Abstract
Background and aims
Hepatic macrophages (Kupffer cells) are involved in the immunopathology of alcoholic liver disease (ALD). The mannose receptor (MR, CD206), expressed primarily by macrophages, mediates endocytosis, antigen presentation and T-cell activation. A soluble form, sMR, has recently been identified in humans.
We aimed to study plasma sMR levels and its correlation with disease severity and survival in ALD patients.
Methods
We included 50 patients with alcoholic hepatitis (AH), 68 alcoholic cirrhosis (AC) patients (Child-Pugh A (23), B (24), C (21)), and 21 healthy controls (HC). Liver status was described by the Glasgow Alcoholic Hepatitis Score (GAHS), Child-Pugh (CP) and MELD-scores, and in AC patients the hepatic venous pressure gradient (HVPG) was measured by liver vein catheterisation. We used Kaplan-Meier statistics for short-term survival (84-days) in AH patients and long-term (4 years) in AC patients. We measured plasma sMR by ELISA.
Results
Median sMR concentrations were significantly elevated in AH 1.32(IQR:0.69) and AC 0.46(0.5) compared to HC 0.2(0.06) mg/L; p<0.001 and increased in a stepwise manner with the CP-score (p<0.001). In AC sMR predicted portal hypertension (HVPG ≥10 mmHg) with an area under the Receiver Operator Characteristics curve of 0.86 and a high sMR cut-off (>0.43 mg/l) was associated with increased mortality (p = 0.005).
Conclusion
The soluble mannose receptor is elevated in alcoholic liver disease, especially in patients with AH. Its blood level predicts portal hypertension and long-term mortality in AC patients.
Introduction
The immunopathology of alcoholic liver disease (ALD) is incompletely understood and treatment options are limited. Therefore, identification of key pathophysiological components and possible targets for intervention is a priority in ALD.
Resident hepatic macrophages (Kupffer cells) are central in the current immunopathological understanding of ALD and we have previously shown that soluble CD163, the hemoglobin-haptoglobin scavenger receptor and a specific marker of macrophage activation, is highly elevated in ALD and originates from Kupffer cells[1–3]. This study focuses on the functionally different macrophage receptor, the mannose receptor (MR, CD206, MRC1) and its relation to clinical endpoints in ALD. The MR is expressed primarily by subsets of macrophages and by the comparatively scarcer dendritic cells, a soluble form (sMR) has recently been identified in humans [4]. MR is a pattern recognition receptor with a broad range of ligands including mannose, sulphated carbohydrates and collagen. The functions of the mannose receptor include endocytosis of microorganisms as well as facilitation of antigen presentation and subsequent induction of the associated immune responses[5, 6]. Furthermore, sMR has a metabolic role in the clearance of glycoproteins with degradation of endogenous glycoproteins such as β-glucuronidase and procollagen, which are up-regulated during inflammation[7]. In mice macrophages increase expression of the MR and its soluble form upon stimulation with IL-4 and IL-10, but the exact mechanism of sMR release in humans is unknown[8, 9]. sMR levels are elevated in critically ill patients receiving intensive care and predict survival in patients with pneumococcal bacteraemia and patients with acute on chronic liver failure[10–12].
In ALD, Kupffer cell activation likely occurs as a consequence of bacterial translocation from the gut to the liver. Early animal studies have shown that ethanol-mediated leakage of gut endotoxins to the liver leads to activation of Kupffer cells and initiation of hepatic inflammation [13–15]. This mechanism seems to be important in humans as well, and both increased gut permeability as well as increased serum levels of bacteria and bacterial products have been demonstrated in human ALD [1, 16–20]. The exact role of Kupffer cells remains controversial with different studies showing booth beneficial and deleterious effects of the cells. [21, 22]
This study was conducted to pursue our previous findings of Kupffer cell activation in ALD, and to obtain corroborative and functionally independent confirmation of it´s role. We hypothesize that plasma concentrations of sMR are higher in ALD patients compared with healthy controls, that sMR is associated with ALD disease severity and portal hypertension, and that it can predict clinical endpoints. For this purpose we investigated plasma levels of sMR in ALD patients with alcoholic hepatitis, alcoholic cirrhosis, and healthy controls and measured the hepatic venous pressure gradient (HVPG) by liver vein catheterization, and investigated the association with short- and long-term survival in AH and AC patients,
Methods
Patients
We conducted a cohort study on three groups: 1) 50 patients admitted with acute AH; 2) 68 patients with alcoholic cirrhosis (AC) with different CP-score; and 3) 21 healthy persons with no history of liver or other diseases.
The AH patients were consecutively included using the following inclusion criteria: A first time diagnosis of AH by a combination of physical and laboratory criteria: A history of excessive alcohol ingestion (10 units or more per day) until at least three weeks before admission; acute jaundice (developed over at most 2 weeks, serum bilirubin > 80 μmol/L). The diagnosis was verified by liver biopsy in 10 patients. Exclusion criteria were: viral hepatitis, autoimmune liver disease, bile duct obstruction, liver tumors or any other cancer, presence of an infectious focus (either clinically assessed or based on chest x-ray, urine samples or ascites puncture), age below 18 or above 75 years, ongoing gastrointestinal bleeding or bleeding within the previous three months, or any prior immune-modulating therapy. Patients were recruited from four hospitals in the Aarhus region in Denmark. The patients were stratified according to the Glasgow Alcoholic Hepatitis Score (GAHS) [23]. Those with a GAHS < 9 were given moderate hyper-alimentation (a daily energy consumption of 35–49 Kcal/kg body weight and a daily protein intake of 1.2–1.5 g/kg) and standard supportive medical care, and those with a GAHS ≥ 9 were treated orally with pentoxifylline 400 mg TID (Trental®, Sanofi Paris, France) according to the national guidelines at the time of the study. Clinical status was also assessed according to the Model for End-stage Liver Disease (MELD) and the Child-Pugh score[24–26]. The infectious status at baseline was recorded. The patients were followed-up for 30 days and blood samples were collected at study entry and at day 7, 14, 21, & 30. Entry blood samples were secured before initiation of treatment. The mortality by day 84 (12-weeks) was recorded in the AH patients. Baseline data and baseline sMR levels from this cohort have previously been reported [1, 4]. No patients received blood transfusions during the study period.
The AC patients (n = 68 Child-Pugh A: n = 23, B: n = 24, C: n = 21) were all set up for clinical routine liver vein catheterization. The HVPG was determined using Swan-Ganz balloon catheters as previously described [27]. Blood was collected during catheterisation. Baseline data has partly been reported previously [2]. None of the patients had signs of alcoholic hepatitis, infection or had fever at examination and none of the patients were treated with antibiotics. The diagnosis of cirrhosis was based on a combination of clinical and biochemical parameters as well as ultrasound or CT-scan. The patients underwent routine clinical assessment of the severity of liver disease by CP-class and MELD score. The galactose elimination capacity (GEC) as a measure of metabolic liver function was determined as previously described [28]. Clinically significant portal hypertension (CSPH) was defined by a HVPG ≥10 mmHg measured during hepatic vein catheterization.
Survival data was collected after four years.
The 21 control persons were referred for hemodynamic investigation to exclude splanchnic perfusion disorders, for which no evidence was found. The HVPG was determined by the same procedure as in the AC patients.
All participants gave informed consent in accordance with the Helsinki Declaration, and the study was approved by the National Committee on Health Research Ethics and the Danish Data Protection Agency (J-No. 2008-41-2020) & (J.nr. 2008-41-2836).
Biochemical analysis of sMR
The plasma concentration of sMR was determined by ELISA as previously described.[4]
The maximum inter-assay coefficient of variation was 15%, and the intra-assay coefficient of variation was below 10%.
Biochemical analysis of soluble cytokines interleukin 17a (IL-17a) & interleukin 22 (IL-22)
IL-22 has a protective role in AH, and to investigate the relationship between sMR and other parts of the immune system we measured IL-22 and the related pro-inflammatory cytokine IL-17a in 20 randomly selected AH patients.
The plasma concentrations of IL-17A, IL-22 were measured with ELISA kits (eBioscience) as previously described[29]. The samples and standards were analysed in duplicate, and the average optical density, minus the average value of the blanks, was used to calculate the concentration of a given sample based on the standard curve. The lower detection limit was determined as the value of the average of the blanks plus 2 standard deviations, which resulted in cut-off values of 2.72 pg/ml (IL-17A), 9.04 pg/ml (IL-22). The values below the detection limit were assigned the cut-off value.
Flow cytometry
To investigate whether peripheral monocytes express MR and therefore could be a source of sMR, we studied the MR expression using flow cytometry. 20 patients with AH, 10 patients with AC, and 10 HCs was studied. Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque (GE Healthcare Bio-sciences, Uppsala, Sweden) from EDTA whole blood samples. Samples were stored at -140°C until en bloc analyses. Cells were thawed in PBS containing 20% heat-inactivated pooled human AB-serum and blocked with 10 μl heat-inactivated mouse serum (Invitrogen, Carlsbad, California, USA) before staining with optimized amounts of fluorescent-conjugated antibodies; CD14-APC (clone MFP9; BD Bioscience, San Diego, CA), CD206-FITC (clone 19.2; BD Bioscience, San Diego, CA). Relevant isotype controls were included. The samples were analysed within 24 hours of preparation using a FACSCanto instrument (BD Bioscience). Data analyses were carried out using FlowJo v10.1 (Tree Star Inc., Ashland, OR). We report the relative fluorescent intensity (MFI) of sMR on the CD14+ monocytes.
Statistical analyses
We used the Kruskal-Wallis test followed by Mann-Whitney test to study differences between groups. The relationships between sMR and clinical and biochemical variables were analysed by Spearman's rank correlation. We examined if sMR was independently associated with CSPH by multiple logistic regression analyses, including the MELD-score, GEC and C-reactive protein (CRP) in the model.
We used Receiver Operator Characteristics (ROC) analyses to evaluate the overall capability of the sMR to predict clinically significant portal hypertension (CSPH) defined as a HVPG ≥10 mmHg. Using the ROC curve, a cut-off point was selected for predicting CSPH. The diagnostic accuracy of the cut-off was determined by calculating sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Healthy persons were excluded for ROC-analysis. In the AH patients, an analysis of variance for repeated measurements with Greenhouse–Geisser correction for sphericity was performed for a comparison of protein levels over time. To ensure normally distributed variables, logarithmic transformation was used where appropriate. Histograms and Q–Q plots were used to check normality.
All data are expressed as median with inter-quartile range, and P-values ≤0.05 were considered statistically significant. STATA version 11.2 ®StataCorp LP was used for the data analyses.
Results
Patients
Age and gender distributions were similar in the three study groups (Table 1). As expected, the patients with AH displayed a more severe liver dysfunction than the AC patients. No patient displayed signs of infection at study entry; however, eight AH patients (16%) developed blood culture positive infection during the 30-day follow up.
Table 1. Study entry characteristics of patients with alcoholic hepatitis and patients with stable alcoholic liver cirrhosis and healthy persons (Data are median (interquartile range)).
| |
Alcoholic Hepatitis | Alcoholic cirrhosis | Healthy Controls | ||
|---|---|---|---|---|---|
| Gender: F/M | 16 / | 34 | 16 / | 52 | 7 / 13 |
| Age (years) | 53 | (11) ++ | 58 | (12) * | 51 (8) |
| Weight (kg) | 75.9 | (25) | 75 | (25) | 58 (25) |
| Height (cm) | 174 | (11) * | 173 | (12) | 166 (16) |
| BMI | 24 | (6) | 25 | (5) | 25 (8) |
| ALT (U/l) | 47 | (39) | 39 | (27) | 30 (10) |
| Sodium (mmol/l) | 132 | (7) * ++ | 139 | (6) * | 143 (3) |
| Bilirubin (μmol/l) | 283 | (255) * ++ | 18 | (14) * | 7 (12) |
| Alkaline phosphatase (U/l) | 203 | (149) * ++ | 169 | (127) | 124 (116) |
| Hemoglobin (mmol/l) | 6.7 | (1) | 7.4 | (2) | NA |
| Creatinine (μmol/l) | 64 | (38) ++ | 78 | (33) | 72 (14) |
| INR | 1.9 | (0.7) * ++ | 1.3 | (0.3) * | 1.05 (0.1) |
| Albumin (g/l) | 27 | (8) * ++ | 34 | (10) * | 41 (7.7) |
| MELD | 21 | (11) | 9 | (6) | |
| GAHS | 9 | (2) | |||
| Child Pugh Score | 11 | (2) | 7 | (4) | |
| Ascites (yes/no) | (31/19) | (37/31) | NO | ||
ALT = Alanine aminotransferase, MELD = Model for End-stage Liver Disease, GAHS = Glasgow Alcoholic Hepatitis Score
INR = International Normalised Ratio.
*p<0.05 pt. vs controls
++p<0.05 AH. vs AC
sMR was elevated in alcoholic liver disease
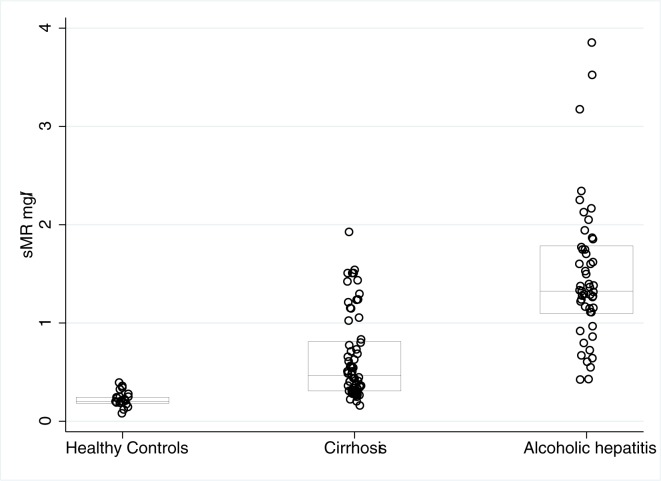
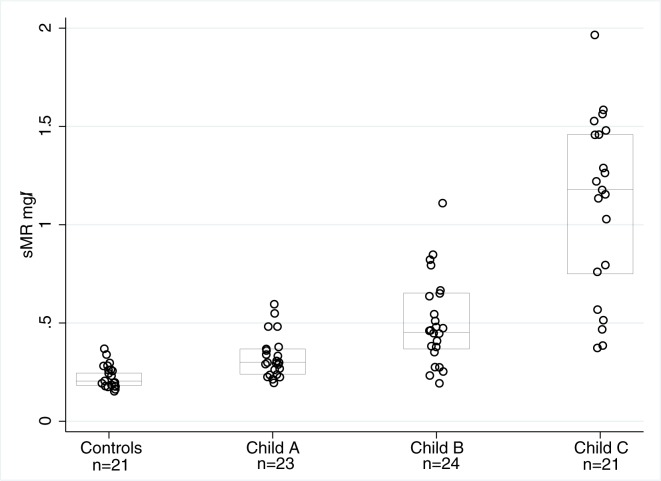
At baseline, the plasma sMR concentration in the AH patients was approximately 3-fold higher than in the cirrhosis patients and nearly 7-fold higher than in the healthy persons (AH 1.32(0.69), AC 0.46(0.5), HC 0.2(0.06) mg/L; p < 0.001, Fig 1). In AC patients, the sMR concentrations increased in a stepwise manner according to the Child-Pugh score. (Fig 2, p<0.05) sMR levels were higher in patients with ascites compared to patients without ascites, and this was the case for both AH patients (1.5 vs 1.1 mg/L; p = 0.003) and AC patients (0.7 vs 0.3 mg/L; p<0.001).
Fig 1. sMR levels in Alcoholic hepatitis, Cirrhosis & healthy control subjects.
Fig 2. sMR in cirrhosis.
sMR (mg/L) levels in healthy control subjects and patients with different degrees of liver cirrhosis. P < 0.05 controls compared to patients with liver cirrhosis. P < 0.05 CP-C compared to CP-A and CP-B.
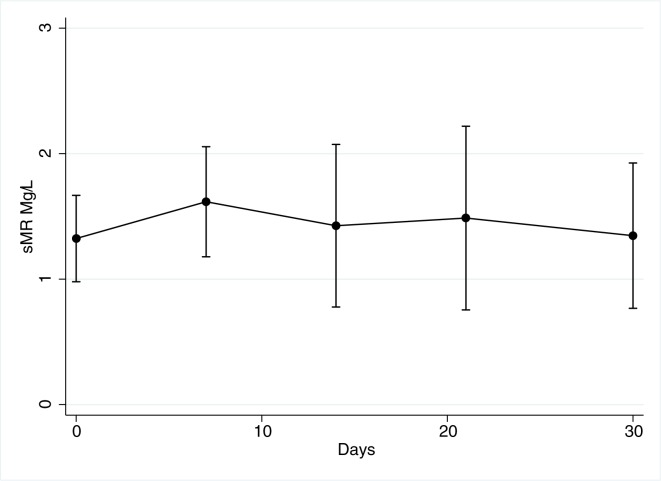
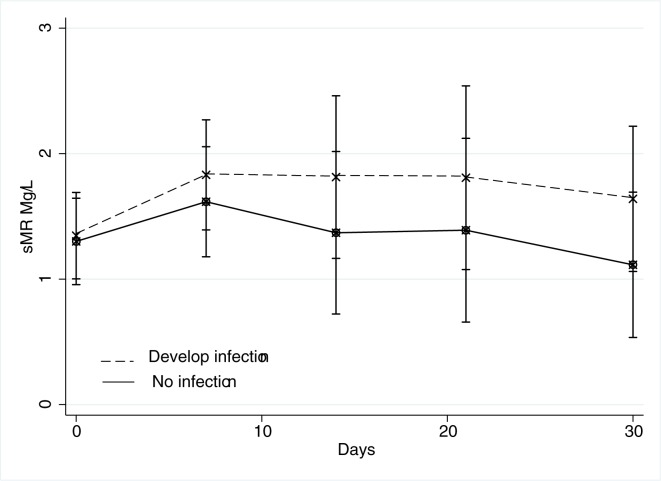
In the AH patients, the sMR concentrations was unchanged during the 30-days follow-up period (p = 0.07; Fig 3). There was no difference at baseline in sMR levels between patients who later developed infection (and subsequently received antibiotic treatment) and those who did not (1.35 vs 1.30 mg/l; p = 0.99). However, sMR levels rose in patients who developed infection, but this rise in sMR level did not reach statistical significance (p = 0.33 repeated measures ANOVA, Fig 4). We observed no differences in sMR levels whether the patients were treated with Pentoxifylline or not (p = 0.9).
Fig 3. sMR in AH patients over time.
sMR levels in Alcoholic hepatitis patients during the 30-day follow-up. (p = 0.07, repeated measures ANOVA).
Fig 4. sMR in AH patients by infection.
sMR levels in Alcoholic hepatitis patients during the 30-day follow-up allocated by patients who later developed infection n = 8 and patients who did not n = 42. (p = 0.07, repeated measures ANOVA).
Internal correlations
In AH patients, baseline analysis showed statistically significant correlations between sMR and Child-Pugh score (r = 0.41; p = 0.003), and albumin (r = 0.37; p = 0.009), but sMR did not correlate to any other clinical or biochemical measures including the GAHS score and cytokines IL-17a (mean 4.0 pg/ml (IQR 2.5) rho -0.2 p = 0.4) and IL-22 (mean 50 pg/ml (IQR 45) rho -0.13 p = 0.58)
At variance, in the AC patients we observed significant correlations between sMR and both clinical and biochemical parameters: The Child-Pugh score (r = 0.71; p < 0.001), the MELD score (r = 0.45; p = 0.002), albumin (r = 0.72; p < 0.001), INR (r = 0.46; p < 0.001), CRP (r = 0.52; p < 0.001), and bilirubin (r = 0.36; p = 0.003).
Relationship between sMR and HVPG (alcoholic cirrhosis patients only)
sMR correlated positively with HVPG (r = 0.52, P<0.001) and sMR was independently associated with CSPH after adjustment for CRP, MELD-score and GEC, (p = 0.03) in multiple logistic regression analysis, Table 2.
Table 2. Multiple logistic regression analyses of the relationships between Clinically significant portal hypertension & sMR, CRP, GEC, and MELD-score.
| N = 60 | ||||
|---|---|---|---|---|
| Dependent Variable = CSPH | Chi square = 0.0001 | |||
| Predictor variables | OR | Std. Error | p-Value | Coeficients |
| sMR | 7379.9 | 29678.0 | 0.027 | 8.90 |
| CRP | 0.9 | 0.1 | 0.286 | -0.06 |
| MELD-score | 1.3 | 0.2 | 0.076 | 0.23 |
| GEC | 0.5 | 0.4 | 0.413 | -0.78 |
CSPH = Clinically significant portal hypertension; GEC = Galactose elimination capacity; CRP = C-Reactive Protein.
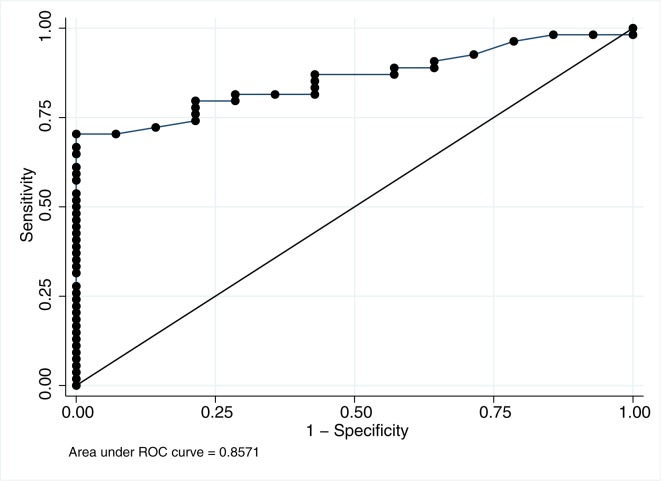
The ROC analysis showed sMR to be a strong predictor of portal hypertension (AUC ROC = 0.86) (Fig 5). For further analysis we chose a cut-off for sMR at 0.43 mg/L equal to the upper normal level. At this cut-off sMR had a sensitivity of 70% and a specificity of 100% for the prediction of CSPH, corresponding to a positive predictive value of 100% and a negative predicted value of 46%.
Fig 5. sMR as a predictor of portal hypertension.
Receiver operating characteristic (ROC) analysis of sMR as a predictor for CSPH in patients with alcoholic cirrhosis.
Mortality
The AH patients were followed up for 84-day mortality and within that period 11 of the 50 (22%) patients died. All but one of the diseased had a GAHS ≥ 9 on admission. We divided the patients into two groups using the median level of sMR (1.3 mg/L) and this analysis showed no difference in mortality between the groups (p = 0.46, log-rank test; data not shown).
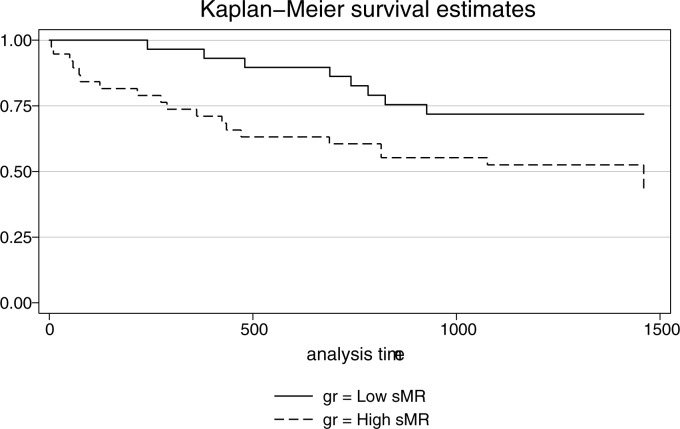
The alcoholic cirrhosis patients were followed up after four years and 41% (28/68) of the patients died. We divided the patients into two groups using the same sMR cut-off of 0.43 mg/Land the AC patients with a sMR above the cut-off had the higher mortality, 53% vs. 27% (p = 0.03, Fig 6).
Fig 6. sMR and survival.
Kaplan-Meier 4-year survival estimates in alcoholic cirrhosis patients according to the plasma level of sMR. The cut-off used was 0.43 (mg/L) (P = 0.03, Log-rank).
Blood monocyte MR & CD14 expression
There was an increase in the numbers of peripheral monocytes in patients with AH compared to both the healthy controls and the AC patients (p = 0.006, data not shown), but we detected no expression of membrane bound MR on CD14+ monocytes in neither patients or controls by flow cytometry.
Discussion
The central finding of our study was the elevated plasma sMR in patients with alcoholic liver disease, most markedly in the patients with alcoholic hepatitis. In the cirrhosis patients, sMR rose with higher Child-Pugh score and with increasing portal pressure. Thus, the levels of sMR accurately predicted if they had clinically significant portal hypertension and their long-term mortality. These findings confirm and support our earlier reports based on sCD163 on the role of the macrophages in ALD, using a new and functionally alternative immune-pathological approach[30] [31].
The major strength of our study is the relevant number of well-characterized patients with ALD. We established the diagnosis of AH in our patients by using a combination of standard clinical and biochemical criteria in accordance with several clinical trials [23, 32]; and the diagnosis was confirmed by liver biopsy in a subset of patients. Likewise, the diagnosis of cirrhosis was made clinically by a combination of clinical, biochemical parameters as well as ultrasound or CT-scan. Interestingly, we could not detect and impact of pentoxifyllin treatment on sMR levels in the AH patients, and this possibly corresponds to the limited clinical impact of pentoxifyllin. It would be interesting to investigate the impact of steroid treatment on sMR levels in AH patients.
A soluble form of the human MR was demonstrated in in vitro experiments in 1999, but it was not till recently that sMR was detected in human blood samples [4, 33]. As a consequence, few studies have investigated sMR in human disease. These studies demonstrated elevated sMR in both critically ill sepsis patients and hepatitis C cirrhosis patients with levels in hepatitis C cirrhosis patients comparable to our AC patients [12, 34]). Of interest, patients with sepsis and liver disease had the highest sMR levels. Recently it was demonstrated that sMR is elevated in patients with acute-on-chronic liver disease (ACLF), and in relation to disease severity; and further, that high sMR levels was an independent predictor for long-term survival in cirrhosis patients without ACLF. Indeed, the addition of sMR to the CLIF-C Acute Decompensation score significantly improved the prognostic performance of the original score[10, 35].
Recent alcohol intake may moderately increase sMR, and it is a limitation that we do not have such information prior to study entry. However, if alcohol should be important we would expect a rapid decline in sMR during the 30-day follow-up of the AH patients. More likely, the florid hepatic inflammation in AH has passed a threshold where alcohol cessation alone will not be enough to dampen inflammation. It is also a limitation that we do not know to which extent the dendritic cells contribute to the generation of sMR, and what pathophysiological implications this might have. However, in comparison to Kupffer cells, dendritic cells are sparse in the liver, and primarily located to the portal regions [36]. Very little is known about clearance of sMR but renal excretion is unlikely owing to the molecular size of the protein (approximately 170 KD). Decreased hepatic metabolism because of shunting might contribute to the elevated levels seen in this study, but we don’t have any specific data to support this. We believe that the origin of the high levels of sMR measured in our study is the liver and predominantly the Kupffer cells. In line with a previous study we found that blood monocytes did not express MR and are an unlikely source of sMR [12].
The functions of sMR are not completely understood, but the soluble part of the receptor retains its binding capacity and thus may serve as a shuttle targeting circulating antigens to the lymphoid organs [37, 38]. There seems to be a slight constitutive shedding of the MR and certain inflammatory mediators elicit extensive shedding. However, LPS is not among these mediators and conversely appears to decrease the expression and shedding of the MR[33]. This is noteworthy because translocated bacteria and LPS presented to the liver is believed to play a key role in the pathogenesis of ALD. Our results with an increased sMR thus cast doubts on the importance of this mechanism. On the other hand sCD163, another marker of macrophage activation, increases its expression and shedding by LPS stimulation and is markedly increased in ALD[1, 39]. These seemingly contradictory results may be reconciled by the assumption that hepatic macrophage activation is effectuated by a variety of stimuli among which LPS is an identified part.
The diversity of the stimulatory pathways notwithstanding, our study supports the occurrence of Kupffer cell activation in ALD and confirm that this may play a role in the immunopathology which may also be corroborated by the repeatedly demonstrated associations of macrophage activation markers, in this case sMR, with clinically important endpoints. Thus, in the AC patients, the ROC-analysis showed a very high positive predictive value of an increased sMR for clinically significant portal hypertension (>10 mmHg). It is well known that hepatic inflammation leads to portal hypertension and we believe the sMR correlation with CSPH mirrors this. Several non-invasive methods for identifying CSPH are under investigation and sMR measurements may contribute to this field alone or in combinations[30]. Given its properties as an endocytic receptor, MR might also be a potential target for intervention with antibody bound compounds. Such proof of concept already exists for the endocytic macrophage receptor CD163. [40, 41]
In conclusion, the blood concentration of the soluble mannose receptor sMR is elevated in ALD, especially in patients with AH. Its level is independently associated with severe portal hypertension and long-term mortality in cirrhosis. Our study confirms and expands our previous findings of macrophage activation in ALD by a different biological approach and possibly motivates intervention studies.
Supporting information
Project data stata format.
(DTA)
Acknowledgments
The authors express their gratitude to technician Kirsten B. Pedersen, Aarhus University Hospital.
Abréviations
- sMR
Soluble (s) Mannose Receptor
- ALD
Alcoholic liver disease
- AH
Alcoholic hepatitis
- AC
Alcoholic cirrhosis
- HC
Healthy controls
- GAHS
Glasgow Alcoholic Hepatitis Score
- CP
Child-Pugh
- MELD
Model for end stage liver disease
- HVPG
Hepatic venous pressure gradient
- ELISA
Enzyme-linked immunosorbent assay
- GEC
Galactose elimination capacity
- CSPH
Clinically significant portal hypertension
- EDTA
Ethylenediaminetetraacetic acid
- PBS
Phosphate-buffered saline
- MFI
Relative fluorescent intensity
- CRP
C-reactive protein
- ROC
Receiver Operator Characteristics
- PPV
Positive predictive value
- NPV
Negative predictive value
- ACLF
Acute-on-chronic liver disease
- LPS
Lipopolysaccharides
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by an unrestricted grant from The “NOVO Nordisk foundation” (http://novonordiskfonden.dk/en) and The Danish Council for Strategic Research (http://ufm.dk/en/research-and-innovation/councils-and-commissions/former-councils-and-commissions/the-danish-council-for-strategic-research) (TRAIN 10-092797).
References
- 1.Sandahl TD, Gronbaek H, Moller HJ, Stoy S, Thomsen KL, Dige AK, et al. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: a prospective cohort study. The American journal of gastroenterology. 2014;109(11):1749–56. doi: 10.1038/ajg.2014.262 . [DOI] [PubMed] [Google Scholar]
- 2.Gronbaek H, Sandahl TD, Mortensen C, Vilstrup H, Moller HJ, Moller S. Soluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosis. Alimentary pharmacology & therapeutics. 2012;36(2):173–80. Epub 2012/05/18. doi: 10.1111/j.1365-2036.2012.05134.x . [DOI] [PubMed] [Google Scholar]
- 3.Holland-Fischer P, Gronbaek H, Sandahl TD, Moestrup SK, Riggio O, Ridola L, et al. Kupffer cells are activated in cirrhotic portal hypertension and not normalised by TIPS. Gut. 2011;60(10):1389–93. Epub 2011/05/17. doi: 10.1136/gut.2010.234542 . [DOI] [PubMed] [Google Scholar]
- 4.Rodgaard-Hansen S, Rafique A, Christensen PA, Maniecki MB, Sandahl TD, Nexo E, et al. A soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illness. Clinical chemistry and laboratory medicine: CCLM / FESCC. 2013:1–9. Epub 2013/10/12. doi: 10.1515/cclm-2013-0451 . [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92(6):1177–86. doi: 10.1189/jlb.0512231 . [DOI] [PubMed] [Google Scholar]
- 6.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. Journal of immunology. 2006;176(11):6770–6. . [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J, Risteli L, et al. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295(5561):1898–901. doi: 10.1126/science.1069540 . [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Pomares L, Reid DM, Brown GD, Taylor PR, Stillion RJ, Linehan SA, et al. Analysis of mannose receptor regulation by IL-4, IL-10, and proteolytic processing using novel monoclonal antibodies. J Leukoc Biol. 2003;73(5):604–13. . [DOI] [PubMed] [Google Scholar]
- 9.Linehan SA, Coulson PS, Wilson RA, Mountford AP, Brombacher F, Martinez-Pomares L, et al. IL-4 receptor signaling is required for mannose receptor expression by macrophages recruited to granulomata but not resident cells in mice infected with Schistosoma mansoni. Laboratory investigation; a journal of technical methods and pathology. 2003;83(8):1223–31. . [DOI] [PubMed] [Google Scholar]
- 10.Gronbaek H, Rodgaard-Hansen S, Aagaard NK, Arroyo V, Moestrup SK, Garcia E, et al. Macrophage activation markers predict mortality in patients with liver cirrhosis without or with acute-on-chronic liver failure (ACLF). J Hepatol. 2016;64(4):813–22. doi: 10.1016/j.jhep.2015.11.021 . [DOI] [PubMed] [Google Scholar]
- 11.Rodgaard-Hansen S, Rafique A, Weis N, Wejse C, Nielsen H, Pedersen SS, et al. Increased concentrations of the soluble mannose receptor in serum from patients with pneumococcal bacteraemia, and prediction of survival. Infect Dis (Lond). 2015;47(4):203–8. doi: 10.3109/00365548.2014.984321 . [DOI] [PubMed] [Google Scholar]
- 12.Kjaergaard AG, Rodgaard-Hansen S, Dige A, Krog J, Moller HJ, Tonnesen E. Monocyte expression and soluble levels of the haemoglobin receptor (CD163/sCD163) and the mannose receptor (MR/sMR) in septic and critically ill non-septic ICU patients. PloS one. 2014;9(3):e92331 doi: 10.1371/journal.pone.0092331 ; PubMed Central PMCID: PMCPMC3956910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34(1):101–8. doi: 10.1053/jhep.2001.25350 [DOI] [PubMed] [Google Scholar]
- 14.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, et al. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166(7):4737–42. Epub 2001/03/20. . [DOI] [PubMed] [Google Scholar]
- 15.Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gabele E, et al. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168(6):2963–9. Epub 2002/03/09. . [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcoholism, clinical and experimental research. 2000;24(4 Suppl):48S–54S. . [PubMed] [Google Scholar]
- 17.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: Reevaluation with an improved chromogenic assay. J Hepatol. 1991;12(2):162–9. [DOI] [PubMed] [Google Scholar]
- 18.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4(1):8–14. . [DOI] [PubMed] [Google Scholar]
- 19.Waidmann O, Brunner F, Herrmann E, Zeuzem S, Piiper A, Kronenberger B. Macrophage activation is a prognostic parameter for variceal bleeding and overall survival in patients with liver cirrhosis. J Hepatol. 2013;58(5):956–61. doi: 10.1016/j.jhep.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 20.Rode A, Nicoll A, Moller HJ, Lim L, Angus PW, Kronborg I, et al. Hepatic macrophage activation predicts clinical decompensation in chronic liver disease. Gut. 2013;62(8):1231–2. doi: 10.1136/gutjnl-2012-304135 . [DOI] [PubMed] [Google Scholar]
- 21.Lanthier N, Rubbia-Brandt L, Lin-Marq N, Clement S, Frossard JL, Goossens N, et al. Hepatic cell proliferation plays a pivotal role in the prognosis of alcoholic hepatitis. J Hepatol. 2015. doi: 10.1016/j.jhep.2015.04.003 . [DOI] [PubMed] [Google Scholar]
- 22.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20(2):453–60. Epub 1994/08/01. . [PubMed] [Google Scholar]
- 23.Forrest EH, Evans CDJ, Stewart S, Phillips M, Oo YH, McAvoy NC, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54(8):1174–9. doi: 10.1136/gut.2004.050781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, Ter Borg PCJ. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–71. doi: 10.1053/he.2000.5852 [DOI] [PubMed] [Google Scholar]
- 25.Kamath PS, Kim WR. The Model for End-stage Liver Disease (MELD). Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563 [DOI] [PubMed] [Google Scholar]
- 26.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9. . [DOI] [PubMed] [Google Scholar]
- 27.Groszmann R, Vorobioff JD, Gao H. Measurement of portal pressure: when, how, and why to do it. Clinics in liver disease. 2006;10(3):499–512, viii. doi: 10.1016/j.cld.2006.08.005 . [DOI] [PubMed] [Google Scholar]
- 28.Tygstrup N. THE GALACTOSE ELIMINATION CAPACITY IN RELATION TO CLINICAL AND LABORATORY FINDINGS IN PATIENTS WITH CIRRHOSIS. Acta Medicin Scandinavia. 1964;175:291–300. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen TK, Andersen T, Hvid M, Hetland ML, Horslev-Petersen K, Stengaard-Pedersen K, et al. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J Rheumatol. 2010;37(10):2014–20. doi: 10.3899/jrheum.100259 . [DOI] [PubMed] [Google Scholar]
- 30.Sandahl TD, McGrail R, Moller HJ, Reverter E, Moller S, Turon F, et al. The macrophage activation marker sCD163 combined with markers of the Enhanced Liver Fibrosis (ELF) score predicts clinically significant portal hypertension in patients with cirrhosis. Alimentary pharmacology & therapeutics. 2016;43(11):1222–31. doi: 10.1111/apt.13618 . [DOI] [PubMed] [Google Scholar]
- 31.Steib CJ. Kupffer cell activation and portal hypertension. Gut. 2011. Epub 2011/06/29. gut.2011.242560 [pii] doi: 10.1136/gut.2011.242560 . [DOI] [PubMed] [Google Scholar]
- 32.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: A double-blind, placebo-controlled trial. Gastroenterology. 2000;119(6):1637–48. [DOI] [PubMed] [Google Scholar]
- 33.Jordens R, Thompson A, Amons R, Koning F. Human dendritic cells shed a functional, soluble form of the mannose receptor. International immunology. 1999;11(11):1775–80. . [DOI] [PubMed] [Google Scholar]
- 34.Andersen ES, Rodgaard-Hansen S, Moessner B, Christensen PB, Moller HJ, Weis N. Macrophage-related serum biomarkers soluble CD163 (sCD163) and soluble mannose receptor (sMR) to differentiate mild liver fibrosis from cirrhosis in patients with chronic hepatitis C: a pilot study. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2014;33(1):117–22. doi: 10.1007/s10096-013-1936-3 . [DOI] [PubMed] [Google Scholar]
- 35.Jalan R, Pavesi M, Saliba F, Amoros A, Fernandez J, Holland-Fischer P, et al. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62(4):831–40. doi: 10.1016/j.jhep.2014.11.012 . [DOI] [PubMed] [Google Scholar]
- 36.Bosma BM, Metselaar HJ, Mancham S, Boor PP, Kusters JG, Kazemier G, et al. Characterization of human liver dendritic cells in liver grafts and perfusates. Liver Transpl. 2006;12(3):384–93. doi: 10.1002/lt.20659 . [DOI] [PubMed] [Google Scholar]
- 37.Su Y, Bakker T, Harris J, Tsang C, Brown GD, Wormald MR, et al. Glycosylation influences the lectin activities of the macrophage mannose receptor. The Journal of biological chemistry. 2005;280(38):32811–20. doi: 10.1074/jbc.M503457200 . [DOI] [PubMed] [Google Scholar]
- 38.Zamze S, Martinez-Pomares L, Jones H, Taylor PR, Stillion RJ, Gordon S, et al. Recognition of bacterial capsular polysaccharides and lipopolysaccharides by the macrophage mannose receptor. The Journal of biological chemistry. 2002;277(44):41613–23. doi: 10.1074/jbc.M207057200 . [DOI] [PubMed] [Google Scholar]
- 39.Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA, et al. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol. 2002;72(4):711–7. . [PubMed] [Google Scholar]
- 40.Granfeldt A, Hvas CL, Graversen JH, Christensen PA, Petersen MD, Anton G, et al. Targeting dexamethasone to macrophages in a porcine endotoxemic model. Crit Care Med. 2013;41(11):e309–18. doi: 10.1097/CCM.0b013e31828a45ef . [DOI] [PubMed] [Google Scholar]
- 41.Graversen JH, Svendsen P, Dagnaes-Hansen F, Dal J, Anton G, Etzerodt A, et al. Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone. Mol Ther. 2012;20(8):1550–8. Epub 2012/05/31. doi: 10.1038/mt.2012.103 ; PubMed Central PMCID: PMC3412497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Project data stata format.
(DTA)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.