Abstract
Huanglongbing is a devastating disease of citrus. In this study, a comprehensive profile of phloem sap amino acids (AA) in four permissive host plants of Candidatus Liberibacter asiaticus (CLas) and three non-permissive Rutaceae plants was conducted to gain a better understanding of host factors that may promote or suppress the bacterium. The AA profiles of Diaphorina citri nymphs and adults were similarly analyzed. A total of 38 unique AAs were detected in phloem sap of the various plants and D. citri samples, with phloem sap of young shoots containing more AAs and at higher concentrations than their mature counterparts. All AAs detected in phloem sap of non-permissive plants were also present in CLas -permissive hosts plus additional AAs in the latter class of plants. However, the relative composition of 18 commonly shared AAs varied between CLas -permissive hosts and non-permissive plants. Multivariate analysis with a partial least square discriminant methodology revealed a total of 12 AAs as major factors affecting CLas host status, of which seven were positively related to CLas tolerance/resistance and five positively associated with CLas susceptibility. Most of the AAs positively associated with CLas susceptibility were predominantly of the glutamate family, notably stressed-induced AAs such as arginine, GABA and proline. In contrast, AAs positively correlated with CLas tolerance/resistance were mainly of the serine family. Further analysis revealed that whereas the relative proportions of AAs positively associated with CLas susceptibility did not vary with host developmental stages, those associated with CLas tolerance/resistance increased with flush shoot maturity. Significantly, the proline-to-glycine ratio was determined to be an important discriminating factor for CLas permissivity with higher values characteristic of CLas -permissive hosts. This ratio could be exploited as a biomarker in HLB-resistance breeding programs.
Introduction
Huanglongbing (HLB; citrus greening) disease has long been a serious disease of citrus in Asia [1]. In recent years, the disease has spread throughout major citrus growing areas in the Americas. The substantial economic damage caused by HLB to the Florida citrus industry is well documented [2], and the disease is threatening the sustainability of citrus production in other major citrus producing states such as California and Texas. Considerable efforts and resources are being expended to control the spread of HLB in the Americas using a three-pronged approach of propagation of clean nursery stock, area-wide vector control and rogueing of infected trees [3].
The most prevalent of the three fastidious, phloem-inhabiting putative alpha-proteobacterial agents of HLB in the U.S. is Ca. Liberibater asiaticus (CLas). CLas is spread by the oligophagous Asian citrus psyllid Diaphorina citri Kuwayama, 1908 (Hemiptera: Liviidae) that feeds and develops exclusively on plants within the Rutaceae family including Citrus spp. and Murraya spp. Both D. citri nymphs and adults can acquire and transmit CLas Although many Citrus (sweet orange, grapefruit, limes, lemons, etc.) and non-Citrus (curry leaf, orange jasmine, etc.) rutaceous species are suitable for D. citri reproduction and development [4–6], the performance of CLas varies among Rutaceae plants [7]. Most commercially grown citrus species are permissive hosts, allowing for CLas multiplication and HLB development [1]. In contrast, curry leaf (Murraya koenigii) and orange jasmine (Murraya exotica) are non-permissive to CLas and thus HLB tolerant/resistant [7]. Some non-Citrus rutaceous plants such as white sapote (Casimiroa edulis) are neither hosts to D. citri [6] nor permissive to CLas [7]. Furthermore, the Madagascar periwinkle (Catharanthus roseus (L.) G.Don (Gentianales: Apocynacae), supports CLas replication and growth [8] even though it is a non-host of D. citri. CLas also readily multiplies within the hemolymph and body of D. citri [9–11], indicating further complexity of the HLB pathosystem. Based on observed differential CLas responses reported in numerous [1,7,12] studies, it is reasonable to postulate that phloem saps of CLas -permissive hosts (i.e. Citrus spp. and periwinkle) as well as D. citri hemolymph and tissue contain unique growth factors that facilitate CLas replication. Contrariwise, phloem saps of non-permissive plants (i.e. Murraya spp.) may contain CLas -suppressing factors. Since CLas is a yet-to-be-cultured fastidious bacterium [1], it is important to gain a better understanding of potential factors regulating its growth and replication.
Most bacteria have limited metabolic capabilities and mainly depend on their hosts for energy and growth substrates [13]. The fastidious nature of CLas suggests that it likely relies on its hosts to meet its nutritional needs. Indeed, an analysis of the complete CLas genome indicates that it has a limited ability for aerobic respiration and is likely auxotrophic for at least five amino acids [14]. CLas infection was also reported to differentially affect the expression of heat shock proteins in D. citri adults and nymphs [15]. Furthermore, the observed down-regulation of hexamerin, an amino acid storage protein in insects [16] led Vyas et al. [15] to suggest that CLas may modulate free amino acid (FAA) availability in its host through regulation of expression of amino acid (AA) storage protein genes. Taken together, amino acids appear to play a significant role in host- CLas interactions and they may be required for the bacterium host nutritional exploitation, growth and transmission processes.
The importance of young expanding citrus flush shoots for CLas acquisition [10,17] and transmission [18,19] by D. citri has been well documented. It has also been shown that the phloem sap of young and expanding citrus flush shoots contain significantly greater numbers and concentrations of individual amino acids than mature shoots [20]. While the amino acid composition of citrus phloem sap [20,21], periwinkle [12], psyllid hemolymph [22] and the non-citrus Rutaceae orange jasmine and curry leaf [23] have been documented, it is unclear how the AA profiles of CLas -permissive hosts and non-permissive Rutaceae plants compare to each other.
To address these knowledge gaps, the goal of this study was to perform a comparative analysis of amino acid profiles of CLas -permissive hosts and non-permissive Rutaceae plants to identify key amino acids that may act as CLas growth promoting and/or suppressing factors. Such CLas -promoting, host-encoded amino acids may be utilized for in vitro culturing of the bacterium or exploited for HLB management. In addition, putative discriminating AAs could be harnessed as biomarkers for quick screen of candidate CLas tolerant and/or susceptible Citrus spp. in breeding programs.
Materials and methods
Insects
Diaphorina citri adults and nymphs were obtained from a laboratory-reared colony at the Texas A&M University-Kingsville Citrus Center, Weslaco, Texas. The colony was established using psyllid adults collected from a mature grapefruit block in 2006, prior to the first detection of HLB in Texas [24]. Routine cultures of the psyllid were maintained on a mixture of caged orange jasmine (Murraya exotica L.), grapefruit (Citrus × paradisi Metcalfd.) and sweet orange (C. × sinensis (L.) Osbeck.) plants at 25±5°C with 14 h:10 h L:D cycle and 65±5% RH. No addition of feral psyllids was made to the colony since 2006 and regular PCR testing (twice a year) has established the colony as CLas -negative.
Plants
The analyses were conducted on three commonly grown citrus species and known hosts of the ACP and CLas, two ornamental CLas tolerant/resistant Rutaceae hosts of D. citri, a CLas tolerant/resistant and psyllid non-host Rutaceae plant, and a CLas -permissive non-Rutaceae psyllid non-host plant (Table 1). All experimental plants were grown in 7.6 L pots containing a commercial potting mix (Metro-Mix #2; Sun Gro Horticulture Inc., Agawam, MA) in a greenhouse at Texas A&M University Kingsville Citrus Center facility. All citrus host plants were grafted onto sour orange (Citrus × aurantium L.) rootstock and were ca. 2 year-old at the time of the experiment. Own-rooted curry leaf, orange jasmine and periwinkle plants (12-24-month-old) were purchased from a certified local nursery in McAllen, Texas, then transferred into the 7.6 L rearing pots. All experimental plants were determined to be CLas -negative based on standard qPCR tests [25], then grown for 6 months under similar conditions in the greenhouse (25–40°C) between March and September 2014 prior to phloem sap collection. The plants were watered daily or as needed to prevent any hydric stress, uniformly fertilized with a complete fertilizer (Peters Professional 20-20-20 General Purpose; The Scotts Company, Marysville, OH) at the rate of 5 g/pot monthly, and sprayed as needed with imidacloprid (Admire Pro) or spirotetramat (Movento) (Bayer CropScience, Research Triangle Park, NC) for insect and mite control.
Table 1. List of plant species evaluated in this study and their permissiveness to Candidatus Liberibacter asiaticus (CLas) or the Asian citrus psyllid (ACP).
| Common name | Botanical name | Cultivar | CLas statusa | ACP statusb |
|---|---|---|---|---|
| Grapefruit | Citrus x paradisi | Rio Red | Permissive | Host |
| Sweet orange | Citrus x sinensis | Marrs | Permissive | Host |
| Lemon | Citrus x limon | Valley lemon | Permissive | Host |
| Curry leaf | Murraya koenigii | Unknown | Non-permissive | Host |
| Orange jasmine | Murraya exotica | Lakeview | Non-permissive | Host |
| White sapote | Casimiroa edulis | Unknown | Non-permissive | Non-host |
| Madagascar periwinkle | Catharanthus roseus | Unknown | Permissive | Non-host |
aPermissive, supports CLas growth and multiplication; Non-permissive, suppress CLas growth and multiplication.
bHost, feeding and reproductive host of the ACP; Non-host, not colonized by the ACP.
Phloem sap collection
Phloem saps were extracted from young expanding flush shoots and mature shoots of Citrus and non-Citrus rutaceous plants using the ethylenediaminetetraacetic acid (EDTA) method according to [26] with some modification [20]. Briefly, flush shoots were excised at the point of attachment to the main twig using sterilized pruning shears and immediately immersed into 30 mL of 20 mM EDTA solution in plastic vials. Whole periwinkle plants cut at their base were processed in a similar manner. The vials were then covered with moist paper towels and transported on dry ice to the laboratory to maintain sample integrity. Samples were agitated at 100 rpm on a table shaker in a dark, temperature (21°C) controlled room for 3 hrs as described by Sétamou et al. [20] Ten young flush shoots, four mature shoots of Rutaceae and two whole periwinkle plants were used for phloem sap collection and three replicates of each sample were prepared. Excised flush shoots or periwinkle plants were removed from the tubes and the EDTA solution with phloem sap extracts was transferred into sterile 50 mL centrifuge tubes and stored at -80°C until further processing. The mass of each cut rutaceous flush shoot or periwinkle plant was individually measured using a Mettler Toledo MS104S analytical balance (Mettler Toledo Inc., Greifensee, Switzerland). Prior to free amino acid analysis, tubes containing the frozen phloem sap solutions were retrieved, uncapped, and freeze-dried for 96 hrs. using a benchtop lyophilizer. The freeze-dried EDTA-phloem exudate (Millrock Bench-Top Freeze-Dryer BT48, Millrock Technology, Kingston, NY) were analyzed for amino acid contents at the University of Missouri-Columbia Experimental Station Chemical Laboratories, following previously described procedures [20].
Extraction of D. citri amino acid
Forty live D. citri nymphs and an equal number of adults were collected from a laboratory-reared colony and thoroughly homogenized separately in 15 ml of EDTA in centrifuge tubes. The homogenates were filtered using a Whatman #2 filter paper, and aliquots (~12 ml) of supernatants were centrifuged at 14,000 × g for 5 min. Approximately 10 ml of the supernatant was collected, freeze-dried, and analyzed for amino acid composition as described above. The analyses involved three replicates of each D. citri developmental stage. In addition, the mass of a group of 10 adults or nymphs (n = 10) was measured using the analytical scale and the mass values were used to determine free amino acid contents of D. citri nymphs and adults per gram of body mass.
Statistical analyses
The FAA concentrations in the various plant phloem exudates and whole psyllid samples were calculated by normalizing the level of each FAA per gram of fresh tissue or whole insect per replicate. Mean values were determined for each FAA per host. A Venn diagram was used to compare the FAA composition of the different hosts based on their presence or absence in the sample (http://bioinformatics.psb.ugent.be/webtools/Venn/). An agglomerative hierarchical cluster (ACH) analysis computed with the Euclidian distance as proximity type and the Ward’s agglomeration method with automatic entropy truncation [27] was used to classify the different hosts in a limited number of relatively homogenous groups according to their amino acid profiles. Grouping of FAAs was similarly performed via ACH, and a heatmap was used to graphically represent the relationship between FAA profiles and host clusters generated based on relative FAA concentrations. In addition, a partial least square discriminant analysis (PLS-DA) that generates a supervised pattern recognition matrix was used to extract maximum information on discriminant compounds for the data. Using PLS-DA, the most discriminatory FAAs between CLas -permissive hosts and non-permissive plants were identified using the index scores of their variable importance on the projection (VIP) plot. An FAA with a VIP > 1 score is considered important [28]. The Pearson linear correlation was used to determine relationships between individual FAAs and to identify those FAAs which highly correlated with the host CLas host status. The relative ratios of each FAA between CLas -permissive and non-permissive hosts as determined by the PLS-DA were generated, and these ratios were compared to 1 using the Student’s t-test. All analyses were performed with the XLSTAT software (version 2016, Addinsoft, Paris, France).
Results
Mean values of total amino acids detected in the phloem sap of young and mature shoots of the six Rutaceae plants, whole periwinkle and whole D. citri adults and nymphs (Table 1) are presented in Table 2. More individual FAAs were detected in citrus phloem (35) relative to D. citri nymphs (30) and adults (31), periwinkle (25) and non-citrus Rutaceae (19–28). Across plant species, between one and nine additional FAAs were detected in the phloem sap of young shoots compared to mature shoots. Similarly, significantly higher concentrations of total and individual FAAs were detected in phloem sap of young shoots of each host plant compared to mature shoots of the same plants (5-39-fold range; Table 2) and this disparity was greater in CLas -permissive hosts (21-39-fold range) than in non-permissive hosts (5-10-fold range). The total amount of amino acids detected per gram of tissue in D. citri 5th instars was comparable to that detected in equivalent numbers of adults.
Table 2. Mean amino acid concentration1(μg/g) of leaf tissue of Candidatus Liberibacter asiaticus (CLas)-permissive and non–permissive plants2 and of whole Diaphorina citri (ACP) adults and nymphs analyzed by HPLC.
| CLas-permissive hosts | CLas-non-permissive hosts | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grapefruit | Lemon | Sweet orange | ACP3 | Peri-winkle4 | Curry leaf | Orange jasmine | White sapote | |||||||||
| Code | Y | M | Y | M | Y | M | Ny | Ad | Stem | Y | M | Y | M | Y | M | |
| Mass per sample unit in grams ± SE | 1.4 ±0.1 | 3.1 ±0.1 | 0.5 ±0.1 | 2.4± 0.0 | 0.6 ± 0.0 | 3.5± 0.0 | 0.00013 ±0.00002 | 0.00055 ±0.00009 | 2.9 ± 0.5 | 0.1 ±0.0 | 0.8 ±0.0 | 0.3 ±0.0 | 1.6 ±0.1 | 1.3 ±0.0 | 3.5 ±0.3 | |
| Free amino acid | ||||||||||||||||
| Phosphoserine | SEP | 1.4 | 0.4 | 2.2 | 0.8 | 2.5 | 0.5 | 475.0 | 237.5 | 0.9 | 6.1 | 1.8 | 4.1 | 1.5 | 0.7 | 0.6 |
| Taurine | TAU | 0.9 | - | 1.1 | - | 1.1 | - | - | 37.5 | - | - | - | - | - | - | - |
| Phosphoethanolamine | PHOS | 1.8 | 0.8 | 3.1 | 1.2 | 3.4 | 0.8 | 150.0 | 56.3 | 0.3 | 13.9 | 4.7 | 10.9 | 3.9 | 1.3 | 1.2 |
| Aspartic Acid | ASP | 0.6 | 0.2 | 1.9 | 0.4 | 1.8 | 0.1 | 800.0 | 275.0 | 12.6 | 2.0 | 0.2 | 1.0 | - | 0.6 | - |
| Hydroxyproline | HYP | 0.4 | - | 2.1 | - | 1.2 | - | - | - | 0.2 | - | - | - | - | - | - |
| Threonine | THR | 4.3 | 0.1 | 7.9 | 0.3 | 7.8 | 0.1 | 1175.0 | 1075.0 | 0.9 | 15.7 | 1.6 | 10.1 | 0.3 | 1.7 | 0.2 |
| Serine | SER | 14.7 | 0.4 | 28.8 | 1.2 | 26.8 | 0.7 | 1050.0 | 343.8 | 3.8 | 11.8 | 1.1 | 6.2 | 0.3 | 1.2 | 0.2 |
| Asparagine | ASN | 30.6 | 3.3 | 116.1 | 3.1 | 45.4 | 0.7 | 1625.0 | 425.0 | 2.3 | 12.4 | 0.6 | 9.6 | 0.6 | 1.3 | - |
| Glutamic Acid | GLU | 1.3 | 0.2 | 5.3 | 0.8 | 4.1 | 0.3 | 2125.0 | 1162.5 | 3.8 | 6.5 | 0.5 | 6.3 | 0.3 | 2.5 | 0.4 |
| Glutamine | GLN | 3.1 | 0.3 | 4.8 | 0.6 | 10.8 | 0.3 | 2825.0 | 3143.8 | 1.1 | - | - | 1.9 | - | 0.9 | - |
| Sarcosine | SAR | 2.0 | - | 2.0 | 0.1 | 2.1 | 0.1 | 100.0 | 18.8 | - | - | - | 0.6 | - | 0.8 | - |
| α-amino-adipic acid | AAD | 2.8 | - | 1.9 | 0.1 | 1.7 | - | 75.0 | 25.0 | - | 0.2 | - | 1.0 | - | 0.1 | - |
| Proline | PRO | 73.4 | 3.2 | 168.1 | 6.3 | 141.8 | 4.7 | 3600.0 | 3037.5 | 11.4 | 2.5 | 0.1 | 13.7 | 0.2 | 0.2 | - |
| Glycine | GLY | 2.9 | 0.1 | 5.9 | 0.2 | 4.4 | 0.1 | 575.0 | 168.8 | 1.7 | 9.7 | 1.3 | 2.6 | 0.5 | 0.4 | 0.2 |
| Alanine | ALA | 19.3 | 0.2 | 31.9 | 0.6 | 28.7 | 0.3 | 2025.0 | 725.0 | 1.3 | 8.4 | 0.8 | 3.4 | 0.2 | 1.8 | 0.1 |
| Citrulline | CIT | - | - | - | - | 0.4 | - | - | - | - | 1.2 | 0.1 | - | - | - | - |
| α-amino-n-butyric acid | AABA | 0.4 | - | 0.4 | - | 0.5 | - | 25.0 | 31.3 | 0.1 | 0.6 | 0.2 | 0.5 | 0.1 | 0.1 | - |
| Valine | VAL | 3.6 | 0.1 | 5.9 | 0.3 | 8.5 | 0.2 | 650.0 | 206.3 | 0.7 | 2.6 | 0.3 | 0.8 | 0.1 | 0.2 | - |
| Methionine | MET | 0.7 | - | 1.0 | - | 1.7 | - | 75.0 | 106.3 | 0.1 | - | - | - | - | - | - |
| Cystine | CYS | 0.2 | - | 0.1 | - | 0.3 | - | 100.0 | 18.8 | 1.2 | - | - | - | - | - | - |
| Isoleucine | L-CYS2 | 1.9 | - | 2.7 | 0.1 | 4.1 | 0.1 | 275.0 | 56.3 | - | 1.4 | - | 0.2 | - | 0.1 | - |
| Leucine | LEU | 4.0 | 0.1 | 5.0 | 0.2 | 7.2 | 0.1 | 250.0 | 81.3 | - | 1.6 | 0.1 | 0.5 | - | 0.1 | - |
| Tyrosine | TYR | 4.1 | 0.1 | 6.3 | 0.1 | 5.9 | 0.1 | 1575.0 | 268.8 | - | 3.3 | 0.5 | 1.4 | 0.3 | 0.3 | 0.1 |
| Cystathionine | CYSTA | - | - | - | - | - | - | 25.0 | 50.0 | - | - | - | - | - | - | - |
| Phenylalanine | PHE | 2.1 | 0.1 | 3.6 | 0.1 | 5.3 | 0.1 | 300.0 | 68.8 | 0.5 | 1.5 | 0.2 | 0.6 | - | 0.1 | - |
| β-alanine | β-ALA | 1.5 | - | 1.4 | - | 2.6 | - | 75.0 | 18.8 | - | - | - | 1.4 | - | 0.2 | - |
| β-amino-isobutyric acid | BAIBA | 0.1 | - | 4.4 | - | - | - | - | 18.8 | - | - | - | - | - | - | - |
| γ-amino-butyric acid | GABA | 27.9 | 0.4 | 48.6 | 1.0 | 51.9 | 0.6 | 100.0 | 37.5 | 9.5 | 1.2 | 0.1 | 4.1 | 0.3 | 0.8 | 0.1 |
| Homocysteine | HCY | - | - | - | - | - | - | 75.0 | 50.0 | - | - | - | - | - | - | - |
| Ethanolamine | ETA | 1.9 | 0.1 | 2.5 | 0.2 | 3.4 | - | - | - | - | - | - | - | - | - | - |
| Tryptophan | TRP | 3.4 | - | 6.3 | - | 1.8 | - | 950.0 | - | - | - | - | - | - | - | - |
| Hydroxylysine | HYL | 0.1 | - | 0.1 | - | 0.3 | - | - | - | - | - | - | - | - | - | - |
| Ornithine | ORN | 0.2 | - | 0.4 | - | 0.5 | - | 250.0 | 81.3 | 0.3 | 7.8 | 0.8 | 1.5 | 0.1 | 0.1 | 0.1 |
| Lysine | LYS | 3.5 | 0.1 | 4.7 | 0.2 | 8.6 | 0.2 | 500.0 | 131.3 | 0.4 | 2.3 | 0.2 | 0.9 | 0.1 | 0.3 | - |
| 1-methyl-histidine | 1-MHIS | 1.3 | - | 1.3 | - | 2.6 | - | - | - | - | - | - | - | - | - | - |
| Histidine | HIS | 1.5 | 0.1 | 2.6 | 0.1 | 3.5 | 0.1 | 950.0 | 418.8 | 0.4 | 4.2 | 0.4 | 1.0 | 0.1 | 1.5 | 0.1 |
| 3-methyl-histidine | 3-MHIS | 0.1 | - | 0.1 | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| Carnosine | CAR | - | - | - | 0.4 | - | 0.1 | - | - | - | - | - | - | - | - | - |
| Arginine | ARG | 4.8 | 0.6 | 8.2 | 0.9 | 1- | 0.2 | 1675.0 | 800.0 | 0.4 | 3.7 | 0.4 | 0.9 | 0.1 | 0.2 | - |
| Total FAA | 223 | 11 | 489 | 19 | 403 | 10 | 3843 | 3711 | 54 | 121 | 16 | 85 | 9 | 17 | 3 | |
| Proteinogenic AA (PAA) | 180 | 9 | 417 | 15 | 328 | 8 | 3631 | 3518 | 43 | 90 | 9 | 61 | 3 | 13 | 1 | |
| 6Ratio PAA: FAA (%) | 80.8 | 83.9 | 85.4 | 80.4 | 81.5 | 79.5 | 94.5 | 94.8 | 79.3 | 74.3 | 52.3 | 71.6 | 33.9 | 77.3 | 39.5 | |
| % Change with age | 3.1 | -5.0 | -2.0 | 0.3 | NC5 | -22.0 | -37.7 | -37.8 | ||||||||
1 Mean concentration based on 3 replications per sample.
2 For Rutaceae host plants, phloem sap of young (Y) and mature (M) flush shoots were tested.
3 For D. citri a sample whole samples of 40 nymphs (Ny) and 40 adults (Ad) were pooled and tested as a replicate.
4 Phloem sap was collected from two whole periwinkle plants cut at the base per sample.
5 NC = not calculated.
6 Ratio of Proteinogenic AA to total FAA.
Several proteinogenic and non-proteinogenic FAAs were detected in whole D. citri nymphs and adults and in phloem sap extracts of CLas permissive and non-permissive plants (Table 2). All CLas -permissive plant species (Table 1) and D. citri life stages (nymph and adult) had greater proportions of proteinogenic FAAs (≈80–95%) compared to CLas -tolerant/resistant plants (≈34–77%) (Table 2). The total AA pool of the three Citrus species was dominated (79.5–85.4%) by proteinogenic FAAs regardless of flush shoot maturity status (young vs. old) (Table 2). Adult and immature D. citri samples contained comparably higher numbers of proteinogenic FAAs that represented most (≈95%) of their entire AA pools. Similarly, the AA pool of the CLas-permissive periwinkle was abundant in proteinogenic FAA (79.3%). Although 72–77% of total FAAs in young shoots of non-Citrus rutaceous plants (curry leaf, orange jasmine and white sapote) were proteinogenic, non-proteinogenic FAAs were the most abundant in phloem sap of mature shoots of these plants with the exception of curry leaf (Table 2). While the proportion of proteinogenic FAAs did not vary with flush shoot growth stage in Citrus spp. and between D. citri developmental stages, there was a dramatic decrease in the relative concentrations of this class of FAAs as flush shoots matured in CLas-tolerant/resistant non-Citrus rutaceous plants (Table 2).
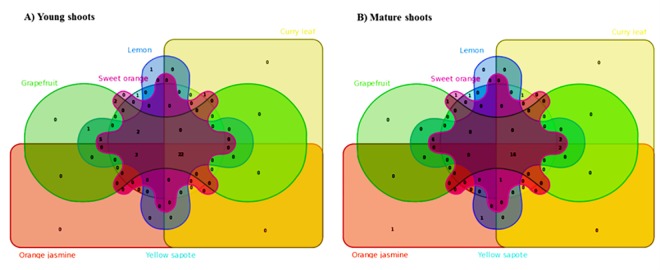
A Venn diagram was used to compare the FAA profiles between the different hosts (Fig 1). Eighteen (18) FAAs (13 proteinogenic: alanine, arginine, asparagine, aspartate, glutamate, glycine, histidine, lysine, phenylalanine, proline, serine, threonine and valine; and five non-proteinogenic: α- amino butyric acid [AAAB], γ-amino butyric acid [GABA], ornithine, phosphoethanolamine and phosphoserine) were common to D. citri (adults and nymphs), periwinkle, and young shoots of all Rutaceae (S1 Table). Phloem sap of young shoots of citrus and periwinkle plants contained cystine that was not detected in sap of young shoots of curry leaf, orange jasmine and white sapote. In addition, tryptophan, hydroxylysine, taurine, carnosine and 1-methyl-histidine were present in phloem sap of young citrus shoots, but absent from sap of young shoots of non-citrus Rutaceae (S1 Table). No FAA was found exclusively in young shoot phloem sap of CLas-tolerant/resistant Rutaceae. With mature shoots, there were 15 FAAs shared by all experimental hosts (Fig 1, S2 Table). Methionine and glutamine were present in phloem sap of mature shoots of citrus, periwinkle, and in whole D. citri (nymphs and adults) but absent in phloem sap of mature shoots of CLas-tolerant/resistant plants. A comparative analysis of mature shoots of rutaceous plants showed that phloem sap of CLas-permissive citrus hosts contained five FAA (glutamine, methionine, carnosine, α-amino-adipic acid and ethanolamine) that were not detected in non-citrus rutaceous plants (Fig 1). With the exception of orange jasmine, citrulline was present only in mature shoots of CLas non-permissive plants (Fig 1).
Fig 1. Venn diagram comparing shared and unique free amino acids present in phloem sap of non-permissive (curry leaf, orange jasmine and white sapote), and permissive (grapefruit, lemon and sweet orange) hosts of Candidatus Liberibacter asiaticus (CLas).
A = young shoots and B = mature shoots.
Concentrations of each AA expressed in micrograms per gram of host tissue (μg/g) were subjected to multivariate analysis. Although CLas-permissive hosts and non-permissive plants shared several FAAs in common, the relative concentrations of individual FAAs varied with the host type, and growth/developmental stage. In general, proline, GABA, aspartate, glutamate, asparagine and glutamine were the most abundant FAAs in all CLas-permissive plants (Fig 2). The six FAAs collectively represented 61 to 71% of the total FAA pool of citrus flush shoot phloem sap and showed no significant variation as the shoots matured. These six FAAs were also dominant in D. citri nymphs (45%), adults (61%) and periwinkle (75%). In contrast, all six FAAs constituted only 9–42% of phloem sap of CLas non-permissive Rutaceae plants (Fig 2). Interestingly, a 2 to 3-fold decrease in total concentrations of the six most abundant FAAs present in permissive hosts was observed in phloem sap of CLas-tolerant/resistant plants with flush shoot maturity (young shoot = 20–42% vs. mature shoot = 9–16%) whereas no such changes occurred between young and mature shoots of CLas-permissive citrus plants. Notably, though abundant in D. citri, glutamine was absent in phloem sap of mature shoots from CLas non-permissive plants and in young shoots of curry leaf. The phloem sap AA pool of CLas tolerant/resistant plants was dominated by glycine, serine, threonine phosphoserine, phosphoethanolamine and ornithine with their total concentrations ranging from 31% in young flush shoots of white sapote, to 73% in mature shoots of orange jasmine (Fig 2). In contrast, these six FAAs abundant in CLas tolerant/resistant plants constituted only 10–21% of total FAA content of CLas-permissive hosts (Fig 2).
Fig 2. Relative concentrations of free amino acids detected in phloem sap of young and mature Rutaceae flush shoots, periwinkle plants, and whole Diaphorina citri nymphs and adults populations.
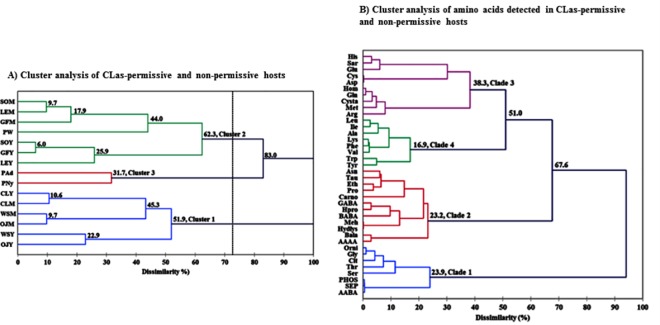
The agglomerative hierarchical cluster (ACH) analysis was used to group the different hosts into three clusters based on their FAA profiles (Fig 3). Cluster 1 comprised both flush growth stages (young and mature) of all three non-citrus plants (curry leaf, orange jasmine and white sapote), and had ≈52% dissimilarity in their amino composition. All CLas-permissive plants (Citrus spp. and periwinkle) segregated into cluster 2 (Fig 3). Within this cluster, phloem sap FAA profiles of mature shoots of Citrus spp. were more similar to that of periwinkle while young shoot phloem sap FAA profiles of the three Citrus species were more similar (only ≈26% dissimilarity). The two life stages of D. citri (nymphs and adults) segregated into cluster 3 with ≈32% dissimilarity between their FAA profiles. Hence, clusters 1, 2 and 3 were designated as non-citrus, citrus and psyllid clusters, respectively. FAA-based ACH analysis resulted in the identification of four distinct clades. Clade 1 contained FAAs that were dominant in CLas-tolerant/resistant plants (e.g. AAAB, phosphoserine, phosphoethanolamine, serine, threonine, citrulline, glycine and ornithine), while Clades 2 and 3 comprised most FAAs present in relatively higher concentrations in CLas-permissive hosts (e.g. arginine, proline, GABA, aspartate, asparagine, glutamate and glutamine and the sulfur-containing FAA methionine, cystine and taurine) (Fig 3). Clade 3 specifically grouped together FAAs that were the most abundant in D. citri and periwinkle plants such as arginine, aspartate, glutamate, glutamine, methionine and cysteine, among others (Fig 3). Clade 4 included FAAs that were present in relatively higher concentrations in D. citri nymphs and moderately present in young flush shoots of Rutaceae plants relative to mature shoots, including alanine, lysine, leucine, isoleucine, phenylalanine, valine, tryptophan and tyrosine.
Fig 3.
Agglomerative hierarchical cluster (ACH); A. Dendrogram depicting clustering patterns of CLas-permissive and non-permissive hosts of Candidatus Liberibacter asiaticus (CLas) into similar groups based on their relative amino acid profiles. Young (Y) and mature (M) flush shoots of were tested for each Rutaceae plant species. SO, sweet orange; GF, grapefruit; LE, lemon; PW, periwinkle plant; CL, curry leaf; OJ, orange jasmine; WS, white sapote; PAd, psyllid adults; PNy, psyllid nymphs. B. Dendrogram depicting clades formed by various free amino acids based on their relative concentrations in the various hosts tested.
A heat map describing the association between FAA content of the different hosts was generated. To enhance readability of the heat map, FAAs with low variability (i.e. interquartile range less than 0.05) were removed from the analysis. The heat map grouped the different hosts into two clusters that strongly correlated with their CLas permissivities (Fig 4). With the exception of young shoot phloem sap of orange jasmine, all CLas-permissive hosts had higher proline concentrations relative to non-permissive plants. Similarly, CLas permissive hosts tended to have higher concentrations of arginine, glutamine and GABA. In contrast, CLas non-permissive plants had higher concentrations of phosphoserine, phosphoethanolamine, glycine, and ornithine relative to CLas-permissive hosts (Fig 4). The ratios of individual AAs were calculated to evaluate the variation in FAA between the two clusters of CLas permissive and non-permissive hosts (Fig 5). Notably, α-amino-n-butyric acid, citrulline, glycine, ornithine, phosphoserine and phosphoethanolamine were present in significantly higher concentrations in CLas non-permissive hosts relative to permissive ones. In contrast, CLas-permissive hosts contained 3 to 16-fold more arginine, asparagine, aspartic acid, ethanolamine, GABA, glutamine, hydroxyproline, proline and the sulfur containing amino acid methionine.
Fig 4. Cluster heatmap describing the relative concentrations of free amino acids in phloem sap of permissive hosts of Candidatus Liberibacter asiaticus, non-permissive plants and whole Diaphorina citri nymphs and adults (OJY = young orange jasmine, OJM = mature orange jasmine, CLY = young curry leaf, CLM = mature curry leaf, WSY = young white sapote, WSM = mature white sapote, GFY = young grapefruit, GFM = mature grapefruit, SOY = young sweet orange, SOM = mature sweet orange, LEY = young lemon, LEM = mature lemon, PW = periwinkle plant, ACPAd = D. citri adults, and ACPNy = D. citri nymphs).
Fig 5. Free amino acid profiles of permissive hosts (P) of Candidatus Liberibacter asiaticus (CLas) and non-permissive (NP) plants.
Permissive hosts comprise phloem sap of young and mature flush shoots of three citrus species, periwinkle plants and whole Diaphorina citri nymphs and adults, while non-permissive host comprise young and mature shoots of curry leaf, orange jasmine and white sapote. Results are based on mean values obtained for growth stages of each plant species (three replicates) and are shown as the ratios of accumulation between the two host categories. Asterisk indicates significant differences according to t-test (P<0.05).
A supervised pattern recognition method or partial least squares-discriminant analysis (PLS-DA) was also used to visualize differences in FAA profiles between the various hosts, between CLas-permissive hosts and non-permissive plants, and to identify specific FAAs that are related to CLas permissivity. A clear discrimination was obtained between CLas permissive and non-permissive hosts with the PLS-DA analysis along the first PLS component (Fig 6), indicating effective removal of FAA variation not correlated to the two CLas response categories. The model discriminating the two CLas response categories had good predictability as shown by a high quality index (cumulative Q2 = 0.774). The cumulative R2Y and R2X that correspond to the correlation between the FAAs (parameter X) and CLas permissivity group (parameter Y) with the PLS components were 0.58 and 0.99, respectively. Simialr analysis using phloem sap FAA of tested plants resulted in similar results (Fig 6). Interestingly, CLas -permissive and non-permissive plants separated quite along PLS component 1, while PLS component 2 discriminated young and mature flush shoots of both types of hosts (Fig 6). Hence both the FAA and host data were well summarized by the four components generated with the PLS-DA analysis (S1 Fig). The FAAs with high variable importance in projection scores were regarded as contributing most significantly to CLas host discrimination. In this regard, twelve (12) FAAs had VIP scores of >1 indicating their importance in discriminating CLas -host status (S1 Fig). Based on their correlation values with CLas -host response categories (Table 3, S1 Fig), these 12 FAAs can be classified as positively (arginine, ethanolamine, methionine, proline, and taurine) or negatively (α-amino-n-butyric acid, β-alanine, citrulline, glycine, ornithine, phosphosethanolamine, phosphoserine and threonine) associated with CLas growth and multiplication. The proportion of all FAAs positively correlated CLas susceptibility in CLas-permissive hosts was 41.9% compared to only 8.1% in CLas-non-permissive plants, suggesting that these FAAs may be positively related to CLas growth. In contrast, non-permissive plants had 5.5-fold more FAAs negatively correlated with CLas growth relative to permissive hosts (52% vs 9.5%). Using the standardized coefficients of the model relating FAAs to CLas host susceptibility (S1 Fig), it was observed that only four FAA namely, GABA, ethanolamine, methionine and proline were positively and significantly related to CLas-susceptibility with 95% confidence limits not encompassing 0. Similarly, six FAAs, AABA, glycine, ornithine, phosphoethanolamine, phosphoserine and threonine were the only amino acids significantly related to CLas non-permissivity. CLas-permissive hosts were characterized by significantly higher proline to glycine, proline to AABA and proline to threonine ratios as compared to non-permissive hosts (Table 4). However, using the 95% jackknife confidence interval as implemented in the PLS-VIP method (Blasco et al. 2015), only two FAAs namely proline and glycine had lower boundaries that did not encompass 1, suggesting that these two variables may be the primary discriminating factors between CLas-permissive and non-permissive hosts. Since these two FAAs were negatively correlated (r = -0.82, P< 0.001, Table 3), the derived proline to glycine ratios varied significantly with CLas-host status with lower ratios in CLas-non-permissive relative to CLas-permissive hosts (Table 4).
Fig 6. Partial least squares-discriminant analysis (PLS-DA).
Correlations between permissive and non-permissive hosts of Candidatus Liberibacter asiaticus (CLas) and their free amino acid content as explanatory variables (X). The ellipse represents the Hotelling T2 with 95% confidence interval (R2Xcum = 0.58, R2Ycum = 0.99, Q2cum = 0.79 for all hosts tested (A); R2Xcum = 0.64, R2Ycum = 1, Q2cum = 0.89 for CLas-permissive and non-permissive plants only (B). Q2cum = cumulative fraction of the total variation of X’s that can be predicted by the extracted components; R2Xcum and R2Ycum represent the fraction of the sum of squares of all X’s and Y’s explained by the current components, respectively. P = CLas-permissive and NP = CLas-non-permissive hosts defined as: OJY = young orange jasmine, OJM = mature orange jasmine, CLY = young curry leaf, CLM = mature curry leaf, WSY = young white sapote, WSM = mature white sapote, GFY = young grapefruit, GFM = mature grapefruit, SOY = young sweet orange, SOM = mature sweet orange, LEY = young lemon, LEM = mature lemon, PW = periwinkle plant, ACPAd = D. citri adults, and ACPNy = D. citri nymphs. The amino acids are defined as: AAAA, α-amino adipic acid; AABA, α-aminobutyric acid; Ala, alanine; Arg, arginine; Asn, asparagine; Asp, aspartate; BABA, β-aminobutyric acid; BALA, β-alanine; Carno, carnosine, Cit, citrulline; Cys, cystine; Cysta, cystathionine; Eth, ethanolamine; GABA, γ-aminobutyric acid; Gln, glutamine; Glu, glutamate; Gly, glycine; His, histidine; Hom, homocysteine; Hpro, hydroxyproline; Hydlys, hydroxyl-lysine; Ile, isoleucine; Leu, leucine; Lys, lysine; Meh, 1-methyl histidine; Met, methionine; Orn, ornithine; Phe, phenylalanine; PHOS, phosphoethanolamine; Orn, ornithine, Pro, proline; Sar, sarcosine; SEP, phosphoserine; Ser, serine; Tau, taurine; Thr, threonine; Trp, Tryptophan; Tyr, tyrosine; Val, valine.
Table 3. Correlation between mean amino acid concentrations and Candidatus Liberibacter asiaticus (CLas) permissivity status of different plants and Diaphorina citri (ACP) adults and nymphs.
| Variables | SEP | TAU | PHOS | ASP | HPRO | THR | SER | ASN | GLU | GLN | SAR | AAAA | PRO | GLY | ALA | CIT | AABA | VAL | MET | CYS | ILE | LEU | TYR | CYSTA | PHE | BALA | BABA | GABA | HMCYS | ETH | TRP | HYDLYS | ORN | LYS | MHIS1 | HIS | CARNO | ARG | NP | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEP | 1.00 | |||||||||||||||||||||||||||||||||||||||
| TAU | -0.48 | 1.00 | ||||||||||||||||||||||||||||||||||||||
| PHOS | 0.97 | -0.45 | 1.00 | |||||||||||||||||||||||||||||||||||||
| ASP | -0.23 | -0.22 | -0.26 | 1.00 | ||||||||||||||||||||||||||||||||||||
| HPRO | -0.47 | 0.38 | -0.44 | 0.32 | 1.00 | |||||||||||||||||||||||||||||||||||
| THR | 0.19 | -0.37 | 0.22 | -0.16 | -0.47 | 1.00 | ||||||||||||||||||||||||||||||||||
| SER | -0.23 | -0.32 | -0.20 | 0.17 | 0.19 | 0.38 | 1.00 | |||||||||||||||||||||||||||||||||
| ASN | -0.44 | 0.56 | -0.37 | -0.21 | 0.29 | -0.39 | -0.05 | 1.00 | ||||||||||||||||||||||||||||||||
| GLU | 0.23 | -0.49 | 0.11 | 0.21 | -0.43 | 0.53 | -0.04 | -0.57 | 1.00 | |||||||||||||||||||||||||||||||
| GLN | -0.34 | 0.26 | -0.40 | 0.01 | -0.21 | 0.14 | -0.52 | -0.21 | 0.37 | 1.00 | ||||||||||||||||||||||||||||||
| SAR | -0.19 | -0.07 | -0.19 | -0.07 | -0.11 | 0.23 | 0.21 | 0.01 | 0.50 | 0.03 | 1.00 | |||||||||||||||||||||||||||||
| AAAA | -0.45 | 0.41 | -0.38 | -0.27 | 0.12 | 0.04 | 0.22 | 0.35 | -0.23 | -0.06 | 0.20 | 1.00 | ||||||||||||||||||||||||||||
| PRO | -0.66 | 0.53 | -0.66 | -0.01 | 0.45 | -0.69 | -0.08 | 0.50 | -0.59 | 0.14 | -0.16 | 0.36 | 1.00 | |||||||||||||||||||||||||||
| GLY | 0.67 | -0.53 | 0.69 | -0.01 | -0.33 | 0.61 | 0.32 | -0.47 | 0.19 | -0.37 | -0.24 | -0.41 | -0.82 | 1.00 | ||||||||||||||||||||||||||
| ALA | -0.42 | 0.16 | -0.42 | -0.21 | 0.17 | 0.35 | 0.32 | -0.09 | 0.26 | 0.21 | 0.59 | 0.32 | -0.13 | -0.08 | 1.00 | |||||||||||||||||||||||||
| CIT | 0.55 | -0.29 | 0.46 | -0.16 | -0.23 | 0.44 | 0.31 | -0.34 | 0.23 | -0.30 | -0.25 | -0.34 | -0.55 | 0.75 | -0.01 | 1.00 | ||||||||||||||||||||||||
| AABA | 0.96 | -0.45 | 0.96 | -0.25 | -0.41 | 0.36 | -0.12 | -0.51 | 0.20 | -0.33 | -0.20 | -0.36 | -0.73 | 0.78 | -0.29 | 0.58 | 1.00 | |||||||||||||||||||||||
| VAL | -0.36 | -0.08 | -0.36 | -0.10 | -0.01 | 0.16 | 0.30 | -0.26 | -0.11 | 0.25 | -0.12 | -0.05 | 0.10 | 0.10 | 0.49 | 0.08 | -0.25 | 1.00 | ||||||||||||||||||||||
| MET | -0.57 | 0.55 | -0.62 | 0.05 | 0.20 | -0.19 | -0.38 | -0.08 | 0.05 | 0.84 | 0.07 | 0.08 | 0.49 | -0.61 | 0.31 | -0.42 | -0.56 | 0.29 | 1.00 | |||||||||||||||||||||
| CYS | -0.24 | -0.17 | -0.27 | 0.98 | 0.39 | -0.23 | 0.12 | -0.24 | 0.14 | 0.03 | -0.17 | -0.24 | 0.04 | -0.03 | -0.20 | -0.17 | -0.25 | -0.03 | 0.10 | 1.00 | ||||||||||||||||||||
| ILE | -0.56 | 0.18 | -0.55 | -0.26 | 0.18 | 0.10 | 0.36 | 0.05 | -0.09 | 0.19 | 0.14 | 0.31 | 0.22 | -0.18 | 0.74 | 0.03 | -0.50 | 0.74 | 0.34 | -0.20 | 1.00 | |||||||||||||||||||
| LEU | -0.59 | 0.50 | -0.53 | -0.39 | 0.33 | -0.04 | 0.37 | 0.30 | -0.51 | 0.01 | 0.00 | 0.51 | 0.43 | -0.24 | 0.60 | -0.06 | -0.47 | 0.64 | 0.30 | -0.32 | 0.81 | 1.00 | ||||||||||||||||||
| TYR | 0.33 | -0.27 | 0.34 | -0.30 | -0.35 | 0.30 | -0.26 | -0.39 | 0.24 | 0.20 | -0.14 | -0.15 | -0.52 | 0.41 | 0.32 | 0.23 | 0.38 | 0.47 | -0.08 | -0.22 | 0.31 | 0.06 | 1.00 | |||||||||||||||||
| CYSTA | 0.14 | 0.13 | 0.16 | -0.09 | -0.26 | 0.08 | -0.69 | -0.32 | 0.19 | 0.73 | -0.20 | -0.24 | -0.12 | -0.06 | -0.09 | -0.22 | 0.13 | -0.02 | 0.54 | -0.06 | -0.08 | -0.24 | 0.34 | 1.00 | ||||||||||||||||
| PHE | -0.36 | -0.04 | -0.30 | 0.00 | 0.13 | 0.05 | 0.51 | -0.02 | -0.38 | -0.16 | -0.08 | 0.00 | 0.15 | 0.15 | 0.34 | 0.04 | -0.25 | 0.85 | 0.01 | 0.04 | 0.58 | 0.64 | 0.21 | -0.37 | 1.00 | |||||||||||||||
| BALA | -0.29 | 0.06 | -0.23 | -0.16 | 0.06 | 0.37 | 0.27 | 0.05 | 0.21 | 0.00 | 0.48 | 0.78 | 0.00 | -0.24 | 0.43 | -0.29 | -0.18 | -0.11 | 0.01 | -0.17 | 0.21 | 0.23 | -0.05 | -0.19 | -0.08 | 1.00 | ||||||||||||||
| BABA | -0.29 | 0.28 | -0.26 | -0.14 | 0.65 | -0.23 | -0.07 | 0.41 | -0.29 | 0.01 | -0.07 | 0.05 | 0.34 | -0.27 | 0.15 | -0.17 | -0.25 | -0.12 | 0.15 | -0.10 | 0.09 | 0.15 | -0.15 | -0.01 | -0.16 | -0.03 | 1.00 | |||||||||||||
| GABA | -0.46 | 0.28 | -0.44 | 0.55 | 0.81 | -0.56 | 0.30 | 0.18 | -0.37 | -0.31 | 0.01 | 0.27 | 0.51 | -0.42 | 0.01 | -0.36 | -0.46 | -0.13 | 0.14 | 0.59 | 0.05 | 0.21 | -0.57 | -0.37 | 0.10 | 0.16 | 0.21 | 1.00 | ||||||||||||
| HMCYS | -0.24 | 0.16 | -0.30 | -0.01 | -0.22 | 0.15 | -0.54 | -0.28 | 0.34 | 0.94 | -0.13 | -0.11 | 0.01 | -0.21 | 0.24 | -0.19 | -0.22 | 0.40 | 0.71 | 0.04 | 0.27 | 0.03 | 0.49 | 0.72 | -0.05 | -0.08 | 0.01 | -0.40 | 1.00 | |||||||||||
| ETH | -0.45 | 0.63 | -0.43 | -0.26 | 0.34 | -0.64 | 0.00 | 0.70 | -0.64 | -0.19 | 0.07 | 0.39 | 0.73 | -0.64 | 0.03 | -0.35 | -0.52 | -0.03 | 0.17 | -0.25 | 0.20 | 0.56 | -0.49 | -0.36 | 0.15 | 0.01 | 0.15 | 0.43 | -0.32 | 1.00 | ||||||||||
| TRP | -0.34 | 0.11 | -0.36 | -0.08 | 0.18 | -0.19 | -0.17 | 0.07 | -0.04 | 0.22 | -0.04 | 0.23 | 0.13 | -0.23 | 0.48 | -0.21 | -0.33 | 0.53 | 0.20 | 0.04 | 0.57 | 0.40 | 0.65 | 0.01 | 0.34 | 0.09 | 0.20 | -0.01 | 0.46 | 0.04 | 1.00 | |||||||||
| HYDLYS | -0.38 | 0.62 | -0.34 | -0.20 | 0.58 | -0.35 | 0.16 | 0.18 | -0.52 | -0.14 | 0.01 | 0.48 | 0.44 | -0.34 | 0.41 | -0.15 | -0.29 | 0.24 | 0.32 | -0.11 | 0.49 | 0.76 | -0.13 | -0.20 | 0.35 | 0.28 | 0.10 | 0.59 | -0.17 | 0.62 | 0.22 | 1.00 | ||||||||
| ORN | 0.39 | -0.42 | 0.42 | -0.11 | -0.34 | 0.74 | 0.49 | -0.34 | 0.13 | -0.25 | -0.23 | -0.26 | -0.66 | 0.91 | 0.08 | 0.77 | 0.54 | 0.32 | -0.48 | -0.14 | 0.07 | 0.03 | 0.35 | -0.14 | 0.34 | -0.16 | -0.22 | -0.48 | -0.11 | -0.53 | -0.18 | -0.28 | 1.00 | |||||||
| LYS | -0.34 | 0.06 | -0.32 | -0.26 | 0.00 | 0.21 | 0.40 | -0.12 | -0.10 | 0.04 | 0.16 | 0.16 | 0.04 | 0.06 | 0.65 | 0.12 | -0.25 | 0.85 | 0.18 | -0.22 | 0.84 | 0.74 | 0.40 | -0.21 | 0.84 | 0.17 | -0.20 | -0.06 | 0.15 | 0.10 | 0.47 | 0.46 | 0.27 | 1.00 | ||||||
| MHIS1 | -0.41 | 0.64 | -0.36 | -0.21 | 0.65 | -0.37 | 0.16 | 0.24 | -0.54 | -0.16 | 0.01 | 0.49 | 0.47 | -0.36 | 0.42 | -0.16 | -0.32 | 0.21 | 0.31 | -0.12 | 0.49 | 0.76 | -0.14 | -0.21 | 0.32 | 0.28 | 0.23 | 0.61 | -0.18 | 0.63 | 0.25 | 0.99 | -0.30 | 0.42 | 1.00 | |||||
| HIS | 0.06 | -0.39 | 0.04 | -0.04 | -0.32 | 0.51 | 0.30 | -0.21 | 0.64 | -0.04 | 0.81 | -0.12 | -0.57 | 0.24 | 0.61 | 0.14 | 0.07 | 0.15 | -0.19 | -0.13 | 0.24 | -0.07 | 0.29 | -0.21 | 0.15 | 0.25 | -0.23 | -0.31 | -0.03 | -0.30 | 0.12 | -0.23 | 0.25 | 0.37 | -0.25 | 1.00 | ||||
| CARNO | -0.06 | -0.23 | -0.11 | -0.07 | -0.19 | -0.30 | 0.06 | 0.17 | -0.14 | -0.04 | -0.05 | -0.06 | 0.35 | -0.27 | -0.26 | -0.16 | -0.17 | 0.04 | -0.09 | -0.12 | -0.07 | 0.02 | -0.29 | -0.14 | -0.04 | -0.22 | -0.10 | -0.01 | -0.13 | 0.44 | -0.15 | -0.15 | -0.21 | -0.21 | -0.16 | -0.20 | 1.00 | |||
| ARG | -0.32 | 0.31 | -0.36 | -0.17 | -0.31 | -0.03 | -0.47 | 0.22 | 0.01 | 0.68 | -0.21 | -0.09 | 0.17 | -0.26 | 0.09 | -0.13 | -0.35 | 0.41 | 0.46 | -0.15 | 0.28 | 0.23 | 0.35 | 0.38 | 0.14 | -0.27 | -0.10 | -0.46 | 0.74 | 0.14 | 0.43 | -0.13 | -0.07 | 0.20 | -0.14 | -0.09 | 0.21 | 1.00 | ||
| NP | 0.71 | -0.56 | 0.75 | -0.22 | -0.46 | 0.73 | 0.27 | -0.41 | 0.43 | -0.37 | 0.22 | -0.14 | -0.87 | 0.79 | 0.04 | 0.55 | 0.79 | -0.23 | -0.64 | -0.29 | -0.26 | -0.36 | 0.29 | -0.05 | -0.18 | 0.22 | -0.29 | -0.47 | -0.32 | -0.60 | -0.38 | -0.36 | 0.67 | -0.06 | -0.38 | 0.49 | -0.27 | -0.46 | 1.00 | |
| P | -0.71 | 0.56 | -0.75 | 0.22 | 0.46 | -0.73 | -0.27 | 0.41 | -0.43 | 0.37 | -0.22 | 0.14 | 0.87 | -0.79 | -0.04 | -0.55 | -0.79 | 0.23 | 0.64 | 0.29 | 0.26 | 0.36 | -0.29 | 0.05 | 0.18 | -0.22 | 0.29 | 0.47 | 0.32 | 0.60 | 0.38 | 0.36 | -0.67 | 0.06 | 0.38 | -0.49 | 0.27 | 0.46 | -1.00 | 1.00 |
AAAA, α-amino adipic acid; AABA, α-aminobutyric acid; Ala, alanine; Arg, arginine; Asn, asparagine; Asp, aspartate; BABA, β-aminobutyric acid; BALA, β-alanine; Carno, carnosine, Cit, citrulline; Cys, cystine; Cysta, cystathionine; Eth, ethanolamine; GABA, γ-aminobutyric acid; Gln, glutamine; Glu, glutamate; Gly, glycine; His, histidine; Hom, homocysteine; Hpro, hydroxyproline; Hydlys, hydroxyl-lysine; Ile, isoleucine; Leu, leucine; Lys, lysine; Meh, 1-methyl histidine; Met, methionine; Orn, ornithine; Phe, phenylalanine; PHOS, phosphoethanolamine; Orn, ornithine, Pro, proline; Sar, sarcosine; SEP, phosphoserine; Ser, serine; Tau, taurine; Thr, threonine; Trp, Tryptophan; Tyr, tyrosine; Val, valine; NP, CLas-non permissive; P, CLas permissive
Table 4. Ratio of mean concentrations between Candidatus Liberibacter asiaticus (CLas)-permissive and non–permissive hosts for key amino acids identified as variables of most importance in partial least squares-discriminant analysis (PLS-DA).
| Amino acid comparison | Category of CLas-host | Ratio of amino acid concentrations | Range of Ratio |
|---|---|---|---|
| Proline to Glycine | Permissive | 24.7 | 6.3–45.2 |
| Non-Permissive | 1.1 | 0–5.2 | |
| Proline to AABA | Permissive | 232.4 | 97.2–3 82.1 |
| Non-Permissive | 6.4 | 0–28.3 | |
| Proline to Threonine | Permissive | 18.3 | 2.8–42.1 |
| Non-Permissive | 0.4 | 0–1.4 |
Discussion
In this study, the hypothesis that FAA contents of plant phloem sap and whole insect samples are strongly associated with CLas host status and could play a role in CLas growth and replication was evaluated. The results showed that FAA composition and relative concentrations greatly varied with host species, flush shoot growth stage and vector developmental stage. In all Rutaceae plants, young shoot phloem sap had higher numbers and concentrations of individual FAAs. In contrast, D. citri nymphs and adults contained the same numbers of individual FAAs and equivalent concentration per g of body weight.
Amino acids have been shown to play a key role in signaling between plants and pathogens in compatible interactions [29–36]. Strong variations in the FAA profiles of HLB-affected citrus tissue have also been reported [37–39]. Although changes in plant FAA profiles occur during plant-pathogen interactions, FAAs required for successful colonization must be present in adequate amounts in healthy plants prior to infection for successful pathogen growth. Concurrently, pathogen-inhibiting factors, including FAA composition must be absent or occur below toxic levels for successful host infection, colonization and establishment. An analysis of the CLas genome recently identified the presence of a tricarboxylic (TCA) cycle indicative of the utilization of a range of AAs as energy sources by CLas [14]. However, CLas is auxotrophic for a number of AAs including proline and must rely on its hosts as the primary sources for these AAs [14,40]. Thus, AA resources present in healthy hosts are a critical factor for successful CLas host colonization. In agreement with findings of the present study, healthy citrus varieties tolerant/resistant to HLB have been reported to contain higher levels of citrulline, glycine and ornithine [37,39] while proline, serine and aspartic acid were present in higher concentrations in most susceptible citrus varieties [37]. This has led to the hypothesis that variation in AA profiles between curry leaf, orange jasmine and citrus (‘Valencia’ sweet orange) may explain their differential responses to D. citri development and CLas growth [23].
Twelve FAAs are significantly related to CLas permissivity, of which seven were positively correlated to CLas non-permissive hosts and five were positively associated with CLas-permissive hosts. Notably, all of these amino acids had aspartate, glutamate or serine as precursors. Interestingly, AAs associated with CLas host susceptibility are either sulfur-containing AAs (methionine and taurine) or known to accumulate during stress (arginine and proline) or following injuries and mechanical damage (GABA), and are direct products of glutamate. The relative concentration of these five AAs did not significantly vary with flush shoot growth stage or D. citri developmental stage in CLas permissive hosts. In addition to their basic role as precursors for protein synthesis, many proteinogenic AAs such as arginine and proline have high nitrogen to carbon ratios and thus can serve as major storage and transport forms of organic nitrogen (especially during periods of stress) and subsequently metabolized for protein synthesis and energy production [32,41]. These AAs can potentially also serve as N and energy substrates for many microorganisms such as bacteria within plants [42]. Genomic analysis indicate that CLas is incapable of synthesizing arginine from glutamate [43] and may depend on its host for acquisition of this AA. Ethanolamine is used by many bacteria as a source of carbon and/or nitrogen [34], and is reported to foster the pathogenicity of many bacteria [44]. For instance, ethanolamine appears to be a key signal for initiation of virulence by Escherichia coli [45]. Sulfur (S) is a key constituent of many indispensable cell components and processes. In bacteria, S-containing AAs are the main pathway of S acquisition and play essential roles in their metabolism [46] including communication and regulation of virulence [47]. Interestingly, S-containing AAs were only present in CLas-permissive hosts and were not detected in non-permissive plants in agreement with a recent study [23]. This indicates that S-containing AAs may be either entirely absent or present below detection levels in CLas-non permissive plants. Nonetheless, such differential levels of S-containing AAs in the two CLas response categories evaluated in this study can contribute to determining their suitability for CLas growth.
In contrast to AAs that are positively related to CLas permissivity, the relative proportions of AAs positively correlated with CLas non-permissive plants (glycine, α-amino-butyric acid, phosphoethanolamine, threonine, serine, ornithine and citrulline) increased with flush maturity in all plant species. Serine and glycine are known to accumulate in plants in response to stress factors such as increased photorespiration or overexpression of proteases and peptidases [48,49]. Serine, derived from 3-phosphoglycerate is also a known precursor of glycine and the sulfur-containing AAs cysteine, cystathionine and methionine. HLB-affected citrus trees showed elevated concentrations of glycine, serine and threonine in phloem sap [37] and, of citrulline, glycine, ornithine and serine in leaf tissue [39], indicating possible roles for these AAs in citrus response to CLas infection. Furthermore, some of the AAs that are upregulated in HLB susceptible cultivars following CLas infection (e.g. citrulline and serine) were also naturally present at higher concentrations in the HLB-tolerant US-897 (Citrus reticulata ‘Cleopatra × Poncirus trifoliata) relative to HLB susceptible cultivars [39] corroborating the findings of this study.
Although many AAs were positively correlated either with CLas non-permissive or permissive hosts, only proline and glycine had PLS-VIP scores greater than the cut-off value of 1 using the 95% jackknife confidence interval. Hence, both AAs could be considered to be among the primary discriminating amino acids between CLas permissive and non-permissive hosts. Remarkably, proline and glycine are osmoprotectants produced in plants under osmotic stress conditions [43,50]. Proline, whose main biosynthetic pathway originates from glutamate as the precursor, is a well-known biomarker of water stress in plants since it accumulates to very high levels under drought conditions [51] and other stresses such as high salinity, heavy metal toxicity and high temperatures [52]. Proline is known to induce antioxidant defense gene expression in many organisms [53] and increasing evidence suggests that this AA plays an important role as a substrate for growth and respiration in bacteria. For Helicobacter pylori, proline is the preferred respiratory substrate during colonization of the human stomach [54]. In E. coli, proline increases oxidative stress tolerance [55]. Low-proline environments impair growth and in vivo survival of Staphylococcus aureus [56]. Many insects contained high levels of proline [57] that is a major fuel source for flight [58]. Therefore, entomopathogenic bacteria species are able to sense and exploit proline for expressing their virulence and initiating metabolism, thus identifying host niche [59].
Proline is the dominant FAA in phloem sap of CLas-permissive citrus ([20, 21], Fig 2) and in D. citri nymphs. Proline was also one of the two most abundant AAs found in periwinkle (along with aspartate) and in D. citri adults (along with glutamine) in this study (Fig 2). It is conceivable that high proline contents in permissive hosts may play a key role as an activator of CLas secondary metabolite virulence factors, but also as an energy source to sustain the pathogen as it establishes, multiplies and spreads. In contrast, very low concentrations of proline (<3%) were detected in CLas non-permissive plants (Fig 2) with the exception of young orange jasmine shoots that contained 16% of proline in their phloem sap. This may explain the unsuitability of these plant species for sustainable CLas establishment and growth.
Orange jasmine has been reported to be a possible host of CLas but considerable variability exists in its infection rates, bacterial titer and persistence due to an apparent lack of CLas fitness in the plant phloem [60]. Lower CLas titer levels have also been reported in orange jasmine and orange jasmine-reared psyllids compared to citrus and citrus-reared psyllid, respectively [61]. It is therefore plausible that CLas successfully colonizes phloem sap of young orange jasmine shoots, but does not persist in the phloem as the flush shoots mature due to rapid metabolic shifts resulting in altered AA profiles, notably a drastic reduction in the proportion of proline, as the shoot matures. Genomic analysis indicate that CLas uses proline as a growth factor [40,62]. Based on reports of increased proline concentrations in HLB-affected trees [39,63], and strong correlations between high proline levels in plant tissue and HLB susceptibility in citrus, Cevallos-Cevalloset al. [37] hypothesized that proline abundance facilitates CLas survival and spread in planta. Taken together, these results point to the critical role played by proline in CLas host recognition, successful colonization and growth. Paradoxically, as proline level increased in plants in response to stress [64] and to CLas infection [39], CLas adaption to the proline-rich environment will also favor post-infection survival/multiplication, thus leading to higher bacterial titer in HLB-affected plants. This pathogen-host dynamic demonstrates how biotrophic organisms exploit their hosts for growth, multiplication and survival.
Glycine is the simplest known AA and, in its methylated form as glycine betaine, is another important osmolyte that adjusts osmotic balance in bacteria, animals, and angiosperms as a common response for host protection against environmental stresses such as salt, drought, and extreme temperatures [50,65]. Exogenous applications of glycine have been shown to increase plant tolerance to abiotic stress [50,66]. Although glycine is used as a metabolic product in some bacteria, a high concentration of glycine is known to have toxic effects that inhibit growth in many bacteria [31]. Glycine is known to inhibit the synthesis of a peptidoglycan component of the bacterial cell wall [67]. As the bacterial cell wall is thinner in gram-negative than in gram-positive bacteria, it is thought that the amount of glycine required to suppress gram-negative bacterial proliferation is lower than that required to suppress proliferation of gram-positive bacteria [31]. Due to its low mammalian toxicity, glycine has been used as an antibacterial agent in foods. Hence, as CLas is a gram-negative bacterium, it is likely that high concentration of glycine in phloem sap of plants can interfere with its metabolism. The percentage of glycine in the AA pool of CLas non-permissive plants was 2.3 to 8-fold higher than that in their CLas-susceptible counterparts in agreement with a previous report [37].
As both CLas promoting and inhabiting AAs occur simultaneously in phloem sap, it is very likely that the outcome of host-CLas interaction will depend on ratios of these two types of AAs. The outcome of this study suggests that the proline-to-glycine ratio may be a critical discriminating factor for host permissivity to CLas, with higher and stable values (6 to 45) characterizing permissive hosts and lower values (<0.6) being emblematic of non-permissive hosts. Using this criterion, orange jasmine could be classified as a transient host of CLas with the phloem sap of its young shoots having a proline-to-glycine ratio of 5.2 whereas its mature shoots had a mean ratio of 0.3.
In conclusion, this study indicates the suitability of FAA profiling for CLas host response discrimination. Although the dimension reduction techniques resulted in clear discrimination of hosts into two groups based on their CLas permissivity and gave a clear lead to AAs correlated with either CLas-permissive or non-permissive hosts, identification of their roles in CLas growth and HLB development in hosts will require additional biological studies. As efforts to identify and develop HLB tolerant/resistant cultivars expand, metabolite profiles such as the proline-to-glycine ratios identified in this study could be exploited as biomarkers for rapid screening of parental and progeny citrus genotypes in resistance breeding programs.
Supporting information
(PDF)
(PDF)
A) VIP scores: Amino acids whose relative concentrations are involved in CLas host discrimination ordered by index score of variable importance on the Protection greater than 1 (VIP-1) criterion.
B) Orthogonal projection coefficients for the comparison between permissive and non-permissive hosts of CLas. Negative values represent FAA positively correlated with CLas-permissive hosts whereas negative values correspond to those with higher concentrations in non-permissive plants.
(TIF)
(XLS)
Acknowledgments
The authors would like to thank Robert R. Saldaña, James H. Hearn, Maggie Garcia and Sri Lakshmi Telagamsetty for their excellent technical assistance in maintaining the plants, collecting the phloem sap and submitting the samples for biochemical analyses. Suggestions and comments from three anonymous reviewers improved an earlier version of the manuscript and are greatly appreciated.
Data Availability
All relevant data are summarized in the paper and supporting information.
Funding Statement
The work was supported by a research grant (TCPB-2013-571213) to Mamoudou Setamou from the Texas Citrus Producers Board. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bové JM. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J Plant Pathol. 2006;88: 427–453. [Google Scholar]
- 2.Hodges AW, Spreen TH. Economic Impacts of Citrus Greening -(HLB) in Florida, 2006/07-2010/11. Univ Florida IFAS Ext; Gainsville; 2015; 7–12. [Google Scholar]
- 3.Grafton-Cardwell EE, Stelinski LL, Stansly PA. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu Rev Entomol. 2013;58: 413–32. doi: 10.1146/annurev-ento-120811-153542 [DOI] [PubMed] [Google Scholar]
- 4.Halbert SE, Manjunath KL. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: a literature review and risk assessment in Florida. Florida Entomol. 2004;87: 330–353. doi: 10.1653/0015-4040(2004)087[0330:ACPSPA]2.0.CO;2 [Google Scholar]
- 5.Hall DG, Richardson ML, Ammar ED, Halbert SE. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol Exp Appl. 2013;146: 207–223. doi: 10.1111/eea.12025 [Google Scholar]
- 6.Sétamou M, da Graça JV, Sandoval JL. Suitability of native North American Rutaceae to serve as host plants for the Asian citrus psyllid (Hemiptera: Liviidae). J Appl Entomol. 2016;140 doi: 10.1111/jen.12300 [Google Scholar]
- 7.Ramadugu C, Keremane ML, Halbert SE, Duan Y, Roose M, Stover E, et al. Long term field evaluation reveals HLB resistance in Citrus relatives. Plant Dis. 2016; PDIS-03-16-0271-RE. doi: 10.1094/PDIS-03-16-0271-RE [DOI] [PubMed] [Google Scholar]
- 8.Garnier M, Bove J. Transmission of the organism associated with citrus greening disease from sweet orange to periwinkle by dodder. Phytopathology. 1983;73: 1358–1363. [Google Scholar]
- 9.Inoue H, Ohnishi J, Ito T, Tomimura K, Miyata S, Iwanami T, et al. Enhanced proliferation and efficient transmission of Candidatus Liberibacter asiaticus by adult Diaphorina citri after acquisition feeding in the nymphal stage. Ann Appl Biol. 2009;155: 29–36. doi: 10.1111/j.1744-7348.2009.00317.x [Google Scholar]
- 10.Ammar ED, Ramos JE, Hall DG, Dawson WO, Shatters RG. Acquisition, replication and inoculation of Candidatus Liberibacter asiaticus following various acquisition periods on huanglongbing-infected citrus by nymphs and adults of the asian citrus psyllid. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogenhout S a, Ammar E-D, Whitfield AE, Redinbaugh MG. Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol. 2008;46: 327–359. doi: 10.1146/annurev.phyto.022508.092135 [DOI] [PubMed] [Google Scholar]
- 12.Killiny N. Generous hosts: What makes Madagascar periwinkle (Catharanthus roseus) the perfect experimental host plant for fastidious bacteria? Plant Physiol Biochem. Elsevier Masson SAS; 2016;109: 28–35. doi: 10.1016/j.plaphy.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 13.Fisher T, Vyas M, He R, Nelson W, Cicero J, Willer M, et al. Comparison of Potato and Asian Citrus Psyllid Adult and Nymph Transcriptomes Identified Vector Transcripts with Potential Involvement in Circulative, Propagative Liberibacter Transmission. Pathogens. 2014;3: 875–907. doi: 10.3390/pathogens3040875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan Y, Zhou L, Hall DG, Li W, Doddapaneni H, Lin H, et al. Complete genome sequence of citrus huanglongbing bacterium, “Candidatus Liberibacter asiaticus” obtained through metagenomics. Mol Plant Microbe Interact. 2009;22: 1011–1020. doi: 10.1094/MPMI-22-8-1011 [DOI] [PubMed] [Google Scholar]
- 15.Vyas M, Fisher TW, He R, Nelson W, Yin G, Cicero JM, et al. Asian citrus psyllid expression profiles suggest candidatus liberibacter asiaticus-mediated alteration of adult nutrition and metabolism, and of nymphal development and immunity. PLoS One. 2015;10: 1–31. doi: 10.1371/journal.pone.0130328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan QW, Melathopoulos AP, Pernal SF, Foster LJ. The innate immune and systemic response in honey bees to a bacterial pathogen, Paenibacillus larvae. BMC Genomics. 2009;10: 387 doi: 10.1186/1471-2164-10-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sétamou M, Alabi OJ, Kunta M, Jifon JL, Da Graça JV. Enhanced Acquisition Rates of “Candidatus Liberibacter asiaticus” by the Asian Citrus Psyllid (Hemiptera: Liviidae) in the Presence of Vegetative Flush Growth in Citrus. J Econ Entomol. 2016;109 doi: 10.1093/jee/tow171 [DOI] [PubMed] [Google Scholar]
- 18.Lee JA, Halbert SE, Dawson WO, Robertson CJ, Keesling JE, Singer BH. Asymptomatic spread of huanglongbing and implications for disease control. Proc Natl Acad Sci. 2015;112: 7605–7610. doi: 10.1073/pnas.1508253112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall DG, Albrecht U, Bowman KD. Transmission rates of “Ca. Liberibacter asiaticus” by Asian citrus psyllid are enhanced by the presence and developmental stage of citrus flush. 2016;109: 558–563. doi: 10.1093/jee/tow009 [DOI] [PubMed] [Google Scholar]
- 20.Sétamou M, Simpson CR, Alabi OJ, Nelson SD, Telagamsetty S, Jifon JL. Quality matters: Influences of citrus flush physicochemical characteristics on population dynamics of the Asian citrus psyllid (Hemiptera: Liviidae). PLoS One. 2016;11 doi: 10.1371/journal.pone.0168997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hijaz F, Killiny N. Collection and chemical composition of phloem sap from Citrus sinensis L. Osbeck (sweet orange). PLoS One. 2014;9: 1–11. doi: 10.1371/journal.pone.0101830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Killiny N, Hijaz F, El-Shesheny I, Alfaress S, Jones SE, Rogers ME. Metabolomic analyses of the haemolymph of the Asian citrus psyllid Diaphorina citri, the vector of huanglongbing. Physiol Entomol. 2016; 134–145. doi: 10.1111/phen.12183 [Google Scholar]
- 23.Killiny N. Metabolomic comparative analysis of the phloem sap of curry leaf tree (Bergera koenegii), orange jasmine (Murraya paniculata), and Valencia sweet orange (Citrus sinensis) supports their differential responses to Huanglongbing. Plant Signal Behav. Taylor & Francis; 2016;11: 00–00. doi: 10.1080/15592324.2016.1249080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Graça J V, Kunta M, Sétamou M, Rascoe J, Li W, Nakhla MK, et al. Huanglongbing in Texas: Report on the first detections in commercial citrus. J Citrus Pathol. 2015; 1–6. [Google Scholar]
- 25.Li W, Hartung JS, Levy L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J Microbiol Methods. 2006;66: 104–115. doi: 10.1016/j.mimet.2005.10.018 [DOI] [PubMed] [Google Scholar]
- 26.King RW, Zeevaart J a. Enhancement of Phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974;53: 96–103. doi: 10.1104/pp.53.1.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miuigan GW, Cooper MC. A study of standardizaction of variables in cluster analysis. J Classif. 1988;204: 181–204. doi: 10.1007/BF01897163 [Google Scholar]
- 28.Mourier PAJ, Agut C, Souaifi-Amara H, Herman F, Viskov C. Analytical and statistical comparability of generic enoxaparins from the US market with the originator product. J Pharm Biomed Anal. Elsevier B.V.; 2015;115: 431–442. doi: 10.1016/j.jpba.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 29.Tonon T, Lonvaud-Funel A. Metabolism of arginine and its positive effect on growth and revival of Oenococcus oeni. J Appl Microbiol. 2000;89: 526–531. doi: 10.1046/j.1365-2672.2000.01142.x [DOI] [PubMed] [Google Scholar]
- 30.Choi YH. Metabolic Discrimination of Catharanthus roseus Leaves Infected by Phytoplasma Using 1H-NMR Spectroscopy and Multivariate Data Analysis. Plant Physiol. 2004;135: 2398–2410. doi: 10.1104/pp.104.041012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minami M, Ando T, Hashikawa SN, Torii K, Hasegawa T, Israel DA, et al. Effect of glycine on Helicobacter pylori in vitro. Antimicrob Agents Chemother. 2004;48: 3782–3788. doi: 10.1128/AAC.48.10.3782-3788.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fagard M, Launay A, Clément G, Courtial J, Dellagi A, Farjad M, et al. Nitrogen metabolism meets phytopathology. J Exp Bot. 2014;65: 5643–5656. doi: 10.1093/jxb/eru323 [DOI] [PubMed] [Google Scholar]
- 33.Bertaccini A, Duduk B. Phytoplasma and phytoplasma diseases: a review of recent research. Phytopathol Mediterr. 2010;48: 355–378. doi: 10.14601/Phytopathol_Mediterr-3300 [Google Scholar]
- 34.Garsin D. Ethanolamine Utilisation in Bacterial Pathogens: Roles and Regulation. Nat Rev Microbiol. 2010;8: 290–295. doi: 10.1038/nrmicro2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kube M, Mitrovic J, Duduk B, Rabus R, Seemüller E. Current View on Phytoplasma Genomes and Encoded Metabolism. Sci World J. 2012;2012: 1–25. doi: 10.1100/2012/185942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haudecoeur E, Planamente S, Cirou A, Tannieres M, Shelp BJ, Morera S, et al. Proline antagonizes GABA-induced quenching of quorum-sensing in Agrobacterium tumefaciens. Proc Natl Acad Sci. 2009;106: 14587–14592. doi: 10.1073/pnas.0808005106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cevallos-Cevallos JM, Futch DB, Shilts T, Folimonova SY, Reyes-De-Corcuera JI. GC-MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus huanglongbing. Plant Physiol Biochem. Elsevier Masson SAS; 2012;53: 69–76. doi: 10.1016/j.plaphy.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 38.Hijaz F, Lu Z, Killiny N. Effect of host-plant and infection with “Candidatus Liberibacter asiaticus” on honeydew chemical composition of the Asian citrus psyllid, Diaphorina citri. Entomol Exp Appl. 2016;158: 34–43. doi: 10.1111/eea.12377 [Google Scholar]
- 39.Albrecht U, Fiehn O, Bowman KD. Metabolic variations in different citrus rootstock cultivars associated with different responses to Huanglongbing. Plant Physiol Biochem. Elsevier Masson SAS; 2016;107: 33–44. doi: 10.1016/j.plaphy.2016.05.030 [DOI] [PubMed] [Google Scholar]
- 40.Wang N, Trivedi P. Citrus Huanglongbing: A Newly Relevant Disease Presents Unprecedented Challenges. Phytopathology. 2013;103: 652–665. doi: 10.1094/PHYTO-12-12-0331-RVW [DOI] [PubMed] [Google Scholar]
- 41.Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15: 89–97. doi: 10.1016/j.tplants.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 42.Halsey CR, Lei S, Wax JK, Lehman MK, Nuxoll AS, Steinke L, et al. Amino Acid Catabolism in Staphylococcus aureus and the Function of Carbon Catabolite Repression. MBio. 2017;8: e01434–16. doi: 10.1128/mBio.01434-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin H, Lou B, Glynn JM, Doddapaneni H, Civerolo EL, Chen C, et al. The complete genome sequence of “Candidatus Liberibacter solanacearum”, the bacterium associated with potato Zebra Chip disease. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertin Y, Girardeau JP, Chaucheyras-Durand F, Lyan B, Pujos-Guillot E, Harel J, et al. Enterohaemorrhagic Escherichia coli gains a competitive advantage by using ethanolamine as a nitrogen source in the bovine intestinal content. Environ Microbiol. 2011;13: 365–377. doi: 10.1111/j.1462-2920.2010.02334.x [DOI] [PubMed] [Google Scholar]
- 45.Kendall MM, Gruber CC, Parker CT, Sperandio V. Ethanolamine controls expression of genes encoding components involved in interkingdom signaling and virulence in enterohemorrhagic escherichia coli O157:H7. MBio. 2012;3: 1–10. doi: 10.1128/mBio.00050-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sperandio B, Polard P, Ehrlich DS, Renault P, Guédon E. Sulfur Amino Acid Metabolism and Its Control in Lactococcus lactis IL1403 Sulfur Amino Acid Metabolism and Its Control in Lactococcus lactis IL1403. J Bacteriol. 2005;187: 3762–3778. doi: 10.1128/JB.187.11.3762-3778.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sperandio V, Mellies JL, Nguyen W, Shin S, Kaper JB. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. PNAS. 1999;96: 15196–15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, Wang X, Zhang H, Yang Y, Ge X, Song F. A rice serine carboxypeptidase-like gene OsBISCPL1 is involved in regulation of defense responses against biotic and oxidative stress. Gene. 2008;420: 57–65. doi: 10.1016/j.gene.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 49.Giri J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal Behav. 2011;6: 1746–1751. doi: 10.4161/psb.6.11.17801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59: 206–216. doi: 10.1016/j.envexpbot.2005.12.006 [Google Scholar]
- 51.Verslues PE, Juenger TE. Drought, metabolites, and Arabidopsis natural variation: A promising combination for understanding adaptation to water-limited environments. Curr Opin Plant Biol. Elsevier Ltd; 2011;14: 240–245. doi: 10.1016/j.pbi.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 52.Sharma SS, Dietz KJ. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot. 2006;57: 711–726. doi: 10.1093/jxb/erj073 [DOI] [PubMed] [Google Scholar]
- 53.Roecker R, Junges GM, Lima DD De. Proline Alters Antioxidant Enzyme Defenses and Lipoperoxidation in the Erythrocytes and Plasma of Rats: In Vitro and In Vivo Studies. 2012; 172–179. doi: 10.1007/s12011-011-9276-6 [DOI] [PubMed]
- 54.Nakajima K, Inatsu S, Mizote T, Nagata Y, Aoyama K, Fukuda Y, et al. Possible involvement of put A gene in Helicobacter pylori colonization in the stomach and motility. Biomed Res. 2008;29: 9–18. doi: 10.2220/biomedres.29.9 [DOI] [PubMed] [Google Scholar]
- 55.Zhang L, Alfano JR, Becker DF. Proline metabolism increases katG expression and oxidative stress resistance in Escherichia coli. J Bacteriol. 2015;197: 431–440. doi: 10.1128/JB.02282-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwan WR, Wetzel KJ, Gomez TS, Stiles MA, Beitlich BD, Grunwald S. Low-proline environments impair growth, proline transport and in vivo survival of Staphylococcus aureus strain-specific putP mutants. Microbiology. 2004;150: 1055–1061. doi: 10.1099/mic.0.26710-0 [DOI] [PubMed] [Google Scholar]
- 57.Pennington JE, Goldstrohm DA, Wells MA. The role of hemolymph proline as a nitrogen sink during blood meal digestion by the mosquito Aedes aegypti. J Insect Physiol. 2003;49: 115–121. doi: 10.1016/S0022-1910(02)00267-6 [DOI] [PubMed] [Google Scholar]
- 58.Scaraffia PY, Wells MA. Proline can be utilized as an energy substrate during flight of Aedes aegypti females. J Insect Physiol. 2003;49: 591–601. doi: 10.1016/S0022-1910(03)00031-3 [DOI] [PubMed] [Google Scholar]
- 59.Crawford JM, Kontnik R, Clardy J. Regulating Alternative Lifestyles in Entomopathogenic Bacteria. Curr Biol. 2010;20: 69–74. doi: 10.1016/j.cub.2009.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damsteegt VD, Postnikova EN, Stone a L, Kuhlmann M, Wilson C, Sechler a, et al. Murraya paniculata and related species as potential hosts and inoculum reservoirs of “Candidatus Liberibacter asiaticus”, causal agent of Huanglongbing. Plant Dis. 2010;94: 528–533. doi: 10.1094/PDIS-94-5-0528 [DOI] [PubMed] [Google Scholar]
- 61.Walter AJ, Hall DG, Duan YP. Low Incidence of “Candidatus Liberibacter asiaticus” in Murraya paniculata and Associated Diaphorina citri. Plant Dis. 2012;96: 827–832. doi: 10.1094/Pdis-08-11-0668 [DOI] [PubMed] [Google Scholar]
- 62.Hartung JS, Shao J, Kuykendall LD. Comparison of the “Ca. liberibacter asiaticus” genome adapted for an intracellular lifestyle with other members of the rhizobiales. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cevallos-Cevallos JM, García-Torres R, Etxeberria E, Reyes-De-Corcuera JI. GC-MS analysis of headspace and liquid extracts for metabolomic differentiation of citrus huanglongbing and zinc deficiency in leaves of “Valencia” sweet orange from commercial groves. Phytochem Anal. 2011;22: 236–246. doi: 10.1002/pca.1271 [DOI] [PubMed] [Google Scholar]
- 64.Verslues PE, Sharma S. Proline Metabolism and Its Implications for Plant-Environment Interaction. Arab B. 2010;8: e0140 doi: 10.1199/tab.0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen THH, Murata N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant, Cell Environ. 2011;34: 1–20. doi: 10.1111/j.1365-3040.2010.02232.x [DOI] [PubMed] [Google Scholar]
- 66.Chen THH, Murata N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2008;13: 499–505. doi: 10.1016/j.tplants.2008.06.007 [DOI] [PubMed] [Google Scholar]
- 67.Hishinuma F, Izaki K, Takahashi H. Effects of glycine and d-amino acids on growth of various microorganisms. Agric Biol Chem. 1969;33: 1577–1586. doi: 10.1080/00021369.1969.10859511 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
A) VIP scores: Amino acids whose relative concentrations are involved in CLas host discrimination ordered by index score of variable importance on the Protection greater than 1 (VIP-1) criterion.
B) Orthogonal projection coefficients for the comparison between permissive and non-permissive hosts of CLas. Negative values represent FAA positively correlated with CLas-permissive hosts whereas negative values correspond to those with higher concentrations in non-permissive plants.
(TIF)
(XLS)
Data Availability Statement
All relevant data are summarized in the paper and supporting information.