Abstract
While aging is associated with increased knowledge, it is also associated with decreased semantic integration. To investigate brain activation changes during semantic integration, a sample of forty-eight 25–75 year-old adults read sentences with high cloze (HC) and low cloze (LC) probability while functional magnetic resonance imaging was conducted. Significant age-related reduction of cloze effect (LC vs. HC) was found in several regions, especially the left middle frontal gyrus (MFG) and right inferior frontal gyrus (IFG), which play an important role in semantic integration. Moreover, when accounting for global gray matter volume reduction, the age-cloze correlation in the left MFG and right IFG was absent. The results suggest that brain structural atrophy may disrupt brain response in aging brains, which then show less brain engagement in semantic integration.
Introduction
Language comprehension is a critical ability for daily life. Semantic integration is a key process in understanding meaning from any given flow of information. Differing from word recognition and retrieval, semantic integration combines small pieces of word-level information into larger message-level representations [1]. While aging is associated with increased vocabulary and augmented stores of world knowledge [2], cumulative evidence has revealed age-related declines in semantic integration during sentence comprehension [3,4].
Event-related potential (ERP) studies have shown that in younger adults, semantic integration is associated with an N400 effect [5,6], with more negative deflection occurring when semantic integration difficulty increases. Semantic integration is also associated with brain activation in multiple brain regions, including the bilateral inferior/middle frontal gyrus (I/MFG), the bilateral middle temporal gyrus (MTG), and the anterior temporal lobe (ATL). This activation has been demonstrated in functional magnetic resonance imaging (fMRI) studies [1,5,7–10].
ERP studies have revealed a much smaller N400 effect in older adults than in younger adults [3,11], with delayed onset of the N400 effect having been reported [12]. What is more, the N400 effect appears to decline linearly with age in older adults. For instance, one study showed that among older adults in their fifties, sixties, and seventies, the smallest N400 effect occurred in people in their seventies [13]. Taking advantage of high temporal resolution, ERP studies have thus provided clear evidence of age-related decline in semantic integration ability. However, the relationship between semantic integration decline and brain activation change in aging still remains unclear. While fMRI studies have focused mainly on semantic processing at the word level [14–19] and on syntactic processing [20–23], a handful of studies have investigated brain function changes in semantic integration during sentence comprehension [22,24].
Erb and Oblesor (2013) presented both degraded and clear auditory sentences to younger and older adults. While younger and older adults showed largely overlapping activation during degraded speech processing, comprehension scores were positively correlated with brain activity in the right MFG in older adults only, and in the left fusiform gyrus, cerebellum, and posterior cingulate cortex in younger adults only. The degrade effect (degraded versus clear speech) was negatively correlated with hearing loss in the bilateral insula. This manifested as an increased blood-oxygen-level dependent (BOLD) signal for clear relative to degraded speech being associated with greater hearing loss. The comprehension score was positively correlated with brain activity in the anterior cingulate cortex (ACC) in both groups, with older adults showing worse performance and lower activity. However, the results revealed that comprehension of degraded speech greatly relied on cognitive control, for which there is a declined capacity in older adults [2,25].
One more study [26] relevant to semantic integration compared brain activation of seniors with lower versus higher accuracy during grammatically simple sentence reading. Seniors with poorer comprehension showed higher activation in the bilateral I/MFG and in the ACC than those with better comprehension. However, the group comparison in this study was based on the entire simple sentence (relative to the baseline), which could not separate semantic integration from syntax processing. Both age-related change and preservation have been reported when syntax complexity increases during speech comprehension [20,21,27,28]. Again, syntax-complexity-induced comprehension difficulty is beyond difficulty in semantic integration per se.
Therefore it is necessary to identify the age-related brain function changes that are responsible for semantic integration, rather than other cognitive processes that may accompany semantic integration. To this end, we constructed high cloze (HC) and low cloze (LC) sentences [29]. High-cloze sentences were sentences in which a noun at a given position (the critical word) was highly expected. In contrast, low-cloze sentences were semantically correct, but the noun at the critical word position was unexpected. Semantic integration appears to be more difficult in LC than in HC sentences, which is evident from larger N400 amplitudes [1,5]. However, compared with reading congruent sentences, reading violation sentences not only increases the integration difficulty but also involves other cognitive processes such as error monitoring and repairing [30,31]. To avoid tapping processes other than semantic integration, the present study focused on the differences between LC and HC sentences (the cloze effect). By employing a sample with an age range from 25 to 75 years old, age-related changes associated with LC versus HC differences were investigated. Since aging has been associated with a smaller N400 effect, it is possible that a smaller cloze effect would be manifested in regions that contribute to semantic integration, including left prefrontal cortex and left temporal cortex.
Moreover, cumulative evidence has demonstrated age-related cerebral gray matter shrinkage [32,33]. Recent studies have shown that reduced gray matter also contributes to language decline [19,34,35]. An interesting question is whether age-related functional alteration could also be explained by gray matter reduction, or if age itself is rather the main cause of brain activity change. According to the mediation hypothesis [36], the association between age and brain function change would disappear if the gray matter reduction fully mediated the age-related function decline; otherwise the association would persist if the gray matter reduction is independent of the age-related function decline [37].
Methods
Participants
A total of 48 right-handed, monolingual Chinese speakers (28 males) participated in this study. Mean age was 49.7 years old (SD 15.3, range 25–75). The number of people in their twenties, thirties, forties, fifties, sixties, and seventies was 8, 8, 6, 13, 8, and 5, respectively, with no significant gender ratio difference across these age subgroups, X2 = 8.86, df = 5, p = 0.15). Mean years of education was 11.4 (SD 2.8, range 6–16). The study was approved by the Ningcheng Central Hospital review board, according to the Declaration of Helsinki. Written informed consent was obtained from each participant immediately prior to the study. Participants were residents of the community who had normal or corrected-to-normal visual acuity. Exclusionary criteria for the study included the following: a major head injury, stroke, a neurological or psychiatric disorder, high blood pressure, diabetes, heart disease, the use of psychotropic drugs, or the presence of metal fragments and/or metallic implants contraindicated for MRIs. Before the MRI, the Beijing version of the Montreal Cognitive Assessment (MoCA, http://www.mocatest.org/) was administered to test short-term memory recall, visuospatial skill, executive functioning, language, orientation, attention, concentration, and working memory. The mean score on the MoCA was 27.2 (SD 1.7, range 25–30), indicating that the participants were cognitively healthy.
Stimuli
To manipulate semantic prediction, we used high cloze (HC) and low cloze (LC) sentences that were modified from a previous study [1]. First, we constructed 144 sentences (each 8 to 13 words in length) with highly constraining contexts. The critical word (CW) appeared at the end of the Chinese sentence (e.g., 放学的小明正背着书包/After school, Peter was carrying his backpack). The CW was then replaced with a semantically unexpected noun (足球/football) that was nevertheless semantically congruent within the context of the LC condition (Table 1). The cloze probability was rated by another 58 participants from the same participant pool. The cloze probabilities of the HC condition (the ratio of the most frequently appearing word in all self-generated responses) ranged from 59% to 100% (mean ± SD, 89.5 ± 10.7%). The cloze probabilities of the high cloze condition were significantly higher than the LC condition (1.0 ± 1.8%, ranging from 0 to 8.6%, t (143) = 91.7, p < 0.001). The CWs were matched across conditions for frequency (log frequency for HC and LC were 2.4 ± 0.7 and 2.4 ± 0.6, respectively, p > 0.5) [38] and the number of character strokes controlling for visual complexity (HC and LC were 16.3 ± 4.5 and 15.9 ± 4.3, respectively, p > 0.5), which is akin to word length in English. Semantic acceptability for all sentences was rated on a 6-point Likert scale (1 = entirely unacceptable, 6 = fully acceptable) by 18 participants from the same pool. The average ratings for the HC and LC conditions were 5.3 ± 0.4 and 5.2 ± 0.4. The acceptability ratings were comparable between HC and LC conditions (t (143) = 1.27, p = 0.21). Sentence presentation was counterbalanced across participants.
Table 1. Stimuli examples and control data (mean ± SD).
| Conditions | Sentences | Frequency | Number of Strokes | Semantic Acceptability |
|---|---|---|---|---|
| High cloze (HC) | 刚放学的小明背着书包。 After school, Peter was carrying his backpack. |
2.4 ± 0.7 | 16.2 ± 4.3 | 5.3 ± 0.4 |
| Low cloze (LC) | 刚放学的小明背着足球。 After school, Peter was carrying his football. |
2.4 ± 0.6 | 15.9 ± 4.4 | 5.2 ± 0.4 |
Note. Critical words were underlined.
Procedure
All stimuli were presented using E-Prime (version 1.1) software. The sentences were presented word by word, with words displayed for 500 ms, followed by a 100 ms blank. There was a 200 ms fixation screen prior to presentation of the first word. The words were presented in Song font with a font size of 40, and the words were black against a gray background. After the sentence presentation, participants were asked to press a button within a 4000 ms time window to indicate whether the sentence was semantically acceptable. The durations of the inter-trial intervals were randomly selected between 200–6200 ms. The sentences were presented in two runs.
fMRI data acquisition
Data acquisition was performed using a Philips 1.5 T MR scanner. Whole-brain echo-planar images (EPIs) were acquired in an interleaved manner with ascending slice order (TR = 2000 ms, TE = 30 ms, flip angle = 77°, 40 slices, voxel size = 3.5 × 3.5 × 3.5 mm3). A high-resolution T1-weighted scan was acquired for each participant after the functional runs using an MPRAGE sequence (192 slices, TE = 2.93 ms; slice thickness = 1mm; voxel size = 1 × 0.875 × 1 mm3).
fMRI preprocessing
The fMRI Expert Analysis Tool (FEAT) v5.0.8, part of the FMRIB Software Library (FSL) was used in the preprocessing and statistical analyses of the fMRI data. Data preprocessing steps were similar to those used in our previous work [39]. Data were first motion corrected, smoothed with an 8 mm Gaussian kernel, and highpass filtered at 100 s. fMRI data were then co-registered to each individual’s high resolution structural scan using boundary-based registration. The high resolution structural image was co-registered to MNI 2 × 2 × 2 mm3 space using an initial linear registration followed by nonlinear warping (using FNIRT). These transformation parameters were then applied to the functional data, which was re-sliced to 2 mm isotropic voxels during non-linear warping into MNI space.
Functional data was first modelled at the individual subject level by fitting a voxel-wise General Linear Model (GLM) to the BOLD data acquired from each run. Task regressors for HC and LC, and wrong trials across conditions, were modelled as a box-car function and convolved with a canonical double gamma hemodynamic response function for each run. Using a fixed-effects model, in the 2nd level model, maps for each condition were averaged across the two runs within each participant.
fMRI statistical analyses
Group analyses focused on the cloze effect (LC-HC). Monte Carlo simulations using the AlphaSim program were used to determine the appropriate combination of significance level and cluster threshold required to reach a corrected significance level of p < 0.05. This took into account both native space voxel dimensions and the effective smoothness estimated directly from our preprocessed data (http://www.restfmri.net). The Monte Carlo simulations used 1000 iterations and indicated a significance level of p < 0.005 and a cluster threshold of 60 voxels in order to reach a corrected significance level of p < 0.05. This threshold was applied to each of the contrasts described in the study.
Voxel-based morphometric analyses
To expose age-related gray matter reduction, each participant’s T1 image was used to perform voxel-based morphometric (VBM) analysis according to the procedure in FSL-VBM [40]. The structural images were brain-extracted and gray matter was segmented before being registered to the MNI 152 standard space using non-linear registration. The resulting images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific gray matter template. All native gray matter images were then non-linearly registered to this study-specific template and "modulated" to correct for local expansion (or contraction) due to the non-linear component of the spatial transformation. Finally, the modulated gray matter images were smoothed with an isotropic Gaussian kernel with a sigma of 3 mm. After preprocessing, a GLM analysis was conducted using age as a predictor, gender and education as covariates, and gray matter reduction as the dependent variable.
Voxel-wise age-cloze correlation
In order to reveal age-related changes in the cloze effect, the cloze effect contrast (LC-HC) of each subject underwent a covariate analysis at the group level, with age as a predictor and education and gender as covariates. To further explore whether age-related brain function changes associated with the cloze effect were influenced by brain volume reduction, a voxel-wise general linear model analysis was conducted. Age and global regional volume reduction were used as regressors, and brain function as the dependent variable. Education and gender were used as covariates, but they were not of primary interest.
ROI analysis
Because previous studies suggested that the left prefrontal cortex (PFC) plays an important role in sentence-level semantic integration, a region of interest (ROI) analysis was also conducted to test the relationship between age and the cloze effect in the left PFC. ROIs in the left middle frontal gyrus (MFG, MNI coordinates: -40 24 24) and the inferior frontal gyrus (IFG, MNI coordinates: -38 40–10) were utilized from our previous study [1], which demonstrated the cloze effect in both explicit and implicit semantic tasks. The significant correlation identified in previous step was then further tested after the VBM was added into the regression model.
Results
Behavioral results
Behavioral results revealed a significant cloze effect in terms of both accuracy and response time (RTs). The participants comprehended the HC sentences with high accuracy (92.6 ± 8.0%); however, they performed significantly worse on the LC sentences (68.5 ± 12.0%), F (1, 47) = 180.4, p < 0.001. The RTs in the LC condition (1382 ± 290 ms) were also significantly slower than those in the HC condition (1716 ± 393 ms), F (1, 47) = 109.4, p < 0.001.
The cloze effect for accuracy (r = -0.10, p = 0.52) and RTs (r = -0.06, p = 0.68) were not significantly correlated with age. However, the accuracy increase in HC (r = -0.32, p = 0.027) and LC (r = -0.28, p = 0.05) was negatively correlated with age. The RTs increase in HC (r = 0.47, p = 0.001) and LC (r = 0.29, p = 0.046) was positively correlated with age. The results indicated that within each condition, an increase in age was associated with worse performance.
fMRI results
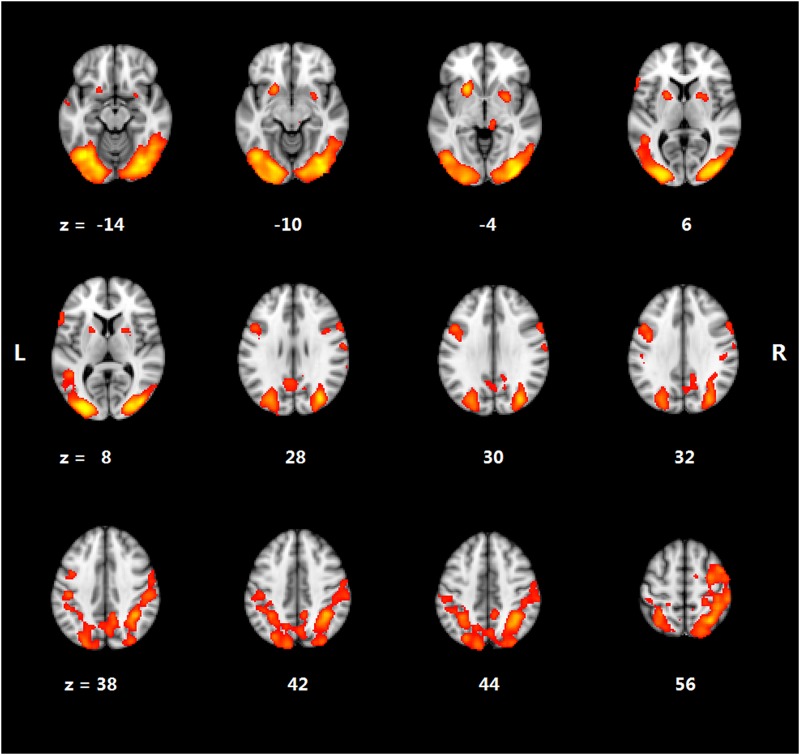
Significant activation occurred in the frontal, temporal, and parietal regions in both the HC and LC conditions. For the cloze effect, Table 2 and Fig 1 depict significantly higher activation in the LC than in the HC condition in several regions. This includes the left middle frontal gyrus (MFG), the left inferior frontal gyrus (IFG), the left anterior temporal lobe (ATL), the right supramarginal gyrus (SMG), the right thalamus, the bilateral occipital/temporal cortex, and the bilateral putamen.
Table 2. Cloze effect on brain activation.
| Region | Hem | Voxels | Z-score | X | Y | Z |
|---|---|---|---|---|---|---|
| LC > HC | ||||||
| Middle frontal gyrus/Precentralgyrus | Left | 361 | 3.70 | -42 | 0 | 34 |
| Inferior frontal gyrus | Left | 95 | 3.21 | -56 | 20 | 10 |
| Occipital/Temporal cortex/Superior parietal lobule | Bilateral | 6235 | 5.51 | -30 | -88 | 14 |
| Anterior temporal lobe | Left | 178 | 4.06 | -56 | 8 | -24 |
| SupramarginalGyrus | Right | 73 | 3.13 | 62 | -38 | 20 |
| Putamen | Left | 516 | 4.70 | -22 | 14 | -4 |
| Putamen | Right | 373 | 3.79 | 28 | 4 | -2 |
| Thalamus | Right | 92 | 3.28 | 12 | -28 | -4 |
| HC > LC | N.S. |
Note. LC: low cloze sentences; HC: high cloze sentences; Hem: hemisphere. X, Y and Z indicate coordinates in MNI standard space. N.S., no significant difference.
Fig 1. Brain activation for cloze effect.
Age-VBM correlation
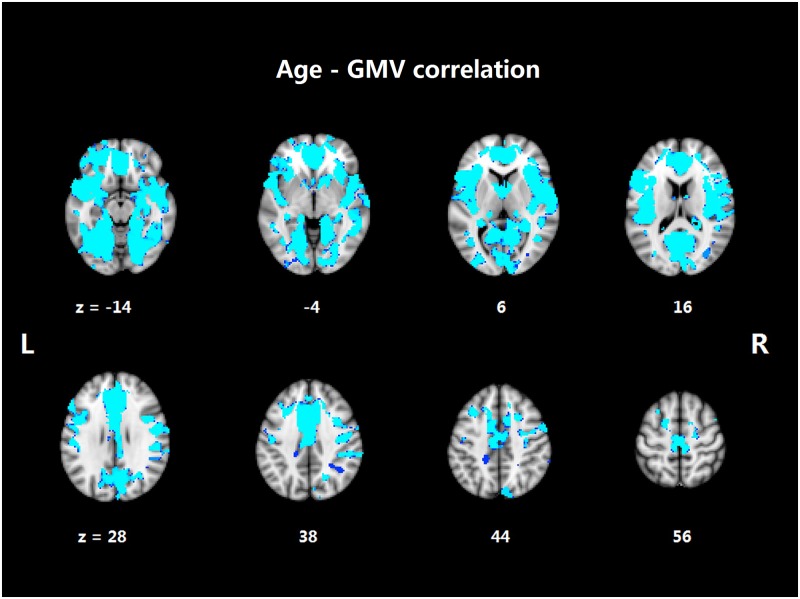
Fig 2 shows the significant negative correlation between age and gray matter volume in widespread regions across the whole brain. These regions included the bilateral IFG and MFG, the bilateral superior/middle/inferior temporal gyrus (S/M/ITG), the bilateral hippocampus, the bilateral occipital and parietal lobe, the cingulate cortex, the bilateral insula, and the basal ganglia.
Fig 2. Aging-associated gray matter volume (GMV) reduction.
Age-cloze correlation
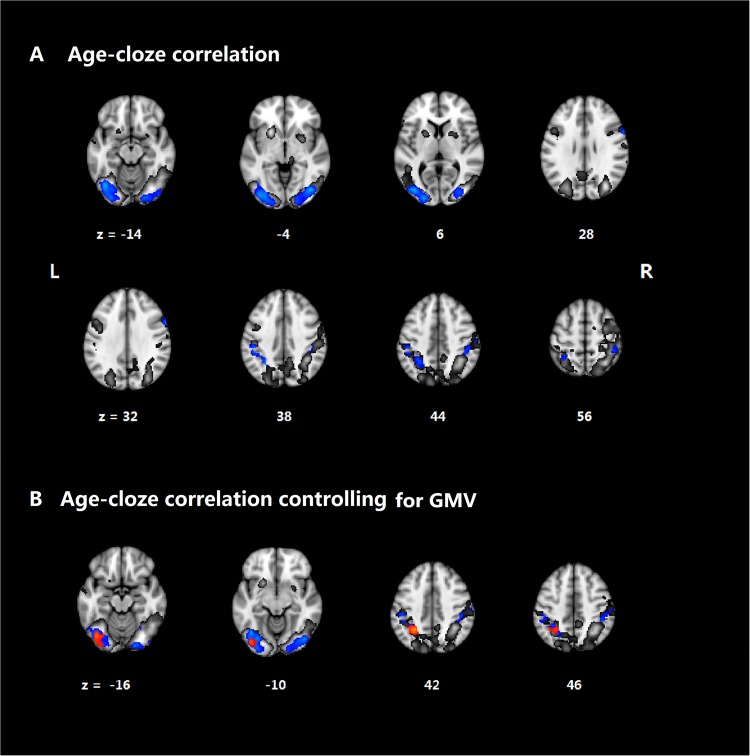
The cloze effect seen in BOLD was significantly reduced in age. As shown in Table 3 and Fig 3A, significant negative BOLD-age correlations were found for the right IFG, the bilateral inferior occipital cortex (IOC), the bilateral SMG, and the right cerebellum. No significant positive correlations were found.
Table 3. Regions showing significant age-cloze effect correlation.
| Region | Hem | Voxels | Z-score | X | Y | Z |
|---|---|---|---|---|---|---|
| Negative correlation | ||||||
| Inferior frontal gyrus/Precentralgyrus | Right | 73 | 3.44 | 60 | 12 | 32 |
| Inferior occipital gyrus | Left | 1372 | 4.24 | -22 | -94 | 2 |
| Inferior occipital gyrus | Right | 1352 | 4.27 | 42 | -76 | -6 |
| Superior parietal lobule/supramarginalgyrus | Left | 526 | 3.72 | -28 | -52 | 40 |
| Supramarginalgyrus | Right | 297 | 3.48 | 44 | -38 | 50 |
| Cerebellum | Right | 117 | 3.39 | 32 | -64 | -24 |
| Cerebellum | Right | 108 | 3.16 | 44 | -44 | -32 |
| Cerebellum | Right | 80 | 3.48 | 52 | -64 | -24 |
| Positive correlation | N.S. |
Note. Hem: hemisphere. X, Y and Z indicate coordinates in MNI standard space. N.S., no significant difference.
Fig 3. Aging-cloze correlation.
(A) Aging-related cloze effect controlling for gender and education (blue), within the cloze effect regions (gray). (B) Aging-related cloze effect controlling for gender, education and gray matter volume (GMV) (red) within the cloze effect regions (gray) and age-cloze effect presented in (A) (blue).
Controlling for gray matter reduction, the significant correlation between age and cloze effect was still observed in the left IOC, the left SMG and the right cerebellum, but not in the right IFG, right IOC, or right SMG. This is shown in Fig 3B and Table 4.
Table 4. Significant age-cloze effect correlations after controlling for gray matter volume.
| Region | Hem | Voxels | Z-score | X | Y | Z |
|---|---|---|---|---|---|---|
| Negative correlation | ||||||
| Inferior occipital cortex | Left | 374 | 3.44 | -40 | -76 | -14 |
| Superior Parietal Lobule | Left | 199 | 4.08 | -28 | -52 | 38 |
| Cerebellum | Right | 68 | 2.95 | 32 | -74 | -22 |
| Positive correlation | N.S. |
Note. Hem: hemisphere. X, Y and Z indicate coordinates in MNI standard space. N.S., no significant difference.
For the ROI analysis, age was significantly correlated with the cloze effect in the left MFG (r = -0.26, p < 0.05) but not in the left IFG (r = 0.01, p = 0.96). For the left MFG, the age-cloze effect correlation was not significant after controlling for global volume reduction (t = -0.38, p = 0.71).
Discussion
Two main findings were discovered in the current study. First, age-related functional and structural alteration was found. Age-related volume reduction occurred in widespread regions, and a smaller cloze effect occurred in the frontal, parietal, and temporal regions. Second, the age-cloze correlation was significant in the left IOC and SMG, but not in the left MFG, right IOC, or right SMG, after controlling for whole brain volume reduction. These results shed light on our understanding of the neural underpinnings of age-related changes during comprehension.
The cloze effect has been frequently implicated in the examination of semantic integration during sentence comprehension. In the present study, in line with previous studies [4,13], there was a significant cloze effect across younger and older adults but no significant age-cloze effect correlation for accuracy or RTs. In contrast, increases in age were associated with lower accuracy and longer RTs. The RT-age correlation suggests that reduced processing speed may contribute to the reading comprehension aging effect [41]. However, as accuracy was also worse in the older adults than younger adults, the result suggests more difficulty in reading comprehension for older adults than younger adults. Previous studies found that the cloze effect related to BOLD activation increases in frontal and temporal regions in young adults [1,42,43]. In line with the literature, the present study revealed higher activation in the LC condition than in the HC condition, for the frontal, temporal-occipital, and parietal regions.
Although we mainly relied on ERPs to investigate age-related changes during semantic integration, the present age-cloze effect correlation in brain activity provides evidence helpful to understanding the brain regions associated with semantic integration in aging. Specifically, the fMRI results showed a clear age modulation of the cloze effect. That is, older adults showed a smaller BOLD cloze effect in the bilateral IOC, SMG, and right IFG in voxel-wise analysis, and in the left MFG in ROI analysis. These results align well with previous ERP studies that have revealed a smaller N400 effect in older adults than younger adults during sentence comprehension [4,44,45]. Previous studies have demonstrated that the bilateral prefrontal cortex, including the left MFG and right IFG reported on here, play an important role in sentence-level semantic integration. However, verbal working memory has been associated with the SMG [46]. The correlation between age and cloze effect thus suggests that aging is not only involved in a decline in semantic integration per se, but also in a decline in supportive processes such as verbal working memory and visual processing.
Under-recruitment in older adults is common during receptive linguistic processing. Pertaining to this, the results found in the present study are consistent with other research. For instance, under-recruitment in the left frontal-temporal regions was found more in poor older readers than in good older readers [22]. It was also found in older adults more than in younger adults during grammatically complex sentence reading [23] and speech perception [19]. Similarly, previous studies have also found age-related under-recruitment in lexical semantic processing [17], word recognition [47], and semantic coding [48]. Under-recruitment may reflect not enough resources [2] in older adults engaged in high demand tasks. Due to the fact that the critical words in the present study did not match the context, the LC sentences may not only have demanded more semantic integration processing in the frontal cortex, but may also have taxed verbal working memory in the SMG [46]. LC sentences might also have elicited more visual processing in the bilateral IOC than the HC sentences. However, older adults may not be able to effectively recruit the integration and supportive processes in comprehension and thus under-recruit brain activations.
Such under-recruitment may also be due to structural decline. Our study, consistent with the literature [33,35,49], found that smaller gray matter volume in older adults was widespread in the cortical and subcortical cortex. Although the age-related volume reduction did not perfectly coincide with the age-related cloze effect, volume reduction was clearly observed in brain regions that are involved in sentence comprehension. These areas include the bilateral frontal-temporal cortex and the temporal-occipital cortex. Because previous studies found a high consistency in global age-related decline, but not in single region atrophy, and the present study found a single widespread cluster after multiple comparison correction, the mean value from the whole volume reduction mask was used in further analysis. When the whole brain volume reduction was added to the regression model, the age-related cloze effect was significantly different than it was without the volume reduction. Specifically, the age-cloze effect correlation in the left IOC and left SMG was still significant. However, that correlation in the left MFG, right IFG, right IOC and right SMG was not significant after the whole brain volume reduction was added in the model.
Thus, the results here suggest that whole brain volume reduction did in fact explain age-related brain functional changes during sentence comprehension. The smaller cloze effect for older adults than younger adults in the specified regions suggests that the brain is less likely to be modulated during semantic integration in aging. The reduction may be due to the globally decreased gray matter. Such a function-structural correlation underscores the findings of recent studies showing that decreased gray matter volume is associated with decreased connectivity within key language regions [19,35]. The age-cloze effect and structural correlations may also explain the previous findings in ERPs. Smaller N400 effects have been demonstrated more in older adults than in younger adults, suggesting that these effects in older adults are less likely to predict upcoming words during language comprehension [3]. Since the previously absent age-cloze correlation was found in the present study in core language regions including the left MFG and right IFG, the results imply that less utilizing prediction by older adults may be due to their having fewer adaptive responses than younger adults.
The left IOC and SMG however, still showed an age-cloze effect with the brain cluster extension more restricted, after volume was controlled for. The IOC and SMG were not part of expected regions for semantic integration. The activation in left IOC during sentence comprehension [31] was associated with reading related visual processing, and the activation in left SMG was associated with working memory demand [50], not semantic integration per se [1,5]. So while the regions could exhibit structural-function correlations [51] in other cognitive functions, they may not necessarily do so in sentence comprehension. Together with the results showing that gray matter reduction explained the age-cloze correlation, these findings suggest heterogeneity of the brain regions in the relationship between age, brain function, and structural change.
One limitation of the present study is that structural changes should be interpreted in the context of gray matter volume reduction. Beyond gray matter volume, structural alteration can also be measured by cortical thickness for gray matter and diffusion tensor imaging [47] and kurtosis imaging [52] for white matter. The latter indices have contributed to understanding functional changes in aging brains, and further investigation is needed to elucidate their specific nature. Moreover, it has been found that older and younger adults might be different in using context constraints during comprehension. Future studies should try to manipulate context constraints to reveal the brain functional and structural basis for sentence comprehension in aging.
In summary, the present study examined brain activity patterns in aging during the comprehension of sentences with varied semantic integration difficulty. The results revealed an age-related decline in brain adaptive changes for integration difficulty. Part of such a decline can be explained by brain volume reduction, suggesting that disrupted brain structures may induce less brain functioning.
Supporting information
ID, subject ID used in the present study; Age, years of age; Gender, 1 for male and 2 for female; MoCA, score for MoCA test; Yr_edu: years of education; Acc_HC, accuracy for High Colze condition; Acc_LC, accuracy for Low Cloze condition; RT_HC, response time for High Colze condition; RT_LC, response time for Low Cloze condition; VBM, mean of VBM in the whole brain masked regions.
(XLSX)
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China (NSF 31571156), the "333" Project of Jiangsu Province and Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions (2nd round), Jiangsu. We thank Mr. Daiqing Shi for his assistent in data collection and analyses, and the reviewers for the helpful comments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the Natural Science Foundation of China (NSF 31571156), the "333" Project of Jiangsu Province and Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions (2nd round), Jiangsu. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhu Z, Feng G, Zhang JX, Li G, Li H, Wang S. The role of the left prefrontal cortex in sentence-level semantic integration. NeuroImage 2013; 76: 325–331. doi: 10.1016/j.neuroimage.2013.02.060 [DOI] [PubMed] [Google Scholar]
- 2.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Ann Rev Psychol 2009; 60: 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wlotko EW, Lee CL, Federmeier KD. Language of the aging brain: Event-related potential studies of comprehension in older adults. Lang Linguist Compass 2010; 4: 623–638. doi: 10.1111/j.1749-818X.2010.00224.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Federmeier KD, Kutas M, Schul R. Age-related and individual differences in the use of prediction during language comprehension. Brain Lang 2010; 115: 149–161. doi: 10.1016/j.bandl.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Z, Hagoort P, Zhang JX, Feng G, Chen HC, Bastiaansen M, et al. The anterior left inferior frontal gyrus contributes to semantic unification. NeuroImage 2012; 60: 2230–2237. doi: 10.1016/j.neuroimage.2012.02.036 [DOI] [PubMed] [Google Scholar]
- 6.Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP). Ann Rev Psychol 2011; 62: 621–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagoort P, Baggio G, Willems RM. Semantic unification In: Gazzaniga MS, editor. The Cognitive Neurosciences. London: MIT Press; 2009. pp. 819–836. [Google Scholar]
- 8.Lau EF, Phillips C, Poeppel D. A cortical network for semantics: (de)constructing the N400. Nature Rev Neurosci 2008; 9: 920–933. [DOI] [PubMed] [Google Scholar]
- 9.Feng G, Chen Q, Zhu Z, Wang S. Separate brain circuits support integrative and semantic priming in the human language system. Cereb Cortex 2016; 26: 3169–3182. doi: 10.1093/cercor/bhv148 [DOI] [PubMed] [Google Scholar]
- 10.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 2009; 19: 2767–2796. doi: 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Federmeier KD, Van Petten C, Schwartz TJ, Kutas M. Sounds, words, sentences: age-related changes across levels of language processing. Psychol Aging 2003; 18: 858–872. doi: 10.1037/0882-7974.18.4.858 [DOI] [PubMed] [Google Scholar]
- 12.Federmeier KD, Kutas M. Aging in context: age-related changes in context use during language comprehension. Psychophysiology 2005; 42: 133–141. doi: 10.1111/j.1469-8986.2005.00274.x [DOI] [PubMed] [Google Scholar]
- 13.Faustmann A, Murdoch BE, Finnigan SP, Copland DA. Effects of advancing age on the processing of semantic anomalies in adults: evidence from event-related brain potentials. Exp Aging Res 2007; 33: 439–460. doi: 10.1080/03610730701525378 [DOI] [PubMed] [Google Scholar]
- 14.Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJ, et al. Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiol Aging 2008; 29: 436–451. doi: 10.1016/j.neurobiolaging.2006.10.024 [DOI] [PubMed] [Google Scholar]
- 15.Shafto MA, Burke DM, Stamatakis EA, Tam PP, Tyler LK. On the tip-of-the-tongue: neural correlates of increased word-finding failures in normal aging. J Cogn Neurosci 2007; 19: 2060–2070. doi: 10.1162/jocn.2007.19.12.2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacombe J, Jolicoeur P, Grimault S, Pineault J, Joubert S. Neural changes associated with semantic processing in healthy aging despite intact behavioral performance. Brain Lang 2015; 149: 118–127. doi: 10.1016/j.bandl.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 17.Gold BT, Andersen AH, Jicha GA, Smith CD. Aging influences the neural correlates of lexical decision but not automatic semantic priming. Cereb Cortex 2009; 19: 2671–2679. doi: 10.1093/cercor/bhp018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peelle JE, Chandrasekaran K, Powers J, Smith EE, Grossman M. Age-related vulnerability in the neural systems supporting semantic processing. Front Aging Neurosci 2013; 5: 46 doi: 10.3389/fnagi.2013.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay P, Dick AS, Small SL. Functional and structural aging of the speech sensorimotor neural system: functional magnetic resonance imaging evidence. Neurobiol Aging 2013; 34: 1935–1951. doi: 10.1016/j.neurobiolaging.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell KL, Samu D, Davis SW, Geerligs L, Mustafa A, Tyler LK, et al. Robust resilience of the frontotemporal syntax system to aging. J Neurosci 2016; 36: 5214–5227. doi: 10.1523/JNEUROSCI.4561-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA. Preserving syntactic processing across the adult life span: the modulation of the frontotemporal language system in the context of age-related atrophy. Cereb Cortex 2010; 20: 352–364. doi: 10.1093/cercor/bhp105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossman M, Cooke A, DeVita C, Chen W, Moore P, Detre J, et al. Sentence processing strategies in healthy seniors with poor comprehension: An fMRI study. Brain Lang 2002; 80: 296–313. doi: 10.1006/brln.2001.2581 [DOI] [PubMed] [Google Scholar]
- 23.Peelle JE, Troiani V, Wingfield A, Grossman M. Neural processing during older adults’ comprehension of spoken sentences: age differences in resource allocation and connectivity. Cereb Cortex 2010; 20: 773–782. doi: 10.1093/cercor/bhp142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erb J, Obleser J. Upregulation of cognitive control networks in older adults’ speech comprehension. Front Syst Neurosci 2013; 7: 116 doi: 10.3389/fnsys.2013.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Z, Johnson NF, Kim C, Gold BT. Reduced frontal cortex efficiency is associated with lower white matter integrity in aging. Cereb Cortex 2015; 25: 138–146. doi: 10.1093/cercor/bht212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, et al. Age-related changes in working memory during sentence comprehension: An fMRI study. NeuroImage 2002; 15: 302–317. doi: 10.1006/nimg.2001.0971 [DOI] [PubMed] [Google Scholar]
- 27.Davis SW, Zhuang J, Wright P, Tyler LK. Age-related sensitivity to task-related modulation of language-processing networks. Neuropsychologia 2014; 63: 107–115. doi: 10.1016/j.neuropsychologia.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeCaro R, Peelle JE, Grossman M, Wingfield A. The two sides of sensory-cognitive interactions: Effects of age, hearing acuity, and working memory span on sentence comprehension. Front Psychol 2016; 7: 236 doi: 10.3389/fpsyg.2016.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagoort P, Brown C. Brain responses to lexical ambiguity resolution and parsing In: C C Jr, Frazier L, Rayner K, editors. Perspectives on Sentence Processing: Hilsdale NY: Lawrence Erlbaum Associates; 1994. pp. 45–80. [Google Scholar]
- 30.Kaan E, Swaab TY. Repair, revision, and complexity in syntactic analysis: an electrophysiological differentiation. J Cogn Neurosci 2003; 15: 98–110. doi: 10.1162/089892903321107855 [DOI] [PubMed] [Google Scholar]
- 31.Zhu Z, Zhang JX, Wang S, Xiao Z, Huang J, et al. Involvement of left inferior frontal gyrus in sentence-level semantic integration. NeuroImage 2009; 47: 756–763. doi: 10.1016/j.neuroimage.2009.04.086 [DOI] [PubMed] [Google Scholar]
- 32.Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging 2005; 26: 1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023 [DOI] [PubMed] [Google Scholar]
- 33.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 2005; 15: 1676–1689. doi: 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- 34.Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci 2011; 31: 12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meunier D, Stamatakis EA, Tyler LK. Age-related functional reorganization, structural changes, and preserved cognition. Neurobiol Aging 2014; 35: 42–54. doi: 10.1016/j.neurobiolaging.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 36.Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull 2011; 137: 753–784. doi: 10.1037/a0023262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Z, Hakun JG, Johnson NF, Gold BT. Age-related increases in right frontal activation during task switching are mediated by reaction time and white matter microstructure. Neurosci 2014; 278C: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai Q, Brysbaert M. SUBTLEX-CH: Chinese word and character frequencies based on film subtitles. PLoS One 2010; 5: e10729 doi: 10.1371/journal.pone.0010729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hakun JG, Zhu Z, Johnson NF, Gold BT. Evidence for reduced efficiency and successful compensation in older adults during task switching. Cortex 2015; 64: 352–362. doi: 10.1016/j.cortex.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 2007; 130: 2375–2386. doi: 10.1093/brain/awm184 [DOI] [PubMed] [Google Scholar]
- 41.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev 1996; 103: 403–428. [DOI] [PubMed] [Google Scholar]
- 42.Huang J, Zhu Z, Zhang JX, Wu M, Chen HC, Wang S. The role of left inferior frontal gyrus in explicit and implicit semantic processing. Brain Res 2012; 1440: 56–64. doi: 10.1016/j.brainres.2011.11.060 [DOI] [PubMed] [Google Scholar]
- 43.Wu CY, Ho MHR, Chen SHA. A meta-analysis of fMRI studies on Chinese orthographic, phonological, and semantic processing. NeuroImage 2012; 63: 381–391. doi: 10.1016/j.neuroimage.2012.06.047 [DOI] [PubMed] [Google Scholar]
- 44.DeLong KA, Groppe DM, Urbach TP, Kutas M. Thinking ahead or not? Natural aging and anticipation during reading. Brain Lang 2012; 121: 226–239. doi: 10.1016/j.bandl.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu N, Hou X, Zhao B, Zhu Z, Yang Y. Age-related temporal-spatial dynamic ERP changes during sentence comprehension. Neurosci Lett 2017; 645: 74–79. doi: 10.1016/j.neulet.2017.02.074 [DOI] [PubMed] [Google Scholar]
- 46.Deschamps I, Baum SR, Gracco VL. On the role of the supramarginal gyrus in phonological processing and verbal working memory: Evidence from rTMS studies. Neuropsychologia 2013; 53C: 39–46. [DOI] [PubMed] [Google Scholar]
- 47.Kemmotsu N, Girard HM, Kucukboyaci NE, McEvoy LK, Hagler DJ Jr., Dale AM, et al. Age-related changes in the neurophysiology of language in adults: relationship to regional cortical thinning and white matter microstructure. J Neurosci 2012; 32: 12204–12213. doi: 10.1523/JNEUROSCI.0136-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron 2002; 33: 827–840. [DOI] [PubMed] [Google Scholar]
- 49.Montembeault M, Joubert S, Doyon J, Carrier J, Gagnon JF, Monchi O, et al. The impact of aging on gray matter structural covariance networks. NeuroImage 2012; 63: 754–759. doi: 10.1016/j.neuroimage.2012.06.052 [DOI] [PubMed] [Google Scholar]
- 50.Nardone R, De Blasi P, Zuccoli G, Tezzon F, Golaszewski S, Trinka E. Transient beneficial effects of excitatory theta burst stimulation in a patient with phonological agraphia after left supramarginal gyrus infarction. Brain Lang 2012; 120: 422–426. doi: 10.1016/j.bandl.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 51.Kalpouzos G, Persson J, Nyberg L. Local brain atrophy accounts for functional activity differences in normal aging. Neurobiol Aging 2012; 33: 623.e621–623.e613. [DOI] [PubMed] [Google Scholar]
- 52.Billiet T, Vandenbulcke M, Madler B, Peeters R, Dhollander T, Zhang H, et al. Age-related microstructural differences quantified using myelin water imaging and advanced diffusion MRI. Neurobiol Aging 2015; 36: 2107–2121. doi: 10.1016/j.neurobiolaging.2015.02.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ID, subject ID used in the present study; Age, years of age; Gender, 1 for male and 2 for female; MoCA, score for MoCA test; Yr_edu: years of education; Acc_HC, accuracy for High Colze condition; Acc_LC, accuracy for Low Cloze condition; RT_HC, response time for High Colze condition; RT_LC, response time for Low Cloze condition; VBM, mean of VBM in the whole brain masked regions.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.