caroline S, Ramset Scott D, McDermott Cara L, Carlson Josh J, Basu Anirban, Veenstra David L. Predicting Low Accrual in the National Cancer Institute’s Cooperative Group Clinical Trials. J Nat’l Cancer Inst. 2016;108:1. doi: 10.1093/jnci/djv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Williams Rebecca J, Tse Tony, DiPiazza Katelyn, Zarin Deborah A. Terminated Trials in the ClinicalTrials.gov Results Database: Evaluation of Availability of Primary Outcome Data and Reasons for Termination. PLOS ONE. 2015;10:1. doi: 10.1371/journal.pone.0127242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Unger, Gralow, Albain, Ramsey & Hershman, supra note 11, at 137–138.
- 280.Id. at 138.
- 281.For example, if a pregnancy test says you are pregnant when you actually are not, that is a false positive.
- 282.Emanuel, supra note 157, at 2297.
- 283.Human Subjects Research Protections: Enhancing Protections for Research Subjects and Reducing Burden, Delay, and Ambiguity for Investigators, 76 Fed. Reg. 44,512 (proposed July 26, 2011) (to be codified at 21 C.F.R. 50, 56 & 45 C.F.R. 46, 160, 164), https://www.gpo.gov/fdsys/pkg/FR-2011-07-26/pdf/2011-18792.pdf.
- 284.Henry Leslie Meltzer. Revising the Common Rule: Prospects and Challenges. JL Med & Ethics. 2013;41:386. doi: 10.1111/jlme.12049. 387. describing “pessimism” that progress toward issuing a NPRM was “stalled, at least for the foreseeable future, if not permanently”. [DOI] [PubMed] [Google Scholar]
- 285.Federal Policy for the Protection of Human Subjects, 80 Fed. Reg. 53,933 (proposed Sept. 8, 2015), https://www.gpo.gov/fdsys/pkg/FR-2015-09-08/pdf/2015-21756.pdf
- 286.Office of Human Research Protections, U.S. Dep’t of Health & Human Servs. NPRM. 2015 – Summary, http://www.hhs.gov/ohrp/humansubjects/regulations/nprm2015summary.html [ https://perma.cc/GC38-4WFY]
- 287.Final Rule, supra note 14, at 7203-04.
- 288.National Institutes of Health. Secretary’s Advisory Committee on Human Research Protections – May 2016 (Day 1) 2016 May 18; https://videocast.nih.gov/summary.asp?Live=19186&bhcp=1 [ https://perma.cc/9G4V-ATHA]
- 289.See; Largent Emily A. Recently Proposed Changes to Legal and Ethical Guidelines Governing Human Subjects Research. JL & Biosciences. 2016;3:10. doi: 10.1093/jlb/lsw001. [DOI] [PMC free article] [PubMed] [Google Scholar]


