Abstract
Pheromone cues are an important component of intersexual communication, particularly in regards to mate choice. Caenorhabditis nematodes predominant rely on pheromone production for mate finding and mate choice. Here we describe a new microfluidic paradigm for studying mate choice in nematodes. Specifically, the Pheromone Arena allows for a constant flow of odorants, including pheromones and other small molecules, to be passed in real time from signaling worms to those making a choice without any physical contact. We validated this microfluidic paradigm by corroborating previous studies in showing that virgin C. remanei and C. elegans males have a strong preference for virgin females over mated ones. Moreover, our results suggest that the strength of attraction is an additive effect of male receptivity and female signal production. We also explicitly examine female choice and find that females are more attracted to virgin males. However, a female’s mate choice is strongly dependent on her mating status.
Introduction
A critical component of sexual reproduction is the ability to find and recognize the appropriate individual with which to mate. Individuals must be able to distinguish members of their own species—namely, conspecifics—from those of other species—heterospecifics. Perhaps equally important is the ability to choose high quality individuals that are receptive to mating. The process of mate choice is shaped by sexual selection and relies on communication between the sexes [1–3]. In particular, sex pheromones—small chemicals produced by a signaler to induce a sexual response in a receiver—are a major means of intersex communication across a wide variety of both invertebrate and vertebrate taxa (reviewed in [4]). Some of the best studied sex pheromones are the cuticular hydrocarbon family found in many insect species [5,6]. These pheromones have both species-specific and sex-specific effects [6]. For example, in Drosophilae female hydrocarbons attract males, while male hydrocarbons have an anti-aphrodisiac effect on other males and increase female receptivity [7–9]. While these studies highlight the importance of pheromones in mate choice, many of the taxa studied have additional behaviors and traits that contribute to the mate choice process, potentially confounding the relative reliance on pheromone-based cues.
Caenorhabditis nematodes rely almost exclusively on pheromone signals for mate choice [10,11]. Pheromone signals in Caenorhabditis have traditionally been studied using plate-based chemotaxis assays, where male attraction is quantified based on his ability to discriminate between a control medium and a female-conditioned medium [12,13]. In particular, these studies have identified ascaroside pheromones as important in mate choice signaling due to their sex-specific production and effects on sexually-associated behaviors [14,15]. Female pheromones act as a male attractant in both hermaphroditic C. elegans [12] and gonochoristic C. remanei [16]. This female-based signaling appears to be related to the amount of sperm stored [16–18] and targets male-specific neurons [13,14,16,19,20]. Recent work has turned to male-produced sex pheromones, showing that hermaphrodites exposed to male pheromones alone have a decrease in lifespan [21–24]. However, such male-produced pheromones do not appear to elicit a female mate choice response [12,16,22]. While ascarosides play a predominant role in mate choice, they also influence other population behaviors, thus necessitating a precise combination and concentration of multiple small molecules for signaling specific to male-female interactions [11,14]. Since pheromone cues depend on such a precise mixture, accurate intersex communication assays necessitate a well-controlled environment where the concentration and diffusion of molecules is clearly defined and external signals are limited.
Microfluidic technology has proved to be an excellent method in which to study behavioral responses under precise environmental control [25–27]. Microfluidic devices scale on the nano- to micro-size and thus the small size of Caenorhabditis makes them suitable for manipulation within a microfluidic environment. Microfluidics offers many advantages over traditional plate-based assays, including better control of the concentration of molecules and their diffusion due to the laminar properties of microfluidics [28]. Several microfluidic devices have been designed to study behavioral responses such as chemotaxis, thermotaxis, and electrotaxis [25–27,29,30]. With respect to reproductive behavior, Chung et al. [29] found a behavioral response of individually isolated males when exposed to a uniform concentration of hermaphrodite-conditioned medium. In particular, they showed that males exposed to pre-conditioned medium spent more time performing sexually-associated behaviors than those exposed to a control medium. While their microfluidic device overcomes the issues of pheromone diffusion on agar plates, it cannot be used to study mate-choice searching patterns [20,31] or direct choice comparisons between different attractants. Additionally, the pheromone signal contained in the pre-conditioned media is likely to decrease over the time required to study such locomotion patterns. Moreover, conditioned medium may be difficult to accurately reproduce as the concentration of small molecules will depend on the density of worms used to produce pheromones as well as the time spent in liquid culture. Given the limitations of exposure to a single pre-conditioned medium, the relationship between a pheromone signal and the elicited sexual response should be studied in real time.
We describe a new microfluidic paradigm, using the Pheromone Arena microfluidic device, that overcomes the current limitations of traditional plate-based assays and existing microfluidic technology to study sex-specific mate choice with constant exposure to pheromones produced in real time. Here we use a broad definition of pheromone to include any odorant signal produced, including but not limited to ascarosides, as used in previous studies [16,24,32]. We show that the Pheromone Arena allows for small-molecule communication alone without a decay of signal over time, thus validating its use for mate choice assays. Using the Pheromone Arena, we show that males are more attracted to virgin females than mated ones in both C. remanei and C. elegans with the degree of attraction being species-dependent. Additionally, we show that females rely on pheromone cues, though their preference is dependent on mating status.
Materials and methods
Worm culture and strains
Wildtype Caenorhabditis remanei (strain EM464) and feminized C. elegans (strain JK574: fog-2(q71) mutation on the standard N2 laboratory background) were used in this study. Feminization of C. elegans is achieved by blocking self-sperm production in hermaphrodites, making them functionally female, and they will be referred to thusly. Use of feminized C. elegans hermaphrodites allowed for direct comparisons between species as well as preventing any potential mate cue effects due to self-sperm [17,18]. Both strains were grown at 20°C on NGM-agar plates seeded with OP50 Escherichia coli bacteria following Brenner [33]. Synchronized cultures of stage 1 larvae were prepared by hypochlorite treatment of gravid females [34]. Larvae were matured to young adulthood in population densities of approximately 1,000 individuals. To maintain virgins, males and females were separated onto sex-specific plates of 40–50 individuals 40–45 hours post-larval stage 1. Mating plates of 25 females and 25 males were created at the same time. At the start of all the choice assays, day 1 adult virgins were 48 hours post-larval stage 1 and day 2 adults were 72 hours post-larval stage 1 (24 hours after separation to virgin or mating plates).
Microfluidic device manufacturing
The Pheromone Arena (final design: v2.1; S1 File) was designed using CAD software (Vectorworks 2013 SP5, Nemetschek Vectorworks, Inc). Single layer devices were fabricated out of polydimethylsiloxane (PDMS) following soft lithography methods [35] and bonded to a glass microscopy slide following exposure to air plasma. Holes for connecting tubing were punched using a 1.25mm biopsy punch.
Microfluidic set-up and experimental protocol
To avoid blocking flow, air was evacuated from each device using a vacuum chamber and replaced with M9 buffer. Worms were loaded into three chambers: ten worms each were loaded into the two upstream “signaling” chambers of the device and 20 to 25 worms were loaded into the single downstream “choice” chamber. To control for environmental and observational biases, worm combinations were alternatively loaded into the left and right upstream chambers.
Liquid was flowed through all inlets continuously throughout the experiment, with the start of flow corresponding to the beginning of an experiment. Flow was maintained at a constant rate using a pressurized air system (S1 Fig). Specifically, air exiting a pressurized one gallon tank was regulated to 1.5 PSI. The air-line running from the tank was bifurcated to two tubing lines, each pressurizing a sealed 500mL bottle of M9. The bottle caps were modified to hold seven pieces of tubing: one for the air-line (terminating at the cap) and six liquid supply lines. The liquid tubing lines extended below the M9 surface, allowing liquid to flow out of the bottle and into the microfluidic device once air pressure was applied from the air-line. Tubing lengths were equal for each partition of the set-up to maintain equal flow through all lines. Pressure could be maintained for three hours off a single air tank.
The head position of each worm in the downstream chamber was counted every 30 minutes and recorded as being located under the left or right upstream chamber. Worms located in the filter separators were not counted. If worms were flushed out of the downstream chamber (and therefore the device), the assay continued without counting these worms and thus some replicates had a decrease in sample size over time. If worms climbed or were flushed into a different chamber from the one into which they were loaded, the experimental run was terminated. Each choice combination was replicated multiple times (n ≥ 3) over multiple days (n ≥ 2). Day 2 adult worms were used for the male choice assays. Day 1 adult worms were used for female choice assays, except when comparing virgin versus mated female choice, which required using day 2 adult females. All data have been made available as supplementary material (S2 File).
Statistical analyses
Data were analyzed in R v3.2.1 (R Core Development Team 2015). Replicates were pooled for each choice assay by time point. An equality of proportions test was performed for each assay individually to determine if: i) males were more attracted to virgin females over mated females or ii) females were more attracted to virgin males over virgin females. The null hypothesis was that of no choice (using a probability of success = 0.5). A chi-square test for homogeneity was preformed to determine if the proportion of males or females choosing virgin females or males, respectively, was equal across species combinations.
Results
Microfluidic design
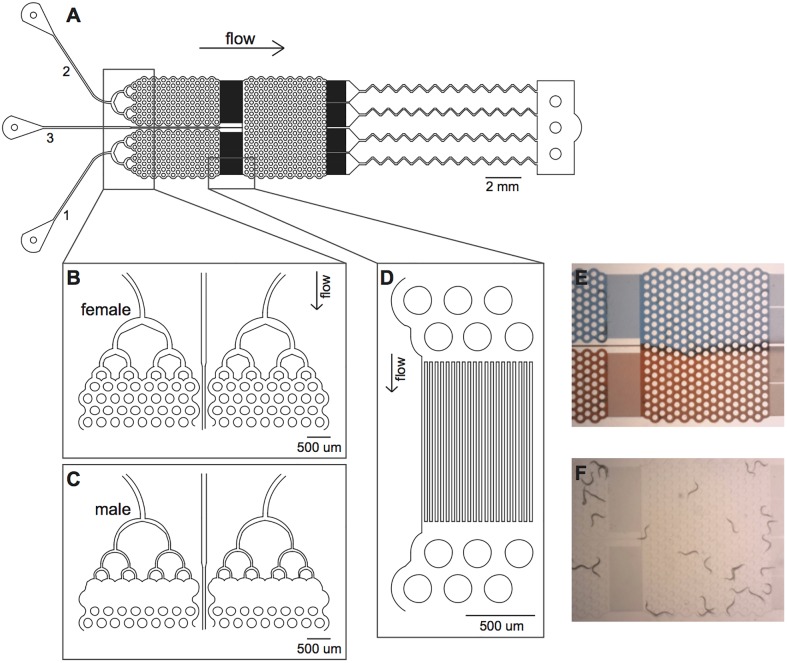
The Pheromone Arena has three sequential components: three inlets with each with a loading network, a main arena, and a single high resistance outlet (Fig 1A). The arena area is further divided into three physically distinct chambers. The chambers all have a pillar-array to facilitate the natural, sinusoidal movement of worms [36,37]. The two upstream chambers hold the pheromone signalers. Two versions of the bifurcating distribution network for loading worms were created to accommodate the size differences between males and females at day 2 of adulthood. Specifically, female signalers have a wider distribution network (Fig 1B), while male signalers have a narrower distribution network coupled with the removal of the first two rows of pillars to prevent males from climbing out of the chamber (Fig 1C). The upstream chambers are separated from the single, large downstream chamber by an 18um filter (Fig 1D). This filter allows for small molecules to pass, but rarely can adult worms pass through. Worms making a choice were loaded into the downstream chamber by a third inlet. Due to continuous laminar flow, two pheromone environments are created in the downstream chamber that mirror the signaling pair in the upstream chambers (Fig 1E). Continuous flow was also maintained through the downstream chamber inlet to prevent any “dead-zones” in the environment. All the chambers were greater than one-by-one worm length, allowing for free movement of individuals without density effects (Fig 1F).
Fig 1. Microfluidic Pheromone Arena for mate choice assays.
(A) Blueprint for the Pheromone Arena (v2.1; S1 File). The three inlets correspond to the three worm chambers: inlets 1 and 2 connect to the upstream chambers and inlet 3 connects to the downstream chamber. All the chambers have a pillar-array (shown as circles) spaced 100um apart to allow for natural worm movement. An 18um filter separates the downstream chamber from the upper two chambers and from the outlet. Extra resistance was added to the outlet distribution to decrease the overall flow rate. (B) Close-up of the distribution network for loading females into the upstream, signaling chambers. (C) An alternative loading distribution network for loading males as the pheromone signalers. (D) Close-up of the filter separating chambers. (E) Visualization of how the laminar flow dynamics create two distinct environments in the downstream chamber using red and blue dyes. (F) Visualization of worms in the device. After being loaded, worms move freely throughout their chamber without passing into another chamber.
No inherent biases were measured in the movement of worms in the downstream chamber. In particular, when no pheromone cue was present, males were equally likely to move to the left of right sides of the chamber in a random fashion (choice of left side: mean = 51.9%, 95% C.I. = 46.9–56.9%, p = 0.45, n = 24 replicates).
Males are attracted to virgin females
Virgin C. remanei and C. elegans males were assayed for their ability to discriminate between conspecific virgin and mated females. Male choice was measured approximately every 30 minutes for 3 hours. Across all replicates, male choice varied little after 60 minutes (S2 Fig). Therefore, we used data from this time point as a single comparative measure of male choice across assays.
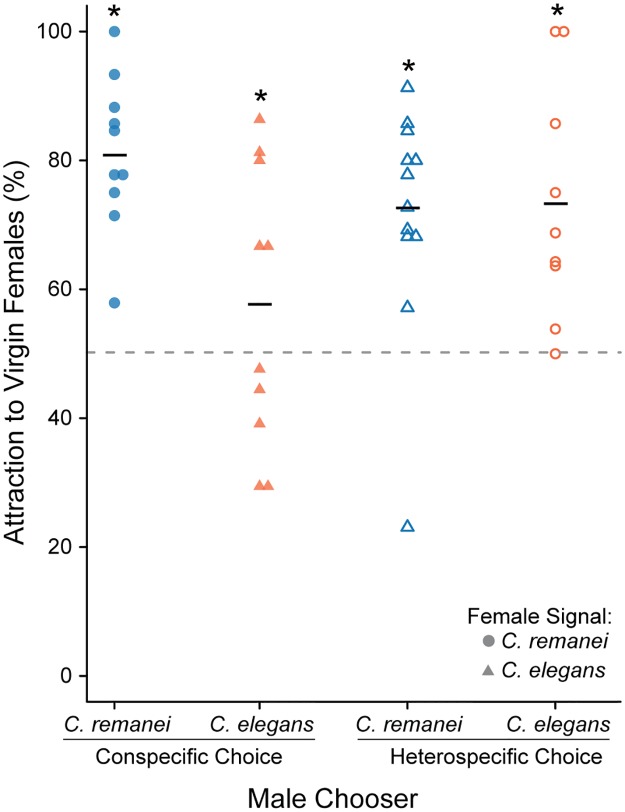
Males were more attracted to virgin females than mated females in both C. remanei (proportions test: χ2 = 46.2, d.f. = 1, p < 0.0001, 95% C.I. of virgin attraction = 72.6–87.1%) and C. elegans (proportions test: χ2 = 4.15, d.f. = 1, p = 0.04, 95% C.I. of virgin attraction = 50.3–64.8%), though the ability to discriminate was much weaker in C. elegans (Fig 2). To better understand this marked difference in male sensitivity, male choice was assayed for the ability to discriminate between virgin and mated heterospecific females. In both heterospecific crosses males were more attracted to virgin females than mated females (C. remanei male proportions test: χ2 = 42.0, d.f. = 1, p < 0.0001, 95% C.I. of virgin attraction = 66.1–78.7%; C. elegans male proportions test: χ2 = 24.2, d.f. = 1, p < 0.0001, 95% C.I. of virgin attraction = 64.1–80.9%). For example, C. remanei males chose virgin C. elegans females more successfully than in the conspecific C. elegans assay. Similarly, C. elegans males had a much high ability to discriminate virgins when they were presented with C. remanei females. The strength of attraction to virgins for both heterospecific assays was between that of the conspecific assays, suggesting species-dependent pheromone effects in both males and females (χ2 = 22.0, d.f. = 3, p < 0.0001).
Fig 2. Males are more attracted to virgin females than mated females within and between species.
Virgin, day 2 adult C. remanei males (blue) and C. elegans males (orange) were given a choice between virgin and mated female pheromone (C. remanei females shown as circles and C. elegans females shown as triangles). Each replicate is represented as an individual point and the mean attraction to virgin females is given by the horizontal bar. Conspecific assays are shown as solid point and heterospecific assays as open points. The null hypothesis of no choice is given by the dashed line. In each assay males were more attracted to virgin females than mated ones. However, the strength of male attraction was dependent on the species of both the chooser and the signaler (Test of homogeneity across all four assays: χ2 = 22.0, d.f. = 3, p < 0.0001). Asterisks denote a significant deviation from the null hypothesis within each signal-chooser combination.
Females choose male pheromone over those of from females
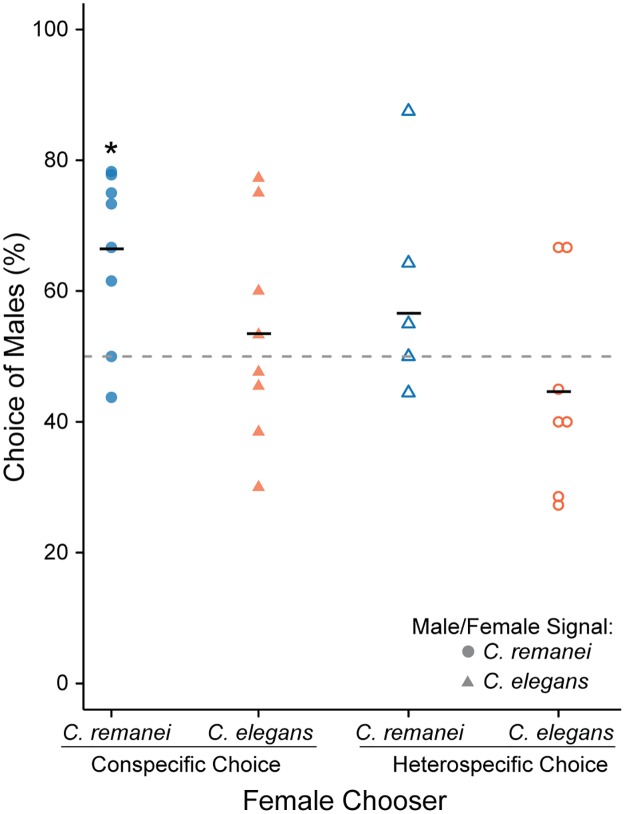
Virgin C. remanei and C. elegans females were assayed for their ability to discriminate between conspecific virgin males and females. Female choice increased slightly over time, but was consistent by 60 minutes, again making this time point a reflective measure of overall female choice (S3 Fig). Interestingly, C. remanei and C. elegans followed a similar trend in choice of males over time, though at very different magnitudes. Specifically, female C. remanei chose conspecific male pheromones over female pheromones (Fig 3; proportions test: χ2 = 14.8, d.f. = 1, p < 0.001, 95% C.I. of male attraction = 58.0–74.0%). When females were given a choice between male pheromone and no pheromone, they still chose the male pheromone more than expected by chance (χ2 = 7.19, d.f. = 1, p < 0.01), suggesting that females are in fact attracted to males and not simply repulsed by other females. However, C. elegans females showed no clear differentiation between conspecific males and females (proportions test: χ2 = 0.563, d.f. = 1, p = 0.45, 95% C.I. of male attraction = 45.0–61.8%). Moreover, the heterospecific assays also showed a lack of discrimination between male and female pheromones, suggesting that female choice may be species-specific, or at least very weak at best within C. elegans. In fact, C. elegans odorant production may be sufficiently weakly or absent, such no discrimination can be made and therefore random choice was measured.
Fig 3. Female choice of virgin males over virgin females is species-specific.
Virgin, day 1 adult C. remanei females (blue) and C. elegans females (orange) were given a choice between virgin male and female pheromone (C. remanei females shown as circles and C. elegans females shown as triangles). Each replicate is represented as an individual point and the mean attraction to males is given by the horizontal bar. Conspecific assays are shown as solid point and heterospecific assays as open points. The null hypothesis of no choice is given by the dashed line. Only when C. remanei females were given a choice of conspecifics were they more attracted to male pheromone than female pheromone (χ2 = 14.8, d.f. = 1, p < 0.001). All other comparisons failed to reject the null hypothesis of no female choice. Asterisks denote a significant deviation from the null hypothesis within each signal-chooser combination.
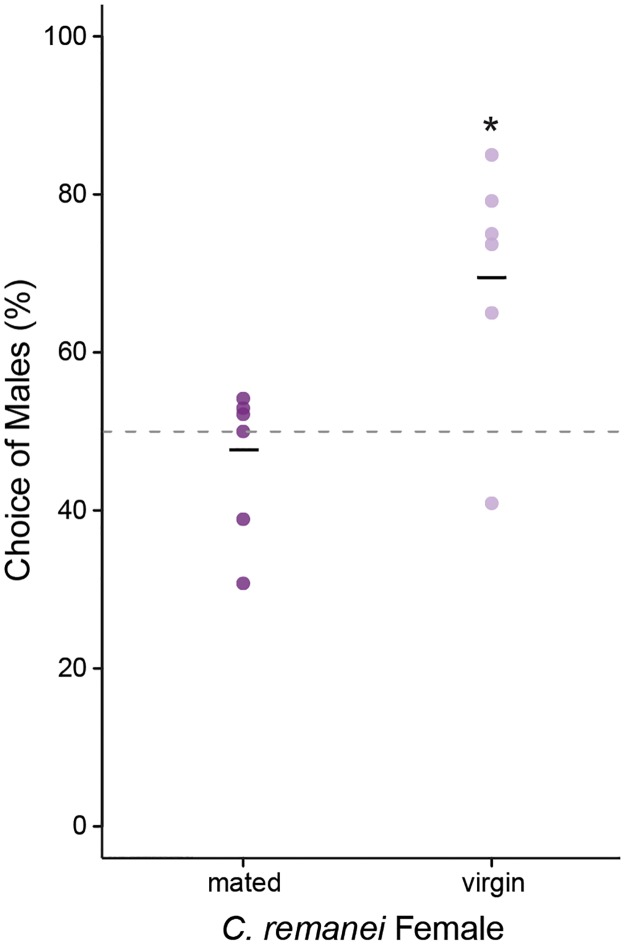
Additionally, we examined if female choice was dependent a female mating status. The female choice assay was replicated using mated and virgin C. remanei females. Virgin females showed a strong preference for virgin male over female pheromones (Fig 4; proportions test: χ2 = 17.5, d.f. = 1, p < 0.0001, 95% C.I. of male attraction = 60.3–69.4%). However, mated females showed no obvious preference (proportions test: χ2 = 0.150, d.f. = 1, p = 0.70, 95% C.I. of male attraction = 38.0–57.5%).
Fig 4. Female attraction depends on mating status.
Virgin (light purple) and mated (dark purple) day 2 adult C. remanei females were given a choice between virgin day 1 adult C. remanei male and female pheromones. Each replicate is represented as an individual point and the mean attraction to males is given by the horizontal bar. The null hypothesis of no choice is given by the dashed line. Virgin females showed a strong preference for males over females (χ2 = 17.5, d.f. = 1, p < 0.0001), while mated females displayed no choice (p = 0.70). Asterisks denote a significant deviation from the null hypothesis within each signal-chooser combination.
Discussion
Mate choice is ubiquitous across metazoans with sexual reproduction. Understanding the signal-receiver dynamics of mate choice—such as pheromones—provides valuable information on how individuals discern high quality mates with a propensity to mate. Here we proposed a new microfluidic paradigm to quantify pheromone communication in nematodes. Our design allows for real time production of pheromones in a controlled environment as well as allowing for the natural searching and choice behaviors of receivers over time. While this design is not the first to use pillared-arenas [36–38], to the best of our knowledge no other worm-specific microfluidic devices have such a physically separated, sequential arena design. Moreover, the Pheromone Arena expands on previous worm choice devices [29,30] by allowing for natural searching behaviors in addition to measuring overall choice. Specifically, we examined male and female mate choice in C. remanei and C. elegans using a combination of conspecific and heterospecific assays to determine how mating status affects attraction. This study is the first use this combinatorial design coupled with real time pheromone signaling and spatial complexity.
The conspecific male choice results support previous studies [16,18] in showing that virgin males are strongly attracted to virgin females over mated females in both C. elegans and C. remanei. Interestingly, males from these species did not discriminate between female mating types with the same intensity [16]. In particular, C. elegans males had a reduced ability to discern virgin females from mated ones. However, when C. elegans males were presented with pheromones from C. remanei females, male attraction to virgins increased. Similary, C. remanei males could distinguish between virgin and mated C. elegans females better than C. elegans males. Therefore, there appears to be a decrease in both female signal intensity as well as male receptor capability in C. elegans, leading to an overall decrease in mate choice ability. This difference in discrimination between species could result from different behavioral or molecular responses within a microfluidic environment, though no obvious differences were observed. A potentially more plausible explanation for this observed discrimination change is the independent lineage transition to self-fertilizing hermaphrodism in C. elegans. Since fertilization is predominantly by selfing and males are rare within populations, sexual selection—apparently including mate recognition dynamics—is greatly reduced.
This hypothesis could be further tested by altering the number of signalers in the upstream chambers. The pheromone arena allows for exact control over the number of worms producing pheromone and, given the constant flow dynamics, altering the number of worms would in effect alter the concentration of pheromone signal in the downstream chamber. Previous studies have used various concentrations of hermaphrodite-conditioned media and found different results in male attraction [16,18]. Our assays used the same number of females in both upstream chambers, however, modulating the number of females in each upstream chamber is promising future work.
Previous work has shown that females are attracted to isolated, male-produced ascaroside cues [15,39], however, this result has not been replicated when the signal is produced in vivo. We took a novel approach comparing female discrimination between male and female produced pheromones to determine if females are truly attracted to males or are simply repulsed by the presence of a high number of other females. A discernable choice of males over females was measured in the C. remanei conspecific assays. Additionally, females were attracted to male pheromone over a no pheromone control, suggesting this choice measured is true attraction to males. Despite making a choice, the intensity with which females choose males was much weaker than seen for the male choice assays. This weak attraction could potentially explain why previous plate-based assays—where male signals can be lost by diffusion or mixed with other signals from the environment—could not measure any female choice of males [13,16]. However, C. elegans females made no choice in the conspecific assays as was also seen for both heterospecific assays, suggesting species-specific effects. Together these results suggest that when sexual selection is strong, as in C. remanei, males take a more active searching approach, such that females are predominantly signalers with males as active receivers and searchers. This signal-receiver dynamic is somewhat of a sex-role reversal from traditional sexual selection models of male signaling and female receiving, though still consistent with anisogamy.
We further examined female choice based on a female’s mating status. Mating status is known to influence remating behavior in many species, such that mated females—or females with sperm—typically have a lower propensity to remate [40,41]. Moreover, mated females will run away from males to avoid remating [42]. These observations are consistent with our results, as virgin females strongly preferred male pheromones while mated females exhibited no clear choice when presented with both male and female pheromones.
While the Pheromone Arena is both an innovative and effective tool, there are several limitations to its use. Importantly, not all odorants produced are necessarily intentional signals or specific to male-female signaling. While we attempted to control for sex-specific effects and our results corroborate previous studies, we cannot exclude the potential that other small molecules were affecting worm position within the arena. This limitation could be overcome by concentrating and isolating small molecules from the device outflow using biochemical techniques and then directly testing the compounds identified, although such analysis was outside the scope of this study. Additionally, the difference in size between older adult males and females poses a problem for intersex comparisons. In particular, the design could be improved to further limit worms from reaching other chambers through the filter separators. This cross-chamber movement was particularly an issue with males, which have a small diameter and a highly-developed searching behavior that leads them to attempt to crawl through the filters to reach the virgin females. However, the filter between the upstream and downstream chambers is currently at the lower limit of what can accurately be manufactured using PDMS-based microfluidics and thus a significant design change would be required to prevent this tenacious behavior.
Despite these limitations, the Pheromone Arena was able to reproduce previously seen sexual behavior responses and go further into the study of species-specific sexual attraction in both males and females. In the future, this type of device could be used to study the effect of density or sex ratio on sexual attraction as well as conduct behavioral analysis of mate choice. Moreover, it would be possible to modify these devices to allow food delivery and perform longer term assays or study the influence of food availability on sexual attraction. Such assays would benefit from being coupled with an automated system [43] or image tracking software [38] to obtain a more accurate counting via recordings and to facilitate high-throughput experiments.
Supporting information
The Pheromone Arena has two versions to account for the difference in size between day 2 adult males and females: male choice of female signalers and female choice of male signalers. In the male choice version, the upstream loading distribution network is sized at 60um to account for the larger diameter of females, while the downstream chamber distribution channel has a final constriction size of 35um to prevent males from climbing back out of the downstream chamber. The female choice version has a smaller upstream distribution network (40um) and a larger downstream chamber distribution (60um). Additionally, in the female choice version the two upstream chambers have the first two rows of pillars removed, again to prevent males from climbing out of the device. Both versions have increased resistance added to the outflow to decreased the overall flow rate through the device. These blueprints are accessible using CAD software. A master height of 65um is recommended.
(EPS)
Raw data for all male choice, female choice, and control experiments.
(XLSX)
A pressurized air system was used to maintain a constant flow rate through the microfluidic devices. A one gallon air tank was regulated to 1.5 PSI was sufficient to run experiments for up to 3 hours. The air-line running from the tank was bifurcated to pressurize two sealed 500mL bottles of M9 buffer. The bottle caps were modified to supply six liquid lines as well as hold the air-line, which terminated at the cap. Since the Pheromone Arena requires three liquid inlet lines, each M9 bottle could run two devices and therefore a single one gallon air tank could simultaneously run four devices. The tubing lengths were equal for each partition of the set-up to maintain equal flow through all lines. The Pheromone Arena was kept on a confocal microscope at 20°C for the duration of each experiment. This figure was modified with permission from Stephen Banse.
(TIFF)
Virgin, day 2 adult C. remanei males were given a choice between virgin and mated conspecific female pheromone (blue) and C. elegans males were given a choice between virgin and mated conspecific female pheromone (orange). The weighted means and standard error are plotted over time. By 60 minutes into the experiment males had made a consistent choice (down triangle). The null hypothesis of no choice is given by the dashed line. In each assay males were more attracted to virgin females than mated ones.
(TIF)
Virgin, day 1 adult C. remanei females were given a choice between virgin conspecific male and female pheromones (blue) and C. elegans males were given a choice between virgin conspecific male and female pheromones (orange). The weighted means and standard error are plotted over time. The null hypothesis of no choice is given by the dashed line. Only C. remanei females made a measurable choice of male pheromone over female pheromone.
(TIF)
Acknowledgments
We would like to thank Nadine Timmermeyer for prototyping Pheromone Arena v1.0, Ben Blue for assistance with microfluidic manufacturing and advice on the pressurized air system, and Michelle Norris for statistical advice. We would also like to thank Nick Stroustrop and an anonymous reviewer for constructive comments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Institutes of Health (training grant T32 GM007413 to KRK and R01 GM102511 to PCP) and the ARCS Foundation Oregon Chapter (KRK).
References
- 1.Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol Evol. 2006;21: 296–302. doi: 10.1016/j.tree.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 2.Jones AG, Ratterman NL. Mate choice and sexual selection: what have we learned since Darwin? Proc Natl Acad Sci USA 2009;106 Suppl 1: 10001–10008. doi: 10.1073/pnas.0901129106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson MB. Sexual selection. Princeton University Press; 1994. [Google Scholar]
- 4.Wyatt TD. Pheromones and animal behavior: communication by taste and smell. Cambridge University Press; 2003. [Google Scholar]
- 5.Howard RW, Blomquist GJ. Chemical ecology and biochemistry of insect hydrocarbons. Annu Rev Entomol. 1982;27: 149–172. [DOI] [PubMed] [Google Scholar]
- 6.Howard RW, Blomquist GJ. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol. 2005;50: 371–393. doi: 10.1146/annurev.ento.50.071803.130359 [DOI] [PubMed] [Google Scholar]
- 7.Grillet M, Dartevelle L, Ferveur J-F. A Drosophila male pheromone affects female sexual receptivity. Proc Royal Soc B. 2006;273: 315–323. doi: 10.1098/rspb.2005.3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferveur J-F. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35: 279–295. doi: 10.1007/s10519-005-3220-5 [DOI] [PubMed] [Google Scholar]
- 9.Brent CS, Byers JA, Levi-Zada A. An insect anti-antiaphrodisiac. eLife 2017;6: 1765 doi: 10.7554/eLife.24063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barr M. Male mating behavior WormBook. 2006: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chute CD, Srinivasan J. Chemical mating cues in C. elegans. Sem Cell Dev Biol 2014;33: 18–24. doi: 10.1016/j.semcdb.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 12.Simon JM, Sternberg PW. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc Natl Acad Sci USA 2002;99: 1598–1603. doi: 10.1073/pnas.032225799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White JQ, Nicholas TJ, Gritton J, Truong L, Davidson ER, Jorgensen EM. The sensory circuitry for sexual attraction in C. elegans males. Curr Biol. 2007;17: 1847–1857. doi: 10.1016/j.cub.2007.09.011 [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PEA, et al. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454: 1115–1118. doi: 10.1038/nature07168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izrayelit Y, Srinivasan J, Campbell SL, Jo Y, Reuss von SH, Genoff MC, et al. Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem Biol. 2012;7: 1321–1325. doi: 10.1021/cb300169c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chasnov JR, So WK, Chan CM, Chow KL. The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc Natl Acad Sci USA. 2007;104: 6730–6735. doi: 10.1073/pnas.0608050104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morsci NS, Haas LA, Barr MM. Sperm status regulates sexual attraction in Caenorhabditis elegans. Genetics. 2011;189: 1341–1346. doi: 10.1534/genetics.111.133603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leighton DHW, Choe A, Wu SY, Sternberg PW. Communication between oocytes and somatic cells regulates volatile pheromone production in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2014;111: 17905–17910. doi: 10.1073/pnas.1420439111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narayan A, Venkatachalam V, Durak O, Reilly DK, Bose N, Schroeder FC, et al. Contrasting responses within a single neuron class enable sex-specific attraction in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2016;113: E1392–401. doi: 10.1073/pnas.1600786113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrios A, Nurrish S, Emmons SW. Sensory regulation of C. elegans male mate-searching behavior. Curr Biol. 2008;18: 1865–1871. doi: 10.1016/j.cub.2008.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aprison EZ, Ruvinsky I. Sexually antagonistic male signals manipulate germline and soma of C. elegans hermaphrodites. Curr Biol. 2016. doi: 10.1016/j.cub.2016.08.024 [DOI] [PubMed] [Google Scholar]
- 22.Shi C, Runnels AM, Murphy CT. Mating and male pheromone kill Caenorhabditis males through distinct mechanisms. eLife. 2017;6: S62 doi: 10.7554/eLife.23493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong L, Cornaglia M, Lehnert T, Gijs MAM. On-chip microfluidic biocommunication assay for studying male-induced demise in C. elegans hermaphrodites. Lab Chip. Royal Society of Chemistry; 2016;16: 4534–4545. doi: 10.1039/C6LC01005A [DOI] [PubMed] [Google Scholar]
- 24.Maures TJ, Booth LN, Benayoun BA, Izrayelit Y, Schroeder FC, Brunet A. Males shorten the life span of C. elegans hermaphrodites via secreted compounds. Science. 2014;343: 541–544. doi: 10.1126/science.1244160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta BP, Rezai P. Microfluidic approaches for manipulating, imaging, and screening C. elegans. Micromachines. 2016;7: 1–26. doi: 10.3390/mi7070123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakhtina NA, Korvink JG. Microfluidic laboratories for C. elegans enhance fundamental studies in biology. Rsc Advances. 2014;4: 4691–4709. doi: 10.1039/C3RA43758B [Google Scholar]
- 27.Ben-Yakar A, Chronis N, Lu H. Microfluidics for the analysis of behavior, nerve regeneration, and neural cell biology in C. elegans. Curr Opinion Neurobiol. 2009;19: 561–567. doi: 10.1016/j.conb.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Squires TM, Quake SR. Microfluidics: Fluid physics at the nanoliter scale. Rev Mod Phys. 2005;77: 977–1026. doi: 10.1103/RevModPhys.77.977 [Google Scholar]
- 29.Chung K, Zhan M, Srinivasan J, Sternberg PW, Gong E, Schroeder FC, et al. Microfluidic chamber arrays for whole-organism behavior-based chemical screening. Lab Chip. 2011;11: 3689–3697. doi: 10.1039/c1lc20400a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCormick KE, Gaertner BE, Sottile M, Phillips PC, Lockery SR. Microfluidic devices for analysis of spatial orientation behaviors in semi-restrained Caenorhabditis elegans. PLoS ONE. 2011;6: e25710–8. doi: 10.1371/journal.pone.0025710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipton J, Kleemann G, Ghosh R, Lints R, Emmons SW. Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate. J Neurosci. 2004;24: 7427–7434. doi: 10.1523/JNEUROSCI.1746-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aprison EZ, Ruvinsky I. Sex Pheromones of C. elegans Males prime the female reproductive system and ameliorate the effects of heat stress. PLoS Genetics. 2015;11: e1005729–19. doi: 10.1371/journal.pgen.1005729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenyon C. The nematode Caenorhabditis elegans. Science. 1988;240: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 35.Qin D, Xia Y, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nature Protocols. 2010;5: 491–502. doi: 10.1038/nprot.2009.234 [DOI] [PubMed] [Google Scholar]
- 36.Lockery SR, Lawton KJ, Doll JC, Faumont S, Coulthard SM, Thiele TR, et al. Artificial dirt: Microfluidic substrates for nematode neurobiology and behavior. J Neurophys. 2008;99: 3136–3143. doi: 10.1152/jn.91327.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park S, Hwang H, Nam S-W, Martinez F, Austin RH, Ryu WS. Enhanced Caenorhabditis elegans locomotion in a structured microfluidic environment. PLoS ONE. 2008;3: e2550–5. doi: 10.1371/journal.pone.0002550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albrecht DR, Bargmann CI. High-content behavioral analysis of Caenorhabditis elegans in precise spatiotemporal chemical environments. Nat Meth. 2011;8: 599–605. doi: 10.1038/nmeth.1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srinivasan J, Reuss von SH, Bose N, Zaslaver A, Mahanti P, Ho MC, et al. A Modular Library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012;10: e1001237–14. doi: 10.1371/journal.pbio.1001237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity. 2011;87: 511–521. [DOI] [PubMed] [Google Scholar]
- 41.Yamane T, Goenaga J, Rönn JL, Arnqvist G. Male seminal fluid substances affect sperm competition success and female reproductive behavior in a seed beetle. PLoS ONE. 2015;10: e0123770–14. doi: 10.1371/journal.pone.0123770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia LR, LeBoeuf B, Koo P. Diversity in mating behavior of hermaphroditic and male-female Caenorhabditis nematodes. Genetics. 2007;175: 1761–1771. doi: 10.1534/genetics.106.068304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stroustrup N, Ulmschneider BE, Nash ZM, López-Moyado IF, Apfeld J, Fontana W. The Caenorhabditis elegans lifespan machine. Nat Meth. 2013;10: 665–670. doi: 10.1038/nmeth.2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Pheromone Arena has two versions to account for the difference in size between day 2 adult males and females: male choice of female signalers and female choice of male signalers. In the male choice version, the upstream loading distribution network is sized at 60um to account for the larger diameter of females, while the downstream chamber distribution channel has a final constriction size of 35um to prevent males from climbing back out of the downstream chamber. The female choice version has a smaller upstream distribution network (40um) and a larger downstream chamber distribution (60um). Additionally, in the female choice version the two upstream chambers have the first two rows of pillars removed, again to prevent males from climbing out of the device. Both versions have increased resistance added to the outflow to decreased the overall flow rate through the device. These blueprints are accessible using CAD software. A master height of 65um is recommended.
(EPS)
Raw data for all male choice, female choice, and control experiments.
(XLSX)
A pressurized air system was used to maintain a constant flow rate through the microfluidic devices. A one gallon air tank was regulated to 1.5 PSI was sufficient to run experiments for up to 3 hours. The air-line running from the tank was bifurcated to pressurize two sealed 500mL bottles of M9 buffer. The bottle caps were modified to supply six liquid lines as well as hold the air-line, which terminated at the cap. Since the Pheromone Arena requires three liquid inlet lines, each M9 bottle could run two devices and therefore a single one gallon air tank could simultaneously run four devices. The tubing lengths were equal for each partition of the set-up to maintain equal flow through all lines. The Pheromone Arena was kept on a confocal microscope at 20°C for the duration of each experiment. This figure was modified with permission from Stephen Banse.
(TIFF)
Virgin, day 2 adult C. remanei males were given a choice between virgin and mated conspecific female pheromone (blue) and C. elegans males were given a choice between virgin and mated conspecific female pheromone (orange). The weighted means and standard error are plotted over time. By 60 minutes into the experiment males had made a consistent choice (down triangle). The null hypothesis of no choice is given by the dashed line. In each assay males were more attracted to virgin females than mated ones.
(TIF)
Virgin, day 1 adult C. remanei females were given a choice between virgin conspecific male and female pheromones (blue) and C. elegans males were given a choice between virgin conspecific male and female pheromones (orange). The weighted means and standard error are plotted over time. The null hypothesis of no choice is given by the dashed line. Only C. remanei females made a measurable choice of male pheromone over female pheromone.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.