Key Points
GL-2045, a recombinant human IgG1-based Fc multimer, binds C1q and inhibits complement-dependent cytotoxicity.
GL-2045 induces self-limited complement activation that is governed by both factors H and I and results in the generation of iC3b.
Abstract
GL-2045 is a recombinant human immunoglobulin G1 (IgG1)–based Fc multimer designed to recapitulate the anti-inflammatory activities of intravenous immunoglobulin (IVIG) on the innate and adaptive immune responses. We used functional in vitro studies to determine if GL-2045 could mimic the modulatory activity of IVIG on complement activation. GL-2045, at log-order lower concentrations than heat-aggregated IgG (HAGG) and IVIG, protected antibody-opsonized cells from complement-dependent cytotoxicity. These protective effects were completely mediated by the higher order multimer fractions of GL-2045 and were partially dependent upon sequestration of C1q. Exposure of serum to GL-2045 and, to a lesser extent, IVIG, resulted in high levels of C4a, limited levels of C3a, and no C5a. In contrast, HAGG induced high levels of C4a, C3a, and C5a. The means by which GL-2045 governed complement activation was dependent on its ability to augment the function of factor H, alone and in combination with factor I, to indirectly limit the alternative form of C3 convertase, with resultant increases in the anti-inflammatory molecule, the “inactive” form of C3b, called iC3b. Although IVIG, like GL-2045, potentiated factor H function, it also directly inhibited the alternative form of C3 convertase. Our findings help elucidate how IVIG, GL-2045, and HAGG regulate complement function. Furthermore, the capacity of GL-2045 to sequester C1q and augment factor H activity, in combination with its ability to generate activation-induced immunomodulatory complement split products, such as iC3b, make it a viable drug candidate for the treatment of diverse complement-mediated diseases.
Visual Abstract
Introduction
Intravenous immunoglobulin (IVIG) can ameliorate specific human diseases that partially rely on aberrant activation of the complement cascade.1-5 Unfortunately, the use of IVIG is hindered by limited supply and high associated costs. Although some of these concerns are mitigated by the development of recombinant complement inhibitors, the specificity of these reagents may also prove to be a liability.6 For example, although the anti-C5 monoclonal antibody (mAb), eculizumab, can effectively treat diseases such as paroxysmal nocturnal hemoglobinuria, by virtue of its specificity, this drug cannot inhibit complement fragments generated upstream of C5 (eg, C3a, C3b), which mediate important biologic effects such as target opsonization, neutrophil chemotaxis, and inflammation.7
Based on the practical and clinical limitations associated with IVIG and a substantial body of evidence suggesting that many of its therapeutic effects are attributable to Fc-bearing aggregates within the preparation,8-11 we developed a drug candidate called GL-2045, composed of recombinant human IgG1 based Fc multimers. The murine immunoglobulin G2a (IgG2a)–based version of this drug, M-045, was effective in the prevention and/or treatment of a variety of rodent models of autoimmunity, including immune thrombocytopenic purpura, collagen-induced arthritis,12 myasthenia gravis,13 and experimental autoimmune neuritis.14 Similarly, GL-2045 was effective in animal models of immune thrombocytopenic purpura and collagen-induced arthritis (X.Z. and J.O., manuscript submitted April 2016).
Given the role of IVIG in the treatment of complement-mediated diseases and the knowledge that a major function of complement is the sequestration/opsonization of immune aggregates, in the present study, we investigated whether and how GL-2045 modulates complement activation. Our data demonstrate that GL-2045 inhibited complement-dependent cytotoxicity (CDC). These inhibitory effects were approximately 1 and 2 to 3 logs more potent than heat-aggregated IgG (HAGG) and IVIG, respectively, and were completely attributable to the higher order multimer fractions. GL-2045–mediated inhibition of CDC was associated with dramatic reductions of C1q, C4b, and membrane attack complex (MAC) deposition on the surface of opsonized cells. In comparison with HAGG and IVIG, GL-2045 induced more potent increases in C4a. However, levels of C3a were significantly lower than those found in HAGG-treated serum, whereas C5a was virtually undetectable. This limited ability of GL-2045 to induce C5a was dependent on the presence of factors H and I, which were potentiated by GL-2045, resulting in high levels of the anti-inflammatory complement cleavage product, the “inactive” form of C3b, called iC3b. These data demonstrate that GL-2045 induces initial activation of the classical pathway of the complement cascade and concomitantly and/or sequentially both inhibits CDC of opsonized targets and generates anti-inflammatory iC3b.
Methods
Cell lines and reagents
SUDHL4 and Ramos cells (ATCC) were maintained in RPMI-1640 (Mediatech Inc, Manassas, VA) supplemented with 5% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA), 1% penicillin/streptomycin, 1% GlutaMAX (Gibco), and 0.01M N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (Mediatech Inc). Antibody-sensitized sheep red blood cells (SRBCs) were purchased from Comp Tech (Tyler, TX).
The expression vector for GL-2045 was developed by seamlessly fusing the complete IgG1 hinge-CH2-CH3 coding region, starting at residue 216 at the C-terminal,15 to the IgG2 12 amino acid residue hinge region (ERKCCVECPPCP). A stable CHO cell line expressing the fusion protein was created using this material. GL-2045 was manufactured by Pfizer using a fed-batch process with chemically defined media. The material was purified using a combination of affinity and ion-exchange chromatography. Analytical characterization ensured the targeted multimer distribution was achieved. The concentration of GL-2045 was 96 g/L. The fractions of GL-2045 were prepared by gel filtration and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).16 IVIG was purchased from Atlantic Biologicals (Miami, FL). HAGG was made by heating 100 mg of IVIG (10 or 50 mg/mL) at 63°C for 30 minutes. Normal human serum (NHS) and rituximab (RTX) were purchased from Cedarlane Labs (Burlington, NC) and from Genentech Inc, respectively. Veronal buffered saline (VBS) was purchased from Lonza (Walkersville, MD). All complement factors as well as factor-depleted sera were obtained from Complement Technology, Inc (Tyler, TX), unless otherwise indicated.
The following reagents were used in flow cytometry and enzyme-linked immunosorbent assays (ELISAs): fluorescein isothiocyanate (FITC)-conjugated anti-C1q antibody (Cedarlane), FITC-C4b (Abcam, Cambridge, MA), FITC-sc5b-9 (LifeSpan Biosciences, Seattle, WA), Annexin V-PE apoptosis detection kit (Biolegend, San Diego, CA), purified anti-C1q and anti-C5b-9 antibodies (Quidel, Athens, OH), goat anti-human IgG (Fc) (Millipore), horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and donkey anti-goat IgG (Santa Cruz Biotechnology Inc., Dallas, TX), C3a and C5a platinum ELISA kits (Affymetrix, San Diego, CA), and an iC3b ELISA kit (Quidel).
CDC and binding of complement activation products to cell surface
GL-2045, HAGG, or IVIG was incubated with NHS for 10 to 15 minutes at 37°C. SUDHL4 or Ramos cells (1.5 × 105/well) were incubated with RTX (10 μg/mL) on ice for 5 minutes in media with 2% fetal bovine serum. NHS/test compound mixtures were added to individual samples to create a final NHS concentration of either 6% or 50% and incubated at 37°C for 45 minutes. Cells were collected and stained with Annexin V PE/7-AAD (BD Biosciences) according to the manufacturer’s instruction, and analyzed by flow cytometry. Live cells were determined by gating on Annexin V−/7-AAD− cells within total population. The percentage of dead/apoptotic cells represents the level of cytotoxicity and was calculated as 100 minus the percent of live cells. Supernatants were collected and analyzed for C3a, C4a, and C5a. To evaluate the binding of complement activation products, these cultures were terminated at 15 minutes for C1q and C4b and 30 minutes for MAC by adding EDTA at a final concentration of 10 mM. The cells were then washed and stained with FITC-conjugated anti-C1q, anti-C4b, and anti-sc5b-9, and analyzed by flow cytometry. Pooled cells from different conditions were used for isotype control staining. Labeled cells were acquired by an LSR II flow cytometer (BD Biosciences) and analyzed using either FACSDiva (BD Biosciences) or FlowJo software (Treestar). The positive gates for anti-C1q, anti-C4b, and anti-sc5b-9 staining were set based on individual isotype controls.
SRBCs were sensitized by incubating with rabbit anti-SRBC IgG (MP Biomedicals, 1:2000) at a concentration of 1 × 109 cells/mL for 30 minutes at 37°C. After 2 washes with VBS, the sensitized cells were incubated for 30 minutes at 37°C with either 1.5% or 50% NHS (pretreated with GL-2045, HAGG, or IVIG at the concentrations indicated for 15 minutes at room temperature). Samples were then centrifuged and a 100 µL/well of supernatant was transferred into a 96-well plate and read at 540 nm with an EPOCH plate reader (BioTek). The percent lysis was calculated as: % lysis = (optical density [OD] test – OD blank)/(OD total lysis – OD blank) ×100.
CBA and iC3b ELISA
GL-2045, HAGG, or IVIG was incubated with pooled NHS or factor H–depleted serum for 90 minutes at 37°C. EDTA was added at a final concentration of 10 mM to terminate complement activation. The previously described treated serum samples or cellular supernatants from the CDC assays (as described previously) were evaluated for the presence of C3a, C4a, and C5a using cytometric bead array (CBA) human anaphylatoxin kit (BD Biosciences) and iC3b by ELISA.
C1q binding assays
The C1q binding assays were performed as previously described.16 A 96-well plate was coated with C1q at 5 μg/mL overnight in phosphate-buffered saline, followed by plate washing and blocking. The plate was then incubated with serial dilutions of test compounds in blocking buffer for 45 minutes and washed 5 times. Goat anti-human IgG (Fc) (1:5000) (Millipore) and donkey anti-goat IgG-HRP (Santa Cruz) were added and incubated for 1 hour at room temperature and washed 5 times. TMB substrate was added and the absorbance was read at 450 nm.
Plate-based complement activation assay
The plate-based complement activation assay was performed and analyzed, as described previously by Jusco et al.17 Briefly, a 96-well microplate was coated with 5 µg/mL HAGG overnight at 4°C. After blocking for 2 hours, GL-2045, HAGG, or IVIG was added and incubated in the presence of 1.5% NHS at 37°C for 30 minutes for C1q deposition or 1 hour for MAC deposition. The plates were then incubated with anti-C1q antibody or anti-C5b-9 antibody for 1 hour, followed by a 1-hour incubation with HRP-conjugated goat anti-mouse IgG, and development with TMB. The absorbance was read at 450 nm.
C3 and C5 convertase assays
C3 and C5 convertase assays were conducted as previously described by Heinen et al and Kerr et al, with slight modifications.18,19 Briefly, C3 convertase was generated either by incubation of C3b (2 μg/mL), factor D (4 μg/mL) and factor B (40 μg/mL) (alternative C3 convertase), or C4 (10 µg/mL), C2 (10 µg/mL), and C1s enzyme (0.1 µg/mL) (classical C3 convertase) in VBS in the presence of GL-2045, HAGG, or IVIG for 15 minutes at 37°C. C3 (80 μg/mL) was added and incubated for an additional 10 minutes. In an alternative approach, C3 was preincubated with GL-2045, HAGG, or IVIG for 15 minutes at 37°C, followed by incubation with the alternative or classical forms of C3 convertase for an additional 10 minutes. C3a concentrations were determined by ELISA.
For the C5 convertase assays, 1 × 108 SRBCs were incubated with C3b (4 μg/mL) overnight at 4°C. The cells were then incubated with C3 (10 μg/mL), factor D (4 μg/mL), and factor B (40 µg/mL) (alternative convertase) at 30°C for 40 minutes. Alternatively, the cells were incubated with C3 (10 μg/mL), C4 (10 µg/mL), C2 (10 µg/mL), and C1s enzyme (0.1 µg/mL) (classical convertase) at 30°C for 40 minutes. To test the C5 convertase activity in the absence of SRBCs, C3b was added directly to the alternative or classical C5 convertase reactions in the absence of C3 and incubated at 30°C for 40 minutes. GL-2045, HAGG, or IVIG was then added and incubated for 15 minutes before addition of 50 μg/mL of C5. C5a levels were examined by ELISA.
Statistical analysis
For each analysis, 3 or 4 independent experiments were conducted, the majority of which were performed on separate days. One-way analysis of covariance (ANCOVA), including experiment (day) as a covariate, was used to compare treatments at various concentration levels. Least squares means were reported. Tukey and Dunnett adjustment procedures were used to control type I errors in the presence of multiple comparisons. For all tests, the response values were log transformed to stabilize variance. Differences were considered statistically significant when *P < .05, **P < .01, or ***P < .001 for multiple comparison-adjusted P values. Log-logistic dose-response 3 and 4 parameter models were fit to compute 50% effective concentration (EC50), 50% inhibitory concentration (IC50), and maximum effect values. The baseline values of response were constrained to be the same for GL-2045, HAGG, and IVIG. In situations when maximum drug effect was not reached, the maximum effect was assumed to be the same across the drugs for estimation of EC50 and IC50. The estimates of EC50, IC50, and maximum effect were reported in text with corresponding standard errors (estimate ± SE). SAS software, version 9.4 (SAS Institute Inc., Cary, NC), and R 3.3.120 drc package21 were used for all reported computations.
Results
GL-2045 protects antibody (Ab)-opsonized cells from CDC
We sought to generate a multivalent IgG1 Fc to mimic the anti-inflammatory properties of pooled human IVIG. It has been observed that human IgG2 naturally dimerizes at a very low level.22 As such, we hypothesized that the IgG2 hinge, in the absence of the IgG2 Fab or CH2/CH3, could multimerize IgG1 Fc. To test this supposition, we generated GL-2045, an IgG1 Fc with the IgG2 hinge at the carboxy end of the linear molecule, devoid of any Fab. Analysis of GL-2045 by SDS-PAGE and chromatography demonstrated that it consists of a homodimer and a consistent multimer distribution of the homodimer that includes the dimer through at least the 12-mer of the homodimer (X.Z. and J.O., manuscript submitted April 2016). These GL-2045 multimers are formed through specific cysteine bonds and are stable, with little batch-to-batch variability (data not shown). This multimerization pattern differentiates GL-2045 from the aggregates in IVIG that form through immunoglobulin idiotype–anti-idiotype interactions.
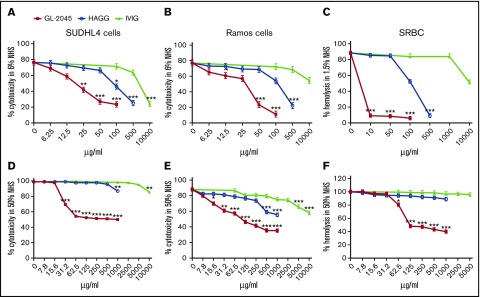
We first evaluated the ability of GL-2045, HAGG, and IVIG to protect Ab-opsonized CD20+FcγRIIB+ SUDHL4 (6% NHS), CD20+FcγRIIB− Ramos (6% NHS), and SRBC from CDC (1.5% NHS) (Figure 1A-C). GL-2045 afforded all 3 cell types the greatest protection against CDC. The IC50s (μg/mL) for GL-2045 vs HAGG vs IVIG, respectively, were: SUDHL4, 19.3 ± 3.7 vs 102.9 ± 10.8 vs 2173.7 ± 579; Ramos, 38.9 ± 11.3 vs 238.9 ± 40.1 vs IC50 not reached; and SRBCs, 3.5 ± 0.7 vs 106 ± 4.5 vs IC50 not reached, respectively. Furthermore, even at the highest concentration (10 000 μg/mL), IVIG did not mediate the same maximal level of protection as GL-2045 on Ab-opsonized Ramos cells or SRBCs (Figure 1B-C).
Figure 1.
GL-2045 protects Ab-opsonized cells from CDC. Ab-opsonized SUDHL4, Ramos, and SRBCs were incubated with either 6% (A-B), 1.5% (C), or 50% (D-F) NHS pretreated with GL-2045, HAGG, or IVIG at indicated concentrations. Cell apoptosis/death was measured by Annexin V/7-AAD staining and analyzed by FACS. SRBC hemolysis was evaluated spectrophotometrically. The results are shown as the least squares mean ± SE estimated by ANCOVA. Natural log variance stabilizing transformation and Tukey procedure for multiple comparisons adjustment were used to test the differences. *P < .05, **P < .01, ***P < .001 compared with no-treatment control.
To attempt to recapitulate more biologically relevant conditions, we performed an analogous set of assays in 50% serum (Figure 1D-F). Under these conditions, although GL-2045 continued to provide potent protection against CDC, the effects of IVIG and HAGG were more limited. The IC50s (μg/mL) of GL-2045 in CDC assays were: SUDHL4, 28.5 ± 1; Ramos, 40.6 ± 10.1; and SRBC, 74.4 ± 4.6. For HAGG and IVIG, the IC50s were not reached at the highest concentrations (Figure 1D,F). Importantly, these results were not secondary to the theoretical potential for GL-2045 to interfere with anti-CD20 opsonization because GL-2045 did not block anti-CD20 binding to either SUDHL4 or Ramos cells (data not shown). Furthermore, despite the fact that GL-2045 avidly binds both to FcγR expressing cells and to C1q, it could not mediate CDC of either SUDHL4 or Ramos cells (supplemental Figure 1). Finally, given the reproducibility of our findings in 6% and 50% serum, we elected to use 6% serum in subsequent experiments.
Only the higher order multimer fractions of GL-2045 protect against CDC
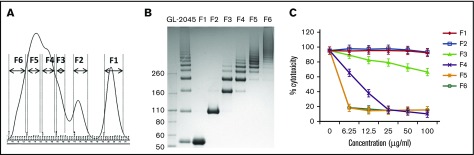
GL-2045 comprises a consistent multimer distribution of human IgG1 Fc multimers (X.Z. and J.O., manuscript submitted April 2016). To determine if the degree of Fc multimerization correlated with the ability to inhibit CDC, we evaluated 6 GL-2045 fractions, each composed of distinct multimers (Figure 2A-B). Fractions 1 and 2, containing the homodimer and the dimer of the homodimer, respectively, did not inhibit CDC. In contrast, fractions 5 and 6, which contained the largest multimers, provided maximal protection against CDC, even at the lowest concentrations tested, with fractions 3 and 4 showing intermediate effects (Figure 2C). These data demonstrate that all of the protective activity of GL-2045 against CDC is contained within the higher order multimer fractions.
Figure 2.
GL-2045 inhibition of CDC is completely dependent on the higher order multimer fractions. (A) GL-2045 was separated into 6 different fractions based on size by gel filtration. (B) SDS-PAGE of GL-2045 and fractions under nonreduced condition. (C) RTX-opsonized SUDHL4 cells were incubated with human complement serum pretreated with different fractions of GL-2045 at indicated concentrations. CDC activity was measured by Annexin V/7-AAD staining and analyzed by flow cytometry. The results are shown as the least squares mean ± SE estimated by ANCOVA. F, fractions.
GL-2045 avidly binds C1q and blocks C1q deposition on Ab-opsonized target cells
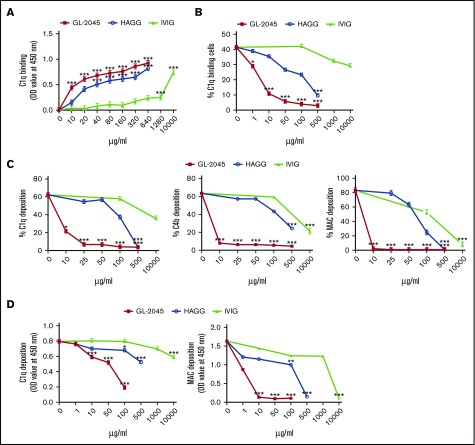
To understand the mechanism(s) by which GL-2045 inhibited CDC, we first tested the hypothesis that the multimerized Fc contents of GL-2045 might directly bind and sequester C1q away from Ab-opsonized target cells. ELISA studies revealed that both soluble GL-2045 (EC50 = 10.3 ± 2.7 μg/mL) and HAGG (EC50 = 60.2 ± 16.2 μg/mL) bound C1q more efficiently than did IVIG (EC50 = 2749.1 ± 280.8 μg/mL) (Figure 3A). To further test whether binding of GL-2045 to C1q could block C1q deposition on mAb-opsonized cells, we exposed anti-CD20–coated SUDHL4 cells to purified C1q in the presence of GL-2045, HAGG, or IVIG (Figure 3B). As anticipated, GL-2045 partially inhibited C1q binding at concentrations as low as 1 µg/mL, with near complete inhibition at 100 µg/mL. Although HAGG mediated similar effects, it was dramatically less potent. The IC50s for GL-2045 vs HAGG were 2.3 ± 0.4 vs 102.1 ± 11.7, respectively. In addition, the IC50 for IVIG was not reached at 10 000 μg/mL.
Figure 3.
GL-2045 sequesters C1q and prevents deposition of C1q, C4b, and MAC on the surface of Ab-opsonized cells. (A) The ability of testing compounds binding to plate-coated C1q was measured by ELISA. (B) GL-2045 blocks RTX-mediated C1q, C4b, and MAC deposition on Ramos cells in a CDC assay. GL-2045, HAGG, or IVIG was incubated in NHS and added to RTX-opsonized Ramos cells. Incubations were carried out for 15 minutes for C1q and C4b deposition or 30 minutes for MAC formation. The cells were then stained with FITC-conjugated anti-C1q, anti-C4b, or anti-C5b-9 mAbs to examine C1q, C4b, or MAC deposition. Data are shown as percent of C1q-, C4b-, or MAC-positive cells within the total cell population. (C) GL-2045 inhibits purified C1q deposition on RTX-opsonized cells. GL-2045, HAGG, or IVIG was preincubated with purified C1q (20 μg/mL) for 10 minutes and the resultant mixture was incubated with RTX-opsonized SUDHL4 cells at 37°C for 15 minutes. C1q deposition was detected with FITC anti-C1q Ab and evaluated by flow cytometry. Data are shown as percentage of C1q-positive cells within the total cell population. (D) GL-2045 inhibits the activation of the classical complement pathway in a plate-based assay. HAGG was coated on a plate to activate the classical pathway. Test compounds were added to the wells and incubated with 1.5% NHS at 37°C for 30 minutes for C1q deposition or 1 hour for MAC deposition. C1q and MAC depositions were determined by ELISA. The results are shown as the least squares mean ± SE estimated by ANCOVA. Natural log variance stabilizing transformation and Tukey procedure for multiple comparisons adjustment were used to test the differences. *P < .05, **P < .01, ***P < .001 compared with no-treatment control.
GL-2045–mediated protection against CDC is associated with reduced C1q, C4b, and MAC deposition on Ab-opsonized cells
Next, to determine whether GL-2045 mediated sequestration of C1q at the recognition phase of the classical pathway inhibited downstream complement activation that mediates target cell killing, we measured the levels of C1q, C4b, and MAC deposition. GL-2045 blocked C1q, C4b, and MAC deposition on RTX-opsonized lymphoma cells (Figure 3C). In contrast, higher concentrations of HAGG and IVIG were required to achieve the comparable levels of inhibition. The IC50s (μg/mL) for the 3 compounds (GL-2045 vs HAGG vs IVIG) for the prevention of C1q, C4b, and MAC deposition were: 6.7 ± 0.9 vs 112.5 ± 8.9 vs IC50 not reached at 10 000μg/mL, 6.9 ± 1.6 vs 228.3 ± 36 vs 2824.3 ± 912.5, and 6 ± 0.9 vs 74.6 ± 5.5 vs 574 ± 206.1, respectively. Interestingly, assessment of C1q binding on live and dead cells in the CDC assay revealed that, consistent with published reports, C1q was deposited on dead cells only, suggesting that the diminished C1q binding observed in this assay could potentially be secondary to the higher percentage of live cells in the GL-2045 treated group.23
Similar results were observed using a plate-based assay of the classical pathway of complement activation (Figure 3D), where the maximum inhibitory doses of GL-2045 for C1q and MAC deposition were 100 and 10 µg/mL, respectively. For HAGG and IVIG, even the highest concentrations tested did not maximally inhibit C1q deposition. Collectively, these data indicate that GL-2045 mediates potent direct inhibition of C1q binding to Ab-opsonized cells and prevents the deposition of complement split products.
GL-2045 drives self-limited complement activation by inducing C4a and C3a, but not C5a
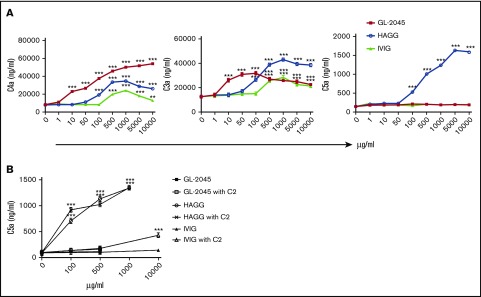
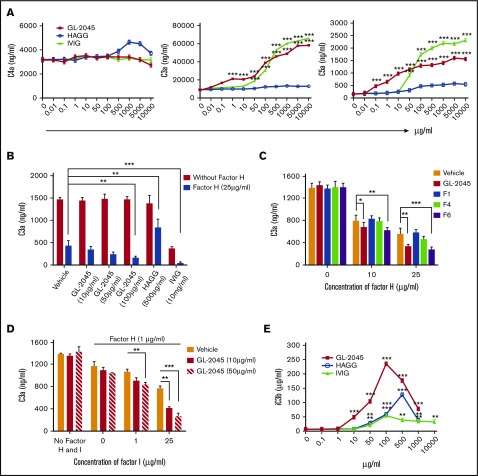
Because GL-2045 is composed of Fc multimers capable of C1q binding, we speculated that it might serve as a platform for initial complement activation. In human serum, GL-2045 mediated significant cleavage of C4 and very modest cleavage of C3, as evidenced by the presence of their split products, C4a and C3a, respectively (Figure 4A). C3a levels in GL-2045–stimulated serum were lower than in HAGG-treated serum and exhibited a dose-dependent bell-shaped curve. Compared with HAGG and GL-2045, IVIG mediated smaller increases in C4a and C3a. The maximal levels of C4a and C3a in samples treated with GL-2045 vs HAGG vs IVIG were: 56 011.4 ± 2489.7 vs 34 306.7 ± 1171.8 vs 24 405.8 ± 2240.1 ng/mL and 31 542.3 ± 874.1 vs 42 520.7 ± 26 27.7 vs 30 036.5 ± 1910.2 ng/mL, respectively. Unlike with HAGG, serum treated with GL-2045 or IVIG did not contain detectable C5a. Although these findings in a cell-free system were largely recapitulated in the presence of opsonized cells, GL-2045 did not increase C3a in this setting (supplemental Figure 3). This lack of increased C3a likely relates to the presence of RTX-opsonized target cells in this model, which induced high levels of C3 cleavage independent of GL-2045.
Figure 4.
GL-2045 drives self-limited complement activation. GL-2045 exposure to NHS results in increased cleavage of both C4 and C3, but does not enhance cleavage of C5. NHS was incubated with various concentrations of GL-2045, IVIG, and HAGG for 90 minutes at 37°C. Levels of C4a, C3a, and C5a were evaluated using the CBA human anaphylatoxin kit. The results are shown as the least squares mean ± SE estimated by ANCOVA. Natural log variance stabilizing transformation and Tukey procedure for multiple comparisons adjustment were used to test the differences. *P < .05, **P < .01, ***P < .001 compared with no-treatment control.
Furthermore, we sought to explore whether the lack of C5a production induced by GL-2045 was secondary to C2 consumption. NHS was incubated with GL-2045, HAGG, or IVIG in the presence of purified C2 at 25 µg/mL (data not shown) or 75 µg/mL for 90 minutes, after which C5a levels were determined by ELISA. Even in the presence of 75 µg/mL purified C2, GL-2045 failed to increase C5a production, whereas HAGG continued to induce high levels of C5a under all conditions tested (Figure 4B).
Taken in concert, our data show that GL-2045 induces activation of the initial step of the classical arm of the complement cascade as evidenced by C4a generation, with limited potential to mediate downstream C3 cleavage and an inability to induce significant C5 cleavage under the conditions tested.
A high concentration of GL-2045 partially inhibits the alternative C3 convertase activity
Based on our knowledge that GL-2045–stimulated serum contained low levels of C3a and no C5a, we postulated that GL-2045 might interfere with generation of the C3 and/or C5 convertases. To test these hypotheses, we first generated the classical and alternative forms of C3 convertase in vitro in the presence of defined concentrations of GL-2045, HAGG, or IVIG. Consistent with the well-documented ability of IVIG to sequester C3b,24,25 IVIG significantly inhibited the activity of the alternative form of C3 convertase, whereas GL-2045 exhibited partial inhibition at a concentration of 10 mg/mL only, which is higher than the effective concentration needed for CDC blockade. HAGG had no effect on the activity of alternative form of C3 convertase (Figure 5A).
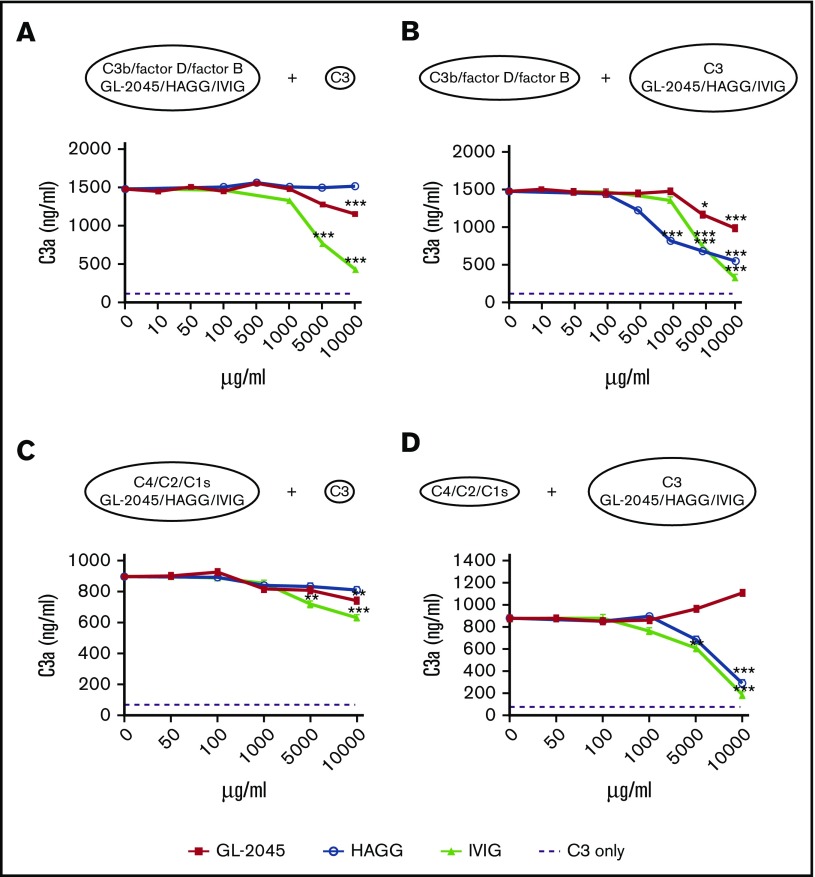
Figure 5.
High dose of GL-2045 partially inhibits the alternative form of C3 convertase. C3a production was examined in the alternative C3 convertase assembly system, generated by incubation of C3b and factors D and B with GL-2045, HAGG, or IVIG for 15 minutes at 37°C. This was followed by the addition of C3 (A) or C3b; factors D and B were incubated for 15 minutes at 37°C followed by addition of C3 preincubated with GL-2045, HAGG, or IVIG (B). For classical C3 convertase assembly, C4, C2, and C1s enzymes were incubated with GL-2045, HAGG, or IVIG followed by the addition of C3 (C). Alternatively, C4, C2, and C1s were incubated with drugs pretreated C3 (D). The results are least squares mean ± SE estimated by ANCOVA. Natural log variance stabilizing transformation and Tukey procedure for multiple comparisons adjustment were used to test the differences. *P < .05, **P < .01, ***P < .001 compared with no-treatment control.
We next determined whether GL-2045 could directly protect C3 from cleavage by C3 convertases. A high concentration of GL-2045 mediated partial protection of C3 from the alternative, but not the classical C3 convertase. In contrast, preincubation of C3 with either IVIG or HAGG protected C3 from cleavage by both forms of C3 convertase (Figure 5B,D). None of the compounds tested directly inhibited the classical form of C3 convertase (Figure 5C) or the C5 convertases (supplemental Figure 4). Collectively, these data are consistent with the published literature suggesting that IVIG inhibits complement activation at the level of the C3 convertase amplification loop.26
Self-limited complement activation by GL-2045 and IVIG is dependent on factor H
Factor H is an important regulator of both classical and alternative pathways of complement activation.27 To understand the mechanisms underlying the self-limited complement activation driven by GL-2045 and IVIG, we evaluated the levels of C4a, C3a, and C5a generated after exposure of factor H–depleted serum to GL-2045, IVIG, or HAGG. In serum lacking factor H, basal levels of C4a were markedly decreased compared with normal serum. No significant increase of C4a was observed after treatment. However, unlike in NHS, in factor H–depleted serum, GL-2045 and IVIG stimulated high levels of C3a and C5a (Figure 6A). Reconstitution of factor H–depleted serum with factor H resulted in a concentration-dependent reduction in levels of C3a and C5a following exposure to GL-2045, confirming the specificity of this response (supplemental Figure 5). Surprisingly, exposure of factor H–depleted serum to HAGG did not increase levels of C3a or C5a. These data suggest that factor H plays an important role in controlling GL-2045–mediated C3a and C5a generation.
Figure 6.
GL-2045 promotes the regulatory function of factors H and I and induces enhanced iC3b generation. (A) Self-limited complement activation by GL-2045 and IVIG is dependent on factor H. Factor H–depleted serum was incubated with various concentrations of GL-2045, HAGG, and IVIG for 90 minutes at 37°C. The sera were then evaluated for the presence of C4a, C3a, and C5a. (B) GL-2045 inhibits the activity of the alternative form of C3 convertase activity in the presence of factor H. GL-2045, HAGG, or IVIG was added to the alternative C3 convertase assembly at indicated concentrations in the presence or absence of factor H (25 μg/mL). C3 cleavage was measured by C3a ELISA. (C) In a different set of experiments, fractions of GL-2045 were added to the alternative C3 convertase assembly in the presence of factor H and C3. C3a generation was evaluated. (D) GL-2045 works in concert with the combination of factors H and I to inhibit the function of alternative form of C3 convertase. The alternative C3 convertase was generated in the presence of combination of GL-2045 and factors H and I. (E) Levels of iC3b were significantly increased in NHS treated with GL-2045. NHS was exposed to GL-2045, HAGG, or IVIG for 90 minutes at 37°C. The levels of iC3b in the serum were determined by ELISA. The results are shown as the least squares mean ± SE estimated by ANCOVA. Natural log variance stabilizing transformation and Tukey procedure (A,E), square root variance stabilizing transformation and Dunnett’s procedure (B), or log variance stabilizing transformation and Dunnett procedure (C-D) for multiple comparisons adjustment were used to test the differences. *P < .05, **P < .01, ***P < .001 compared with no-treatment (A,E) or vehicle (B-D) control.
GL-2045 promotes the regulatory function of factors H and I and enhances iC3b generation
We next sought to investigate whether GL-2045 affects the inhibitory function of factor H. We incubated the alternative form of C3 convertase with a suboptimal concentration of factor H. As anticipated, factor H reduced the ability of the alternative form of C3 convertase to generate C3a. Addition of GL-2045 potentiated the function of factor H in a concentration-dependent manner (Figure 6B). The higher order multimer fractions were completely responsible for this potentiating effect (Figure 6C). IVIG alone, in the absence of factor H, inhibited C3a generation. Furthermore, combination of factor H and IVIG induced a more profound reduction of C3a level. In contrast, HAGG significantly hindered factor H–mediated inhibition of C3 convertase activity.
Factor H is a cofactor for factor I, which inhibits the C3 convertase by converting C3b to iC3b.28 To determine if GL-2045 augmented the function of factor H in the presence of factor I, we used an analogous system with a fixed suboptimal concentration of factor H in the presence of escalating concentrations of factor I. GL-2045 augmented the ability of factors H and I, in combination, to inhibit the alternative form of C3 convertase in a concentration-dependent manner (Figure 6D). Based on these findings, we reasoned that if GL-2045 augments the function of factors H and I, the end product resulting from these interactions, iC3b, should be increased in GL-2045–treated NHS. Indeed, our results demonstrated that GL-2045 was more potent than either IVIG or HAGG in inducing iC3b, with a bell-shaped concentration dependence, and resulted in higher peakiC3b levels (Figure 6E). Collectively, these data demonstrate that GL-2045 potentiates the function of factors H and I, inhibiting the complement cascade at the level of the alternative form of C3 convertase and inducing high levels of iC3b.
Discussion
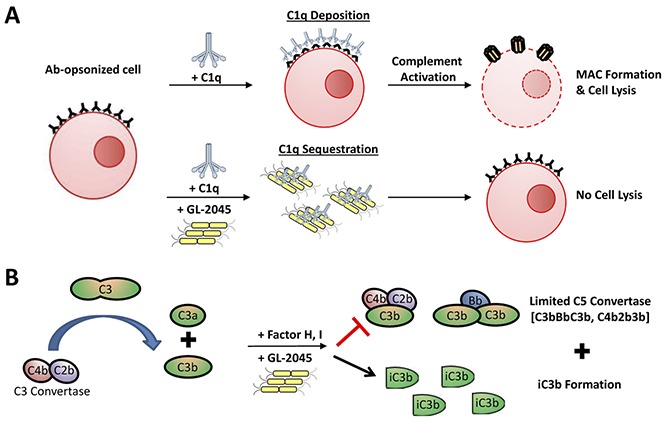
GL-2045 was designed and produced to serve as a recombinant IVIG “mimetic” for the treatment of autoimmune diseases. We compared the ability of GL-2045, IVIG, and HAGG to modulate complement activation. Of the 3 compounds tested, GL-2045 demonstrated the most potent binding to C1q and prevented C1q deposition on anti-CD20 mAb-opsonized B-cell lines. These observations are consistent with the fact that high-affinity binding and activation of C1q requires Fc clustering.29 The blockade of C1q deposition was associated with both the inhibition of CDC and significant reductions in the deposition of downstream complement activation products, such as C4b and MAC, on Ab-coated target cells. This GL-2045–mediated inhibition of CDC was completely dependent on the higher order multimer fractions. Importantly, we acknowledge that potency comparisons between these compounds may be partially confounded by our use of mass vs molar concentrations and the different ratios of larger vs smaller Fc-bearing complexes within each drug preparation. These data suggest that GL-2045 acts as a sink, sequestering C1q away from target cells and preventing the initiation of CDC on the cell surface (Figure 7).
Figure 7.
Proposed model of GL-2045–complement interactions. (A) GL-2045 binds to and sequesters C1q, thus preventing the activation of classical pathway at the site of inflammation. (B) GL-2045 also induces early activation of the classical pathway, resulting in the formation of C4bC2b. Although this molecule can cleave C3, the ability of GL-2045 to potentiate degradation of C3b to iC3b by factors H and I in combination and disassociation of C3bBb by factor H inhibits the C3 convertase amplification loop and the C5 convertases.
As a consequence of C1q binding, GL-2045 induced C4a and C3a, but not C5a, in NHS. In contrast, HAGG was able to drive the complement activation cascade through to completion. RTX infusion into patients with a heavy tumor burden is reported to induce significant complement component C2 deletion.30 Therefore, to understand the potential relevance of C2 consumption on the absence of GL-2045–mediated generation of C5a in our studies, we added excess C2 to GL-2045–treated NHS. Addition of C2 did not induce any C5a production in NHS treated with GL-2045, but did result in small, but statistically significant, increases in C5a in IVIG-treated NHS at high concentrations. These data suggest that the formation of GL-2045/C1q complexes is not able to sustain and amplify subsequent complement activation in vitro in the presence of soluble regulators from human serum.
The alternative pathway largely defines the amplification of complement activation.31 High concentrations (5-10 mg/mL) of IVIG directly interfered with assembly/activity of alternative C3 convertase. In contrast, GL-2045 could not achieve similar effects at concentrations higher than its IC50 for CDC inhibition, whereas HAGG had no effect. These findings are in keeping with prior studies, demonstrating that IVIG blocks complement activation through covalent interactions with C4b32 and C3b,24,25 sequestering them from both the amplification loop and surface of opsonized cells33 and preventing local MAC activation.34-40
Despite differences in their ability to directly inhibit the alternative form of C3 convertase, both GL-2045 and IVIG indirectly govern the alternative form of C3 convertase by potentiating factor H activity through mechanisms still to be determined. These data are consistent with prior reports demonstrating that IVIG modulates C3bBb cleavage by factor H.33 Similarly, pentraxin-3, an antibody-like protein, can both bind and sequester C1q and potentiate factor H function, allowing apoptotic cells to die a “silent death.”41 Recent structural data suggesting that factor H has the potential to form tetramers on polyanionic surfaces, resulting in a functional increase in both its decay accelerating and cofactor activities,42 may explain the ability of the higher order multimer fractions of GL-2045 to enhance factor H activity.
The end result of GL-2045–mediated potentiation of factors H and I is the potent generation of iC3b. As expected, the amount of iC3b generated by GL-2045 is higher than that induced by IVIG because, by inhibiting the alternative form of C3 convertase, IVIG limits the amount of C3b available for “inactivation.” iC3b binding to complement receptor 3 mediates long-lasting tolerogenic properties including, but not limited to, reduced monocyte differentiation into dendritic cells following u irradiation, the generation of myeloid-derived suppressor cells, and induction of transforming growth factor β2 and interleukin-10.43-47 These data demonstrate that the ability of GL-2045 to induce complement split products such as iC3b, which are recognized to play an important role in the generation of tolerance, requires initial activation of the complement cascade and cannot be recapitulated with compounds that simply block the early phases of complement activation.
Because GL-2045 is advanced as a drug candidate, 1 theoretical clinical concern is its ability to induce high levels of C4a and intermediate levels of C3a. In considering this possibility, it is germane that previous reports demonstrate that aggregated IVIG engagement of C1q results in its rapid degradation in vivo, suggesting that the initial stimulus for activation will be self-limited.48 Furthermore, IVIG is also recognized to mediate initial complement activation followed by inhibition.49 Indeed, evaluation of blood samples from patients with recurrent spontaneous abortions of uncertain etiology following IVIG infusion revealed significant increases in Bb, C3bc, C5a, and MAC, with stable levels of C1q, C3, and C4.50 Similarly, administration of high doses of IVIG to patients with dermatomyositis resulted in reduced levels of C4 and enhanced levels of the C3a.26 Finally, despite the fact that GL-2045 induced high levels of C4a and intermediate levels of C3a, these molecules are dramatically less potent anaphylatoxins than C5a,51 and recent reports now acknowledge that C3a should be considered immunomodulatory rather than simply pro-inflammatory because it mediates diverse pro- and anti-inflammatory properties.52,53
As with other drug candidates in development, such as C1INH proteins, anti-C1s mAbs, and anti-MASP2 mAbs, GL-2045 inhibits complement-mediated activation on the cell surface.54 Unlike these more “traditional” drug candidates, GL-2045 induces self-limited complement activation, resulting in the generation of proteins with long-lasting anti-inflammatory properties (Figure 7). Ongoing in vivo studies in nonhuman primates will help further define the influence of GL-2045 on complement function.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This study was supported by Pfizer Inc through a sponsored research agreement between Pfizer Inc and the University of Maryland, Baltimore.
Authorship
Contribution: All authors made substantive intellectual contributions to the experimental design; H.Z. and X.Z. performed the majority of the studies; E.M. performed the sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and fractionated GL-2045; S.E.S., H.Z., X.Z., and E.S. analyzed data and interpreted the studies; D.R. performed all statistical analyses; J.O., G.L., D.S.B., and S.E.S. designed research; S.E.S. and X.Z. supervised the project; and all authors had input into the final version of the manuscript.
Conflict-of-interest disclosure: S.E.S. is a cofounder, consultant, and stockholder in Gliknik Inc, a biotechnology company. He receives royalties for intellectual property, related to B7-H1 (PD-L1), licensed by the Mayo Clinic College of Medicine to third parties. He is a paid consultant to Astra Zeneca. He also receives research support from Pfizer and Gliknik through sponsored research agreements through the University of Maryland, Baltimore. D.S.B. is cofounder of Gliknik Inc. and an employee. H.O. and E.M. are employees of Gliknik. D.R., J.O., and G.L. are employees of Pfizer Inc.
Correspondence: Scott E. Strome, Department of Otorhinolaryngology–Head and Neck Surgery, University of Maryland School of Medicine, Baltimore, MD 21201; e-mail: sstrome@som.umaryland.edu; and Xiaoyu Zhang, Department of Otorhinolaryngology–Head and Neck Surgery, University of Maryland School of Medicine, Baltimore, MD 21201; e-mail: xyzhang@som.umaryland.edu.
References
- 1.van der Meché FGA, Schmitz PIM. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. N Engl J Med. 1992;326(17):1123-1129. [DOI] [PubMed] [Google Scholar]
- 2.Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. Lancet. 1997;349(9047):225-230. [PubMed] [Google Scholar]
- 3.Dalakas MC, Illa I, Dambrosia JM, et al. . A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. 1993;329(27):1993-2000. [DOI] [PubMed] [Google Scholar]
- 4.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971-982. [DOI] [PubMed] [Google Scholar]
- 5.Zinman L, Ng E, Bril V. IV immunoglobulin in patients with myasthenia gravis: a randomized controlled trial. Neurology. 2007;68(11):837-841. [DOI] [PubMed] [Google Scholar]
- 6.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013;190(8):3839-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25(11):1256-1264. [DOI] [PubMed] [Google Scholar]
- 8.Augener W, Friedman B, Brittinger G. Are aggregates of IgG the effective part of high-dose immunoglobulin therapy in adult idiopathic thrombocytopenic purpura (ITP)? Blut. 1985;50(4):249-252. [DOI] [PubMed] [Google Scholar]
- 9.Teeling JL, Jansen-Hendriks T, Kuijpers TW, et al. . Therapeutic efficacy of intravenous immunoglobulin preparations depends on the immunoglobulin G dimers: studies in experimental immune thrombocytopenia. Blood. 2001;98(4):1095-1099. [DOI] [PubMed] [Google Scholar]
- 10.Bazin R, Lemieux R, Tremblay T, St-Amour I. Tetramolecular immune complexes are more efficient than IVIg to prevent antibody-dependent phagocytosis of blood cells. Br J Haematol. 2004;127(1):90-96. [DOI] [PubMed] [Google Scholar]
- 11.Clynes R. Immune complexes as therapy for autoimmunity. J Clin Invest. 2005;115(1):25-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain A, Olsen HS, Vyzasatya R, et al. . Fully Recombinant Murine StradomersTM effectively prevent idiopathic thrombocytopenic purpura and treat arthritis in mice. Arthritis Res Ther. 2012;14(4):R192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiruppathi M, Sheng JR, Li L, Prabhakar BS, Meriggioli MN. Recombinant IgG2a Fc (M045) multimers effectively suppress experimental autoimmune myasthenia gravis [published corrigendum appears online at http://dx.doi.org/10.1016/j.jaut.2014.10.004]. J Autoimmun. 2014;52:64-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niknami M, Wang M-X, Nguyen T, Pollard JD. Beneficial effect of a multimerized immunoglobulin Fc in an animal model of inflammatory neuropathy (experimental autoimmune neuritis). J Peripher Nerv Syst. 2013;18(2):141-152. [DOI] [PubMed] [Google Scholar]
- 15.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of Proteins of Immunological Interest. 5th ed. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health. NIH Publication No; 91. 1991: 3242. [Google Scholar]
- 16.Zhang X, Olsen HS, Chen S, et al. . Anti-CD20 antibody with multimerized Fc domains: a novel strategy to deplete B cells and augment treatment of autoimmune disease. J Immunol. 2016;196(3):1165-1176. [DOI] [PubMed] [Google Scholar]
- 17.Jusko M, Potempa J, Mizgalska D, et al. . A metalloproteinase mirolysin of tannerella forsythia inhibits all pathways of the complement system. J Immunol. 2015;195(5):2231-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinen S, Hartmann A, Lauer N, et al. . Factor H–related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114(12):2439-2447. [DOI] [PubMed] [Google Scholar]
- 19.Kerr MA. The human complement system: assembly of the classical pathway C3 convertase. Biochem J. 1980;189(1):173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R CoreTeam. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 21.Ritz C, Baty F, Streibig JC, Gerhard D. Dose-response analysis using R. PLoS One. 2015;10(12):e0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo EM, Wims LA, Chan LA, Morrison SL. Human IgG2 Can form covalent dimers. J Immunol. 2003;170(6):3134-3138. [DOI] [PubMed] [Google Scholar]
- 23.Pawluczkowycz AW, Beurskens FJ, Beum PV, et al. . Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of b cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183(1):749-758. [DOI] [PubMed] [Google Scholar]
- 24.Brown EJ, Berger M, Joiner KA, Frank MM. Classical complement pathway activation by antipneumococcal antibodies leads to covalent binding of C3b to antibody molecules. Infect Immun. 1983;42(2):594-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadd KJ, Reid KBM. The binding of complement component C3 to antibody-antigen aggregates after activation of the alternative pathway in human serum. Biochem J. 1981;195(2):471-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutz HU, Stammler P, Bianchi V, et al. . Intravenously applied IgG stimulates complement attenuation in a complement-dependent autoimmune disease at the amplifying C3 convertase level. Blood. 2004;103(2):465-472. [DOI] [PubMed] [Google Scholar]
- 27.Kishore U, Sim RB. Factor H as a regulator of the classical pathway activation. Immunobiology. 2012;217(2):162-168. [DOI] [PubMed] [Google Scholar]
- 28.Bajic G, Degn SE, Thiel S, Andersen GR. Complement activation, regulation, and molecular basis for complement‐related diseases. EMBO J. 2015;34(22):2735-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diebolder CA, Beurskens FJ, de Jong RN, et al. . Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343(6176):1260-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy AD, Beum PV, Solga MD, et al. . Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol. 2004;172(5):3280-3288. [DOI] [PubMed] [Google Scholar]
- 31.Harboe M, Mollnes TE. The alternative complement pathway revisited. J Cell Mol Med. 2008;12(4):1074-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell RD, Dodds AW, Porter RR. The binding of human complement component C4 to antibody-antigen aggregates. Biochem J. 1980;189(1):67-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutz HU, Stammler P, Jelezarova E, Nater M, Spath P. High doses of immunoglobulin G attenuate immune aggregate-mediated complement activation by enhancing physiologic cleavage of C3b in C3bn- IgG complexes. Blood. 1996;88(1):184-193. [PubMed] [Google Scholar]
- 34.Berger M, Rosenkranz P, Brown CY. Intravenous and standard immune serum globulin preparations interfere with uptake of 125I-C3 onto sensitized erythrocytes and inhibit hemolytic complement activity. Clin Immunol Immunopathol. 1985;34(2):227-236. [DOI] [PubMed] [Google Scholar]
- 35.Basta M, Dalakas MC. High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. J Clin Invest. 1994;94(5):1729-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basta M. Modulation of complement-mediated immune damage by intravenous immunoglobulin. Clin Exp Immunol. 1996;104(Suppl. 1):21-25. [PubMed] [Google Scholar]
- 37.Arumugam TV, Tang S-C, Lathia JD, et al. . Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci USA. 2007;104(35):14104-14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spycher M, Matozan K, Minnig K, et al. . In vitro comparison of the complement-scavenging capacity of different intravenous immunoglobulin preparations. Vox Sang. 2009;97(4):348-354. [DOI] [PubMed] [Google Scholar]
- 39.Vidarte L, Pastor C, Mas S, et al. . Serine 132 is the C3 covalent attachment point on the CH1 domain of human IgG1. J Biol Chem. 2001;276(41):38217-38223. [DOI] [PubMed] [Google Scholar]
- 40.Sahu A, Pangburn MK. Covalent attachment of human complement C3 to IgG. Identification of the amino acid residue involved in ester linkage formation. J Biol Chem. 1994;269(46):28997-29002. [PubMed] [Google Scholar]
- 41.Inforzato A, Doni A, Barajon I, et al. . PTX3 as a paradigm for the interaction of pentraxins with the Complement system. Semin Immunol. 2013;25(1):79-85. [DOI] [PubMed] [Google Scholar]
- 42.Pangburn MK, Rawal N, Cortes C, Alam MN, Ferreira VP, Atkinson MAL. Polyanion-induced self-association of complement factor H. J Immunol. 2009;182(2):1061-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh C-C, Chou H-S, Yang H-R, et al. . The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood. 2013;121(10):1760-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahara M, Kang K, Liu L, Yoshida Y, McCormick TS, Cooper KD. iC3b arrests monocytic cell differentiation into CD1c-expressing dendritic cell precursors: a mechanism for transiently decreased dendritic cells in vivo after human skin injury by ultraviolet B. J Invest Dermatol. 2003;120(5):802-809. [DOI] [PubMed] [Google Scholar]
- 45.Sohn J-H, Bora PS, Suk H-J, Molina H, Kaplan HJ, Bora NS. Tolerance is dependent on complement C3 fragment iC3b binding to antigen-presenting cells. Nat Med. 2003;9(2):206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verbovetski I, Bychkov H, Trahtemberg U, et al. . Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J Exp Med. 2002;196(12):1553-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amarilyo G, Verbovetski I, Atallah M, et al. . iC3b-opsonized apoptotic cells mediate a distinct anti-inflammatory response and transcriptional NF-κB-dependent blockade. Eur J Immunol. 2010;40(3):699-709. [DOI] [PubMed] [Google Scholar]
- 48.Veerhuis R, van Es LA, Daha MR. Effects of soluble aggregates of IgG on the binding, uptake and degradation of the Clq subcomponent of complement by adherent guinea pig peritoneal macrophages. Eur J Immunol. 1985;15(9):881-887. [DOI] [PubMed] [Google Scholar]
- 49.Levin D, Golding B, Strome SE, Sauna ZE. Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol. 2015;33(1):27-34. [DOI] [PubMed] [Google Scholar]
- 50.Mollnes TE Høgåsen, De Carolis C, et al. High-dose intravenous immunoglobulin treatment activates complement in vivo. Scand J Immunol. 1998;48(3):312-317. [DOI] [PubMed] [Google Scholar]
- 51.Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34-50. [DOI] [PubMed] [Google Scholar]
- 52.Coulthard LG, Woodruff TM. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J Immunol. 2015;194(8):3542-3548. [DOI] [PubMed] [Google Scholar]
- 53.Morgan EL, Weigle WO, Hugli TE. Anaphylatoxin-mediated regulation of the immune response. I. C3a-mediated suppression of human and murine humoral immune responses. J Exp Med. 1982;155(5):1412-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan BP, Harris CL. Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov. 2015;14(12):857-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.