Key points
Finding an inherited complement abnormality in HSCT-associated TMA provides a rationale for the use of a complement inhibitor.
Alternative complement inhibitors such as Coversin should be considered in patients who are resistant to eculizumab.
Introduction
Thrombotic microangiopathy (TMA) is a well-recognized complication of hematopoietic stem cell transplantation (HSCT).1 The incidence ranges from 10% to 30%2 with a poor outcome with proteinuria and complement activation.3 Complement abnormalities identified include factor H autoantibodies and a high prevalence of a deletion that includes the genes encoding factor H–related proteins 1 and 3.4 The involvement of complement in the pathogenesis of HSCT-TMA has led to the use of eculizumab to treat HSCT-TMA5 with evidence of improved survival.6 The dose needed to achieve complement blockade is higher than in atypical hemolytic uremic syndrome (aHUS).5-7 Eculizumab is also used to treat paroxysmal nocturnal hemoglobinuria (PNH), and resistance has been reported in Japanese patients carrying a C5 variant (c.2654G>A; p.Arg885His) that prevents eculizumab binding.8 This variant is found in the Japanese population at an allele frequency of 1.7%. We report here a patient with HSCT-associated TMA resistant to eculizumab who was found to carry a CFH variant known to be associated with aHUS and the C5 variant (c.2654G>A; p.Arg885His). Because of this, the patient was treated with the recombinant C5 inhibitor Ornithodoros moubata complement inhibitor (Coversin).9
Case description and methods
A male child presented at 1 year with a cervical lymph node abscess and recurrent cervical lymphadenopathy, biopsy of which showed granulomatous inflammation. At 20 months, he was found to have liver abscesses and a middle lobe mass of the right lung. On biopsy, these showed granulomas with necrosis and fibrosis (Figure 1A). A nonreactive nitroblue tetrazolium test suggested a diagnosis of chronic granulomatous disease. Screening of the gene (CYBB) encoding cytochrome b(−245)β subunit showed a deletion of exon 4. At the age of 3 years, he underwent an HLA-matched unrelated HSCT. A timeline is shown in Figure 2A. At day+27 (D+27), he developed skin graft-versus-host disease (GVHD), which was treated with topical steroids. At D+36, he developed gastrointestinal (GI) GVHD, which was treated with methylprednisolone and infliximab. He also developed features of HSCT-TMA with microangiopathic hemolytic anemia (MAHA) and hypertension; cyclosporin was withdrawn with improvement. ADAMTS13 activity was 40%. The diarrhea subsided and was replaced by an ileus with recurrent melena. Abdominal computed tomographic scan at D+82 showed thickening of the small bowel (supplemental Figure 1). Upper and lower GI biopsies undertaken by endoscopy showed hyperplastic and regenerative changes in the antrum and duodenum that were not typical of GVHD, and there was no histological evidence for adenovirus or cytomegalovirus. At D+132, he developed seizures with hypertension. Peripheral blood film showed fragmentation. Lactate dehydrogenase (LDH) was elevated (1819 IU/L, normal range 470-900 IU/L); platelet count was decreased (75 × 109/L), and hemoglobin was decreased (8.2 g/dL). Recurrent TMA was diagnosed,10 and eculizumab was started at 600 mg IV weekly. Despite ongoing treatment of GVHD (with more frequent infliximab doses, further pulses of high-dose steroids, basiliximab, and mesenchymal stem cells), he developed a skin rash, which on biopsy suggested grade II skin GVHD (Figure 1B). Repeat GI endoscopies (D+196) for the first time showed GVHD (grade IV) (Figure 1C). Although the rash improved, the GI problems persisted. After 10 weekly infusions of eculizumab with no clinical improvement, mutation screening (of DNA samples extracted prior to HSCT) showed a functionally significant11 heterozygous CFH mutation (c.3356A>G; p.Asp1119Gly) previously found in familial aHUS.12 In addition, he carried the C5 variant (c.2654G>A; p.Arg885His) that confers resistance to eculizumab.8 Factor H autoantibodies were not detected. Multiplex ligation-dependent probe amplification showed 2 copies of CHFR1 and CFHR3. Screening of parental samples showed that he had inherited the CFH and the C5 variant from his father, who has no medical problems. Classical pathway hemolytic assay (CH50) while he was on eculizumab at D+145 and D+160 was 35% and 33% of normal, respectively.
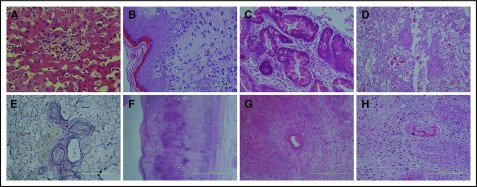
Figure 1.
Histopathology. (A) Preserved liver parenchyma with an inflammatory focus within the lobule, associated with clusters of foamy macrophages containing finely granular golden brown pigment, which was positive with PAS stain and negative with Perls stain for iron. These appearances are characteristic of chronic granulomatous disease (hematoxylin and eosin [H&E] stain). (B) Skin biopsy demonstrating spongiosis of the epidermis with basal vacuolization, dyskeratosis, and a lymphocytic infiltrate of the papillary dermis. Pigment incontinence is also seen within the papillary dermis. These features are consistent with GVHD (H&E stain). (C) Bowel mucosa with crypts shows intraepithelial vacuoles, filled with karyorrhectic debris. These apoptotic bodies are numerous, and intraepithelial lymphocytes are also present. There is scant inflammation within the lamina propria. These features are consistent with GVHD (H&E stain). (D) Postmortem lung parenchyma with muscular arteries shows concentric cellular intimal proliferation. There is almost complete occlusion. Within the airspaces, there is degenerating fibrin. No acute inflammation was present (H&E stain). (E) The corresponding elastic Van Gieson stain of panel D confirms the concentric intimal proliferation. These findings are of severe pulmonary arterial vasculopathy (EVG stain). (F) Postmortem bowel, with extensive necrosis and loss of the normal mucosa. Bacterial colonies are seen, and even at this low power, hemorrhage can be appreciated within the submucosa, which also appears expanded and fibrotic. The muscularis propria appears ragged. These features are of hemorrhagic necrosis, resultant from TMA (H&E stain). (G-H) A high-power view of the submucosa from panel F, highlighting a vessel with eccentric partial occlusion due to thrombus adherent to the wall. A similar vessel is seen in panel H, which is occluded and contains macrophages. These features are related to the underlying vasculopathy (H&E stain). Scale bars, 500 μm (A) and 100 μm (B-H).
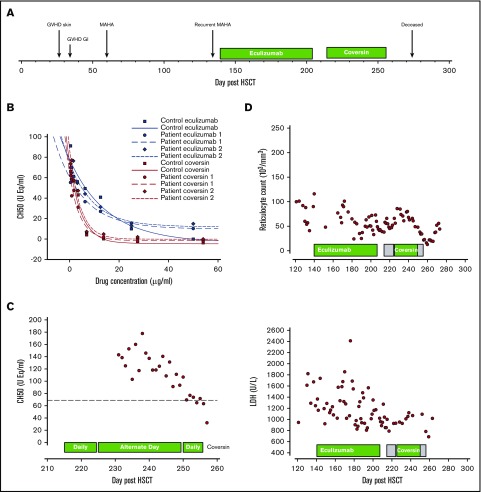
Figure 2.
Timeline and effects of Coversin. (A) Timeline of events including GVHD and MAHA. (B) Effect of increasing concentrations of Coversin and eculizumab on CH50 in patient and normal control serum. (C) CH50 during treatment with alternate day and daily Coversin. (D) LDH levels (U/L) and reticulocyte count (103/mm3) during treatment with eculizumab and Coversin. Shaded areas in the boxes showing the duration of Coversin therapy indicate where daily therapy was given, and unshaded areas in the boxes indicate where alternate day therapy was given.
To determine whether C5 could be blocked by Coversin, ascending doses of eculizumab and Coversin were added to patient serum and pooled control serum. Terminal pathway activity was assessed by measurement of CH50 (Quidel, San Diego, CA). Coversin at ∼15 µg/mL, the known therapeutic concentration, completely blocked terminal complement activity (TCA) in both patient and control serum (Figure 2B). In contrast, eculizumab 50 µg/mL ablated TCA in control serum but was unable to completely inhibit TCA at any concentration tested in patient serum.
A request was made to Akari Therapeutics PLC for Coversin. Following parental consent, Akari supplied Coversin, and eculizumab was discontinued.
The quantity of Coversin available was limited; treatment was commenced on D+214 with a blocking subcutaneous dose of 0.57 mg/kg (7.63 mg) and thereafter a daily maintenance dose of 28% of this (2.18 mg) for the next 10 days until D+224. The initial clinical response was encouraging with an improvement in his GI symptoms with a decrease in the volume of diarrhea and resolution of melena. After D+224, the frequency was decreased to alternate days to conserve the supply. Twelve doses of 2.18 mg were given on alternate days until D+248. CH50 assays showed that despite the clinical improvement blockade of TCA was not complete on alternate day administration (Figure 2C). A further dose of 7.63 mg Coversin was therefore given on D+250 followed by 2.18 mg daily for 6 days at which time no further Coversin was available. With this, the CH50 decreased to beneath the lower limit of normal of 70 equivalent units per milliliter (CH50 UEq/mL). Over the next 21 days, the patient's condition deteriorated, and he died on D+277. At postmortem, there was evidence of severe pulmonary arterial vasculopathy (Figure 1D-E). There was hemorrhagic necrosis of the small bowel with arterial fibrinoid necrosis and intraluminal thrombi resultant from TMA (Figure 1F-H). Because of autolysis, it was not possible to determine whether there was a renal TMA. The lowest estimated glomerular filtration rate estimated using the “bedside” Schwartz equation13 was 38 mL/min/1.73 m2 on D+270. During the 58 days that the patient received Coversin, there were no adverse events and no injection site reactions. Low-titer nonneutralizing anti-Coversin antibodies (immunoglobulin G) were detected after 14 days of treatment. LDH levels and reticulocyte count during the period of time that the patient received both eculizumab and Coversin are shown in Figure 2D. Research within the report was approved by the North East–York Research Ethics Committee and performed in accordance with the Declaration of Helsinki.
Results and discussion
TMA is a well-established complication of HSCT.14 Pivotal to all forms of TMA is endothelial cell activation15; multiple factors, including infections, drugs, and GVHD, are likely responsible in HSCT-TMA. There is interest in the role of complement in all forms of TMA, including HSCT, driven by the finding of complement abnormalities in ∼50% of aHUS patients. The improved outcome in aHUS with eculizumab7 has led to enthusiasm for its use in other forms of TMA. That complement activation should be seen in TMA is not surprising in view of the interaction of the complement and coagulation pathways.16 In such circumstances, complement blockade with agents such as eculizumab may only be appropriate if factors are present that lead to impaired regulation of complement activation. Are such abnormalities present in HSCT-TMA? In 3 of 6 patients with HSCT-TMA, Jodele et al identified factor H autoantibodies.4 An association between factor H autoantibodies and aHUS is well established.17 Jodele et al found that 5 of the 6 patients with HSCT-TMA had a heterozygous deletion, including CFHR1.4 In our patient, we found a functionally significant CFH mutation (c.3356A>G; p.Asp1119Gly).11 Use of eculizumab was therefore appropriate. Because of the lack of response, we looked for factors that might be impairing the activity of the drug. Finding the C5 variant that confers eculizumab resistance was unexpected and led us to seek alternative agents to block C5.
Coversin is a 16.7-kDa recombinant protein derived from a natural protein discovered in the saliva of the Ornithodoros moubata tick.18 In vitro assays suggested that complement blockade could be achieved with Coversin. The crystal structure of Coversin bound to C5 indicates that Arg885His would not inhibit binding of the drug.19 When Coversin enters the circulation, it binds rapidly to circulating C5. Once bound, it assumes the half-life of C5, which in humans is reported to be 63 hours.20 Any free Coversin that is not bound to C5 is rapidly excreted by the kidney because of its low molecular weight. Experiments in C5-deficient mice show that virtually no Coversin can be detected within 30 minutes of IV administration.9 C5 is rapidly synthesized by the liver and other tissues and in the absence of any circulating Coversin will restore hemolytic activity in humans within ∼24 hours of administration of Coversin.
To assess the response to eculizumab and Coversin, we used both the clinical status and the markers of MAHA. The clinical response and improvement in LDH and reticulocyte count suggest that introduction of Coversin was beneficial. However, the supply of Coversin was limited, leaving a small window of opportunity to reverse the TMA. Despite the eventual outcome, this unique case provides proof of principle that alternative C5 inhibitors may be as effective as eculizumab in patients with inherited forms of complement dysregulation. Coversin may have a particular role in those patients who are resistant to eculizumab due to C5 variants such as c.2654G>A; p.Arg885His.
The previous finding of factor H autoantibodies and now in our patient a functionally significant CFH mutation does suggest that some patients with HSCT-TMA will have an underlying complement abnormality. In larger cohorts of HSCT-TMA patients, the prevalence of such abnormalities has been found to be higher,21 emphasizing the potential role for complement inhibitors.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement number 305608 (EURenOmics).
Authorship
Contribution: T.H.J.G. wrote the paper; F.P., J.S., S.J.M., L.P., J.W.P., R.C., P.A., and P.V. contributed vital clinical and pathological information; and W.H.W.-D., J.-i.N., M.A.N., and I.M. performed research and analysed data.
Conflict-of-interest disclosure: Newcastle University has received fees from Alexion Pharmaceuticals and Akari Therapeutics PLC (UK) for lectures and consultancy undertaken by T.H.J.G. University College London has received unrestricted research grants from Akari Therapeutics PLC (UK). M.A.N. and W.H.W.-D. are employees of Akari Therapeutics PLC and hold share options in the company. The remaining authors declare no competing financial interests.
Correspondence: Timothy H. J. Goodship, Institute of Genetic Medicine, Central Pkwy, Newcastle upon Tyne NE1 3BZ, United Kingdom; e-mail: tim.goodship@ncl.ac.uk.
References
- 1.George JN, Li X, McMinn JR, Terrell DR, Vesely SK, Selby GB. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: a diagnostic dilemma. Transfusion. 2004;44(2):294-304. [DOI] [PubMed] [Google Scholar]
- 2.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118(6):1452-1462. [DOI] [PubMed] [Google Scholar]
- 3.Jodele S, Davies SM, Lane A, et al. . Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124(4):645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jodele S, Licht C, Goebel J, et al. . Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood. 2013;122(12):2003-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jodele S, Fukuda T, Vinks A, et al. . Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2014;20(4):518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jodele S, Fukuda T, Mizuno K, et al. . Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(2):307-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legendre CM, Licht C, Muus P, et al. . Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura J, Yamamoto M, Hayashi S, et al. . Genetic variants in C5 and poor response to eculizumab. N Engl J Med. 2014;370(7):632-639. [DOI] [PubMed] [Google Scholar]
- 9.Hepburn NJ, Williams AS, Nunn MA, et al. . In vivo characterization and therapeutic efficacy of a C5-specific inhibitor from the soft tick Ornithodoros moubata. J Biol Chem. 2007;282(11):8292-8299. [DOI] [PubMed] [Google Scholar]
- 10.Ruutu T, Barosi G, Benjamin RJ, et al. ; European Group for Blood and Marrow Transplantation; European LeukemiaNet. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92(1):95-100. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira VP, Herbert AP, Cortés C, et al. . The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J Immunol. 2009;182(11):7009-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards A, Buddles MR, Donne RL, et al. . Factor H mutations in hemolytic uremic syndrome cluster in exons 18-20, a domain important for host cell recognition. Am J Hum Genet. 2001;68(2):485-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Muñoz A, Schneider MF, et al. . New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shulman H, Striker G, Deeg HJ, Kennedy M, Storb R, Thomas ED. Nephrotoxicity of cyclosporin A after allogeneic marrow transplantation: glomerular thromboses and tubular injury. N Engl J Med. 1981;305(23):1392-1395. [DOI] [PubMed] [Google Scholar]
- 15.Ballermann BJ. Endothelial cell activation. Kidney Int. 1998;53(6):1810-1826. [DOI] [PubMed] [Google Scholar]
- 16.Huber-Lang M, Sarma JV, Zetoune FS, et al. . Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682-687. [DOI] [PubMed] [Google Scholar]
- 17.Moore I, Strain L, Pappworth I, et al. . Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood. 2010;115(2):379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunn MA, Sharma A, Paesen GC, et al. . Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J Immunol. 2005;174(4):2084-2091. [DOI] [PubMed] [Google Scholar]
- 19.Fredslund F, Laursen NS, Roversi P, et al. . Structure of and influence of a tick complement inhibitor on human complement component 5. Nat Immunol. 2008;9(7):753-760. [DOI] [PubMed] [Google Scholar]
- 20.Sissons JG, Liebowitch J, Amos N, Peters DK. Metabolism of the fifth component of complement, and its relation to metabolism of the third component, in patients with complement activation. J Clin Invest. 1977;59(4):704-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jodele S, Zhang K, Zou F, et al. . The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood. 2016;127(8):989-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.