Abstract
BACKGROUND:
Nonalcoholic fatty liver disease, the hepatic manifestation of metabolic syndrome, is associated with increased risk of colorectal adenoma, a precursor of colorectal cancer. Because nonalcoholic fatty liver disease and colorectal adenoma share many common risk factors of metabolic syndrome, the association between these 2 pathological findings has been investigated in multiple studies, but the results have been conflicting.
OBJECTIVE:
The present study aimed to assess the relationship between the fatty liver index, a predictor of nonalcoholic fatty liver disease, and the prevalence of colorectal adenomas.
DESIGN:
This is a retrospective observational study.
SETTINGS:
This study was conducted at a single expert center.
PATIENTS:
A total of 2976 consecutive subjects over 40 years of age undergoing routine checkups including abdominal ultrasonography and colonoscopy at Chung-Ang University Hospital Health Care Center were included.
MAIN OUTCOME MEASURES:
The primary outcome measured was the prevalence of colorectal adenomas according to fatty liver index.
RESULTS:
Among these subjects, 932 (31.3%) had colorectal adenoma, 691 (23.2%) had metabolic syndrome, and 1512 (50.8%) had fatty liver on ultrasonography. In multivariate analysis, fatty liver index ≥30 was associated with an increased risk of colorectal adenoma (OR, 1.269; 95% CI, 1.06–1.49; p = 0.008). The fatty liver index-high group (fatty liver index ≥30) had more colorectal adenomas and more advanced colorectal adenomas than the fatty liver index-low group (fatty liver index <30) (p < 0.001 and p = 0.042). The prevalence of colorectal adenomas increased with increasing quartile of fatty liver index (p < 0.05).
LIMITATIONS:
The study was limited by a relatively healthy Asian population.
CONCLUSION:
The high fatty liver index may be a useful predictor of colorectal adenoma. See Video Abstract at http://links.lww.com/DCR/A478.
Keywords: Cancer screening, Colonoscopy, Colorectal adenoma, Fatty liver index
Colorectal cancer (CRC) is the second most prevalent cancer and the leading cause of cancer death worldwide.1 The majority of CRCs develop via the adenoma-carcinoma sequence. Colorectal adenomas are increasingly prevalent in Korea.2,3 Adenomatous colorectal polyps are considered to be precursors of CRC. Because of this, colonoscopy with polypectomy if polyps are identified permits both for screening and prevention of CRC.2,4 Screening for CRC should be initiated at the age of 50 years in an asymptomatic average-risk population. Factors other than age, such as family history, sex, race, smoking, and obesity can also affect CRC risk.5,6
Epidemiological studies have reported metabolic syndrome (MetS) to be associated with an increased colorectal adenoma risk.3 Furthermore, nonalcoholic fatty liver disease (NAFLD) is commonly referred to as the hepatic manifestation of MetS, and its prevalence is almost certainly increasing.7
Nonalcoholic fatty liver disease encompasses a broad array of liver pathology, extending from simple steatosis to nonalcoholic steatohepatitis (NASH); some patients with NAFLD even develop liver fibrosis, with some progressing on to cirrhosis.8
A noninvasive method of predicting NAFLD, the “fatty liver index (FLI)” is calculated by using 4 variables: BMI, waist circumference, triglycerides (TGs), and γ-glutamyltransferase (GGT). The FLI on validation has been found to have a high accuracy rate in detecting NAFLD (0.84; 95% CI, 0.81–0.87).9 This algorithm has already been applied to a large population with high diagnostic accuracy in the detection of fatty liver.10,11
There is consensus among many studies that NAFLD is closely related to MetS, or to single-feature MetS, such as obesity, dyslipidemia, and type 2 diabetes mellitus.12 Because NAFLD and colorectal adenoma share many risk factors, many studies have investigated the association between these 2 entities with conflicting results. Several recent studies have revealed that NAFLD is associated with an increased risk of colorectal adenoma.13,14 One cross-sectional study showed that NASH patients had a higher prevalence of colorectal adenomas (51.0% vs 25.6%; p = 0.005) and advanced colorectal neoplasms (34.7% vs 14.0%; p = 0.011) than those with simple steatosis.13
Therefore, the aim of the present study was to assess the relationship between the FLI, a predictor of NAFLD, and the prevalence of colorectal adenoma, the GI manifestation of MetS. In addition, we intended to elucidate the clinical usefulness of FLI in screening of CRC.
MATERIALS AND METHODS
Study Population
We conducted a study on a consecutive series of subjects over the age of 40 years participating in a routine checkup program at Chung-Ang University Hospital Health Care Center from January 2008 to December 2013. All subjects underwent physical examinations (height, body weight, waist circumference, and blood pressure), blood tests (glucose, TG, high-density lipoprotein cholesterol, aspartate aminotransferase, alanine transaminase, GGT, total bilirubin, albumin, and platelets), abdominal ultrasonography, and colonoscopy. History of alcohol consumption was specifically investigated by extensive review of the patients’ medical records. We defined NAFLD as the presence of hepatic steatosis on ultrasound in the absence of increased alcohol use (>40 g alcohol per day for men or >20 g alcohol per day for women). Exclusion criteria were as follows: a history of colonic disease such as CRC, polyps, or IBD, colorectal surgery, or colonoscopy within the 10 previous years, and a family history of CRC. Subjects taking a statin or aspirin for ≥1 year were excluded to avoid the potential protective effect of aspirin and statins on colorectal adenomas. Finally, any subjects who had serologic evidence of hepatitis virus infection, or history of other liver disease, such as hemochromatosis, α-1 antitrypsin deficiency, Wilson disease, autoimmune hepatitis, primary biliary cirrhosis, or primary sclerosing cholangitis, were also excluded.
The Institutional Review Board approved this study (C2014024(1220)), and all participants provided written informed consent for the use of personal data for research.
Measurement of Anthropometric Parameters
Height and body weight measurements were automated (GL-150; G-Tech International Co, Uijungbu City, Korea, and Inbody720; Biospace Co, Chun-An City, Korea), and BMI was calculated as body weight divided by height squared (kg/m2).15 The waist circumference of each subject was measured by trained nurses at the midpoint between the lower borders of the rib cage and the upper pole of the iliac crest. Blood pressure was measured with a standard mercury sphygmomanometer in seated subjects after 5 minutes of rest. A venous blood sample was drawn after more than 12 hours of fasting, and serum levels of TG, high-density lipoprotein cholesterol, glucose, aspartate aminotransferase, alanine transaminase, GGT, albumin, and platelets were measured.
We calculated the FLI as follows to obtain scores between 0 and 100.9
 |
Abdominal Ultrasonography
Abdominal ultrasound scans were performed on all participants. We used the iU22 system (Philips Ultrasound, Bothell, WA) with a C5-1 pure wave transducer (5–1 MHz) or the LOGIQ E9 system (GE Medical Systems, Inc, Wauwatosa, WI) with a C1-5 transducer (1–6 MHz) to scan the liver.
The diagnosis of hepatic steatosis was made on the basis of 5 known criteria: namely, liver to kidney contrast, brightness of the liver parenchyma, deep beam attenuation, echogenic walls in the small intrahepatic vessels, and definition of the gallbladder wall. Based on these findings, and using a standardized algorithm, we categorized the degree of steatosis as a 4-level variable (none, mild, moderate, or severe).16,17
Colonoscopy
Colonoscopy was performed using a flexible colonoscope (CF-H260AL; Olympus, Tokyo, Japan) after bowel preparation. Various drugs for bowel preparation were used as follows.
We performed colonoscopies that reached the cecum after bowel preparation with 4 L of CoLyte powder (TAEJOON PHARM Co, Ltd, Seoul, Korea). The CoLyte powder was composed of polyethylene glycol (3350.29 g), anhydrous sodium sulfate (2.85 g), sodium hydrogen carbonate (0.84 g), sodium chloride (0.73 g), and potassium chloride (0.37 g). We examined colonoscopic features, including the size, location, number, and histologic findings of polyps. Polyp size was assessed using open colonoscopy biopsy forceps (MTW-Endoskopie Manufaktur, Wesel, Germany). Adenoma size was classified into <10 and ≥10 mm (the largest size was used for multiple adenomas). The location of colorectal adenomas was divided into 3 categories: the proximal colon, including the cecum, ascending colon, and transverse colon; the distal colon, including the splenic flexure, descending colon, sigmoid colon, and rectum; and both sides of the colon. The number of adenomas was classified as either single or multiple (≥2). Histological findings were classified according to their premalignant potential: colorectal adenomas included tubular, villous, or serrated adenomas; and controls were characterized by normal colonoscopic findings and nonpolypoid benign lesions such as nonspecific colitis or histologically confirmed hyperplastic polyps. In addition, advanced adenomas were defined as estimated diameter ≥1 cm, containing ≥25% villous features, and/or high-grade dysplasia.
Statistical Analysis
Categorical variables were evaluated by using the χ2 test or Fisher exact test. Continuous variables were evaluated by using the Student t test. Continuous variables were expressed as means ± SD. To identify the significant risk factors for colorectal adenoma, multiple logistic regression with enter method was used. Because the multicollinearity diagnostic indicated multicollinearity issues among BMI, presence of metabolic syndrome, presence of fatty liver, and FLI (condition indices <30; variation inflation factor values <10), separate multivariable analyses with these variables were performed. A p value below 0.05 was considered statistically significant. The software package used for analysis was SPSS version 20.0 (SPSS Inc, Chicago, IL).
RESULTS
Baseline Demographic and Clinical Characteristics
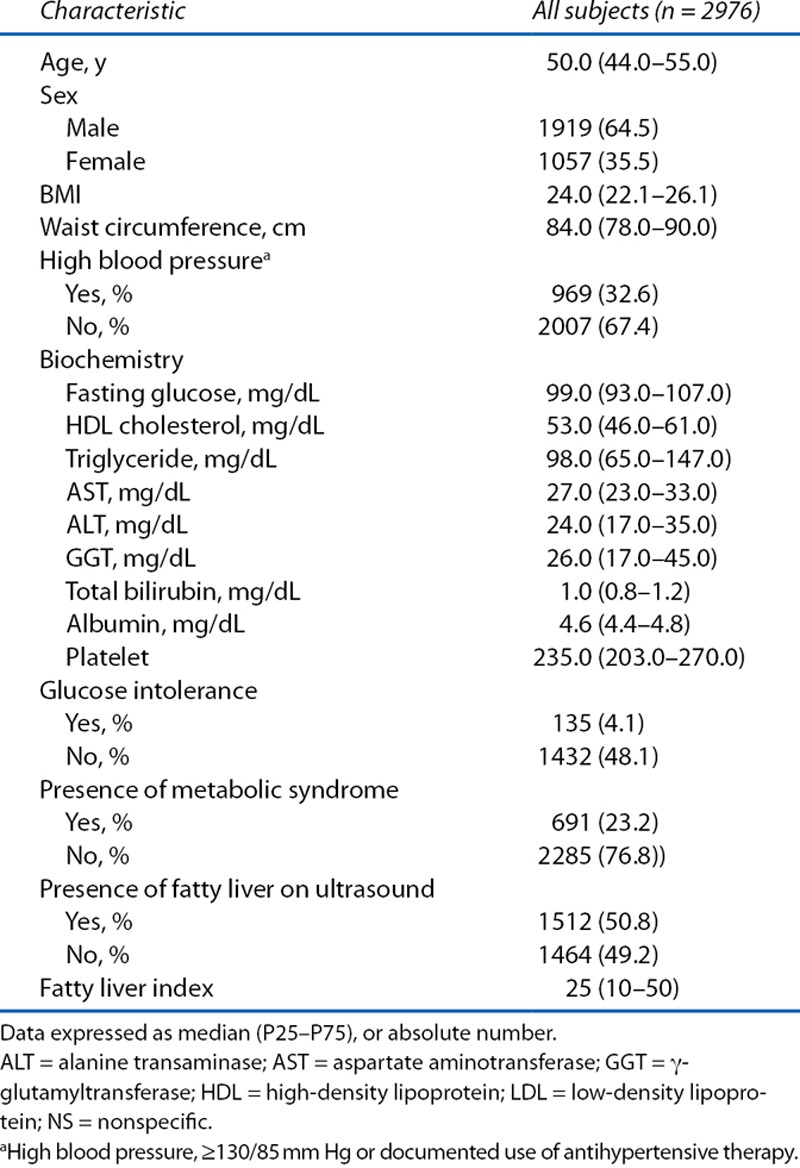
A total of 2976 patients were included in this study. The mean age was 50 years. Among the patients, 1919 (64.5%) were men and 1057 (35.5%) were women. Of these, 691 patients (23.2%) had MetS, and 1512 patients (50.8%) were determined by ultrasound to have fatty liver. The median FLI was 25 (Table 1).
Table 1.
Baseline characteristics of the subjects
Characteristics of Colorectal Adenoma in Subjects
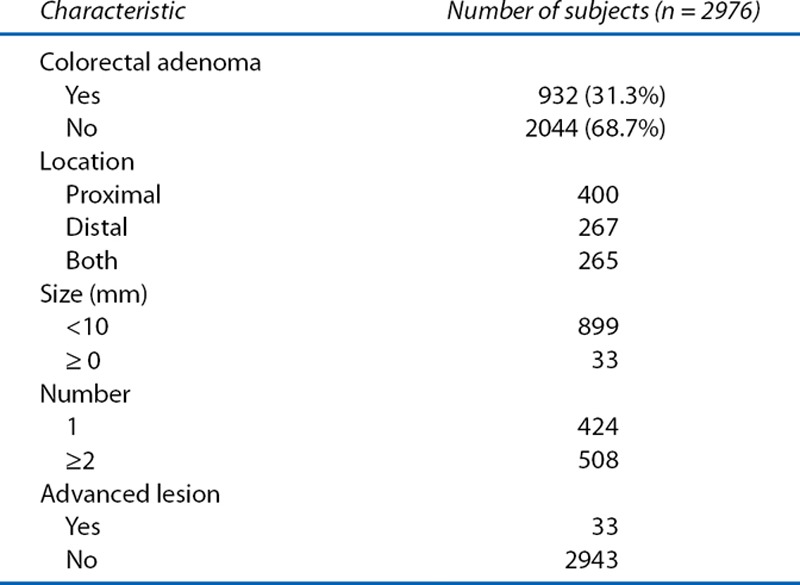
Among the 2976 subjects, 932 patients (31.3%) had colorectal adenoma. Table 2 shows the characteristics of the colorectal adenomas in these 932 patients. Adenomas were more commonly distributed in the proximal side of the colon than on the distal sides of the colon or both (42.9%, 28.7%, and 28.4%). Most adenomas (96.5%) were less than 10 mm in size. Among the patients, 508 (54.5%) had multiple adenomas, and 424 (45.5%) had only 1 adenoma. Pathologically, 33 patients (1.1%) among the total subjects had advanced lesions.
Table 2.
Characteristics of colorectal adenoma in subjects
Univariate and Multivariate Analyses of the Predictive Factors Associated With Colorectal Adenoma
Univariate and multivariate analyses of the risk of colorectal adenoma in the total study population are shown in Table 3. The results of multiple logistic regression analyses showed that older age (≥60 years), male sex, higher BMI (≥ 25), and presence of fatty liver were associated with an increased risk of colorectal adenoma. In particular, a FLI ≥30 was associated with an increased risk of colorectal adenoma (OR, 1.26; 95% CI, 1.06–1.49). Furthermore, when the prevalence of colorectal adenoma based on FLI 30 was examined for groups divided by age into those younger and older than 50 years, the prevalence of colorectal adenoma was significantly higher in all patients with FLI ≥30 regardless of age (Figure 1).
Table 3.
Univariate and multivariate analyses of the factors associated with colorectal adenoma in total subject population
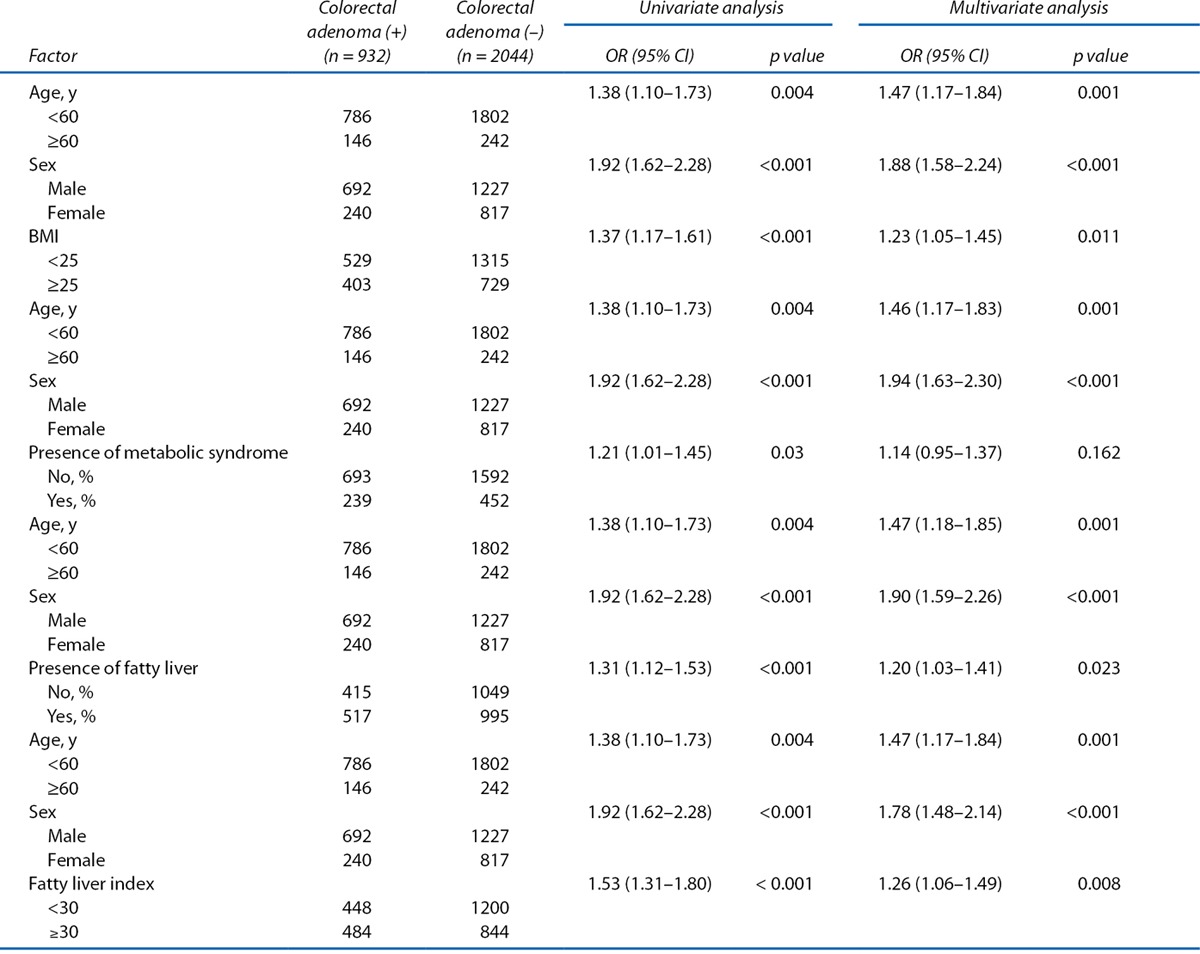
FIGURE 1.
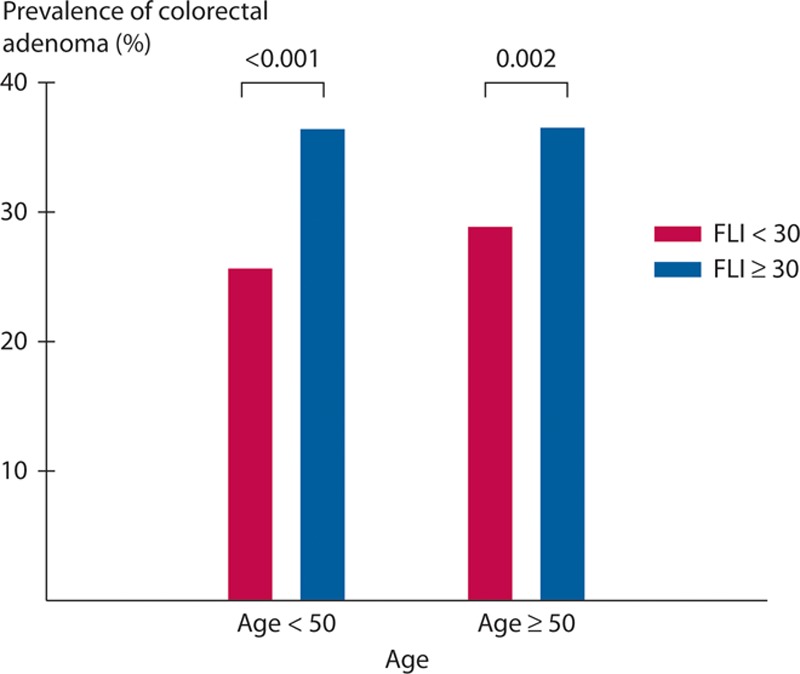
Prevalence of colorectal adenoma according to the age based on the fatty liver index (FLI). When the prevalence of colorectal adenoma based on FLI 30 was examined for 2 groups divided by age into those younger and older than 50 years, the prevalence of colorectal adenoma was significantly higher in all patients with FLI ≥30 regardless of age.
Characteristics of Colorectal Adenoma in FLI-High and FLI-Low Groups
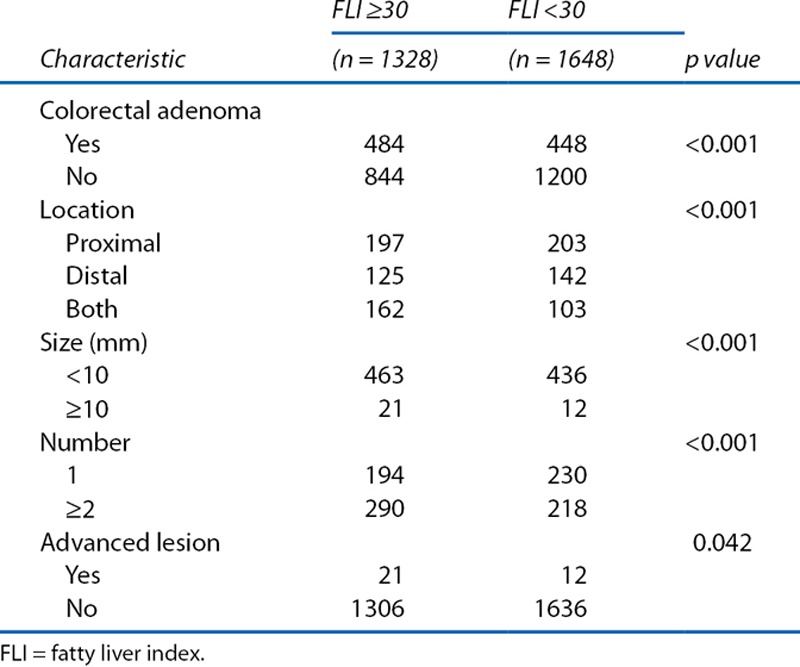
We compared the characteristics of colorectal adenoma in the FLI-high group (FLI ≥30) with those in the FLI-low group (FLI <30) (Table 4). Subjects in the FLI-high group had more colorectal adenomas (p < 0.001). Among patients with colorectal adenomas, the FLI-high group had adenomas that were more frequently distributed in the proximal side of the colon or both sides and were less than 10 mm in size, and this group also had a greater frequency of multiple adenomas than the FLI-low group (p value for each trend <0.001). In addition, the FLI-high group had more advanced lesions than the FLI-low group (p = 0.042).
Table 4.
Characteristics of colorectal adenoma categorized by fatty liver index
Prevalence of Colorectal Adenomas According to Degrees of Steatosis
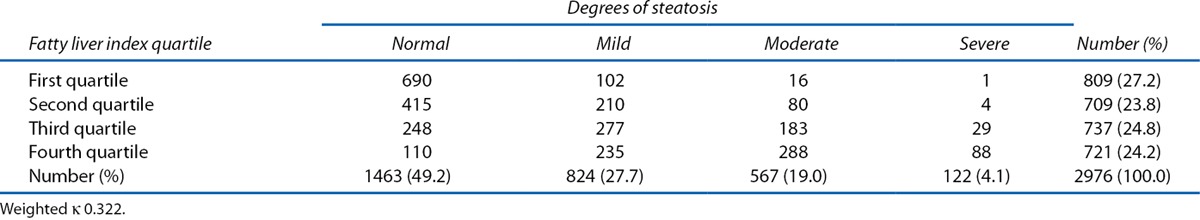
As shown in Table 5, FLI correlated well with degrees of steatosis. As the quartile of FLI increased, the severity of steatosis on ultrasound increased. The interobserver agreement between degrees of steatosis and FLI was fair (weighted κ 0.32; 95% CI, 0.30–0.34).
Table 5.
Interobserver agreement between degrees of steatosis and fatty liver index
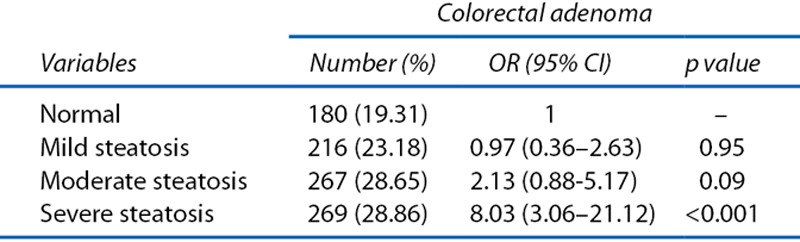
The prevalence of colorectal adenomas according to degree of steatosis in the total study population is shown in Table 6. The prevalence of colorectal adenomas increased in severe steatosis (OR, 8.03; 95% CI, 3.06–21.12; and OR, 6.33; 95% CI, 1.95–20.59).
Table 6.
OR and 95% CI of prevalence of colorectal adenoma (n = 932) and advanced colorectal adenoma (n = 33) according to degrees of steatosis (n = 2976).
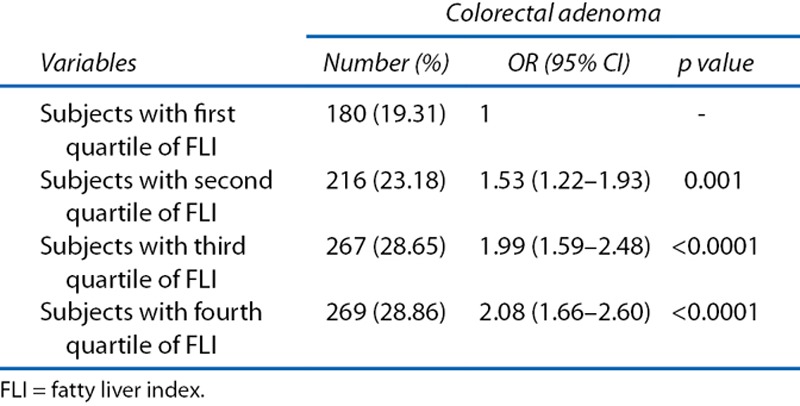
Prevalence of Colorectal Adenomas According to FLI
The prevalence of colorectal adenomas according to quartile of FLI in the total study population is shown in Table 7. The prevalence of colorectal adenomas increased with increasing quartile of FLI (OR, 1.53; 95% CI, 1.22–1.93; OR, 1.99; 95% CI, 1.59–2.48; and OR, 2.08; 95% CI, 1.66–2.08).
Table 7.
OR and 95% CI of prevalence of colorectal adenoma (n = 932) and advanced colorectal adenoma (n = 33) according to fourths of fatty liver index (n = 2,976)
DISCUSSION
This is the first study to evaluate the relationship between FLI and the prevalence of colorectal adenomas. The present study demonstrated that FLI correlated with the development of colorectal adenoma. Therefore, FLI may be useful as a predictor of colorectal adenoma in screening colonoscopy.
Although some studies establish an association between colorectal adenoma and fatty liver, this study investigated a simpler way to demonstrate the association between colorectal adenoma and fatty liver.13,14 We selected FLI, rather than abdominal ultrasound, as a tool for identifying fatty liver. Therefore, the present study assessed the relationship between FLI, a predictor of nonalcoholic fatty liver disease, and the prevalence of colorectal adenomas.
Routine health screenings commonly include colonoscopy and ultrasonography for asymptomatic subjects in Korea. In particular, abdominal ultrasonography and colonoscopy are often included in routine cancer-screening programs for subjects over 40 years of age. Therefore, this study examined voluntary health checkups.
In this study, we found that older age (≥60), male sex, and higher BMI (≥25) were associated with the development of colorectal adenoma when the individual components were analyzed separately. Moreover, previous studies suggested that the presence of MetS, individual components of MetS, or higher BMI is associated with increased risk of colorectal adenoma or CRC.3,18
In addition, high FLI correlated strongly with the severity of steatosis in the present study, showing, in particular, that the presence of fatty liver and a high FLI of ≥30 were associated with the development of colorectal adenoma. Furthermore, the prevalence of advanced colorectal adenomas and the number of colorectal adenomas were increased with increasing FLI.
Prior studies suggest that patients with NASH have a higher risk of colorectal adenomas than those with simple steatosis.13 Another study shows that patients with NAFLD may have a greater polyp burden.7
The aim of this study was not only to investigate the association between NAFLD and colorectal adenoma, but also to quantitatively measure the risk of colorectal adenomas by using the FLI as a predictive tool. Subjects in the FLI-high group (FLI ≥30) had 484 (36.4%) colorectal adenomas, whereas those in the FLI-low group (FLI <30) had 448 (27.2%) colorectal adenomas. In multivariate analysis, classification into the FLI-high group was associated with an increased risk of colorectal adenoma in comparison with classification into the FLI-low group (OR, 1.26; 95% CI, 1.06–1.49).
Comparison of the characteristics of colorectal adenomas in the FLI-high group with those in the FLI-low group revealed that the FLI-high group had more advanced lesions than the FLI-low group (p = 0.042) in the present study. We assume that these results may be attributed to the influence of mechanisms other than NAFLD on the carcinogenesis of CRC.
In addition, the exact mechanisms linking NAFLD and colorectal adenoma remain unclear. Colorectal neoplasm may arise in subjects with NAFLD through either insulin resistance or chronic inflammation.19,20 Insulin resistance, considered a critically important mechanism underlying MetS, is hypothesized to play a major role in the carcinogenesis of colorectal neoplasm.21 Insulin itself is a powerful mitogenic agent with direct in vitro growth-promoting effects.22 Moreover, insulin directly activates insulin-like growth factor-1, which stimulates the proliferation of the colonic epithelium.23 Studies have shown that insulin and insulin-like growth factor-1 increase the risk of colorectal neoplasm by inhibiting apoptosis and promoting proliferation.21 Another mechanism proposed to explain the relationship between MetS and colorectal adenoma is chronic inflammation.24 Chronic inflammation associated with components of MetS involves a network of cellular and systemic responses that integrate many signaling pathways. In particular, inflammatory cytokines, such as tumor necrosis factor-α and interleukin-6, as well as other proinflammatory cytokines, play critical roles in the colorectal adenoma and cancer.25 Furthermore, NAFLD is regarded as a condition of insulin resistance and a systemic low-grade inflammatory state.19 Patients with NAFLD would therefore be expected to have greater numbers of colorectal adenomas developed through the above mechanisms. In addition, increased leptin and decreased adiponectin levels in NAFLD patients have also been suggested in relation to an increased risk of colorectal adenoma.26
Various guidelines regarding CRC screening have been developed in many countries and by many organizations. Most guidelines recommend that screening begin at age 50 in asymptomatic average-risk persons. An “average-risk” individual has no personal history of CRC or adenomatous polyps, family history of CRC, or diseases that increase the risk of CRC, such as IBD.27,28 Recent studies suggested that future risk stratification will involve incorporating risk factors other than age and family history, such as obesity, MetS, type 2 diabetes mellitus, and smoking status.29 Moreover, the 2008 American College of Gastroenterology Guidelines suggest the need for screening of obese individuals at an age younger than 50 years, perhaps as early as 45 years.30 Our results showed that subjects with a high FLI have a higher prevalence of colorectal adenomas. We suggest calculating the FLI before performing a screening colonoscopy. Thus, we expect that FLI is a useful predictor of colorectal adenoma regardless of the presence of MetS. Individuals with a high FLI may need more attention during screening colonoscopy, and this finding may have implications in the future planning for screening of CRC.
The present study had some limitations. First, there may have been a selection bias, because subjects were recruited from among individuals who visited a health center for health examinations, and thus these individuals felt greater concern regarding their health status. However, this study was conducted on a full consecutive series of subjects for 6 years to avoid selection bias. Second, there is a lower prevalence of obesity in Asian populations. Additional large-scale prospective studies are needed to better identify the true role of FLI in the early detection of CRC.
To the best of our knowledge, this is the first study to investigate the predictive value of FLI on the incidence of colorectal adenoma, a precursor of CRC. The present study demonstrated that subjects with a high FLI have a higher prevalence of colorectal adenomas. Therefore, the high FLI may be a useful predictor of colorectal adenoma.
Footnotes
Funding/Support: None reported.
Financial Disclosures: None reported.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D.Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Leslie A, Carey FA, Pratt NR, Steele RJ.The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89:845–860. [DOI] [PubMed] [Google Scholar]
- 3.Kim BC, Shin A, Hong CW, et al. Association of colorectal adenoma with components of metabolic syndrome. Cancer Causes Control. 2012;23:727–735. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G.Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–168. [DOI] [PubMed] [Google Scholar]
- 5.Sung JJ, Lau JY, Young GP, et al. Asia Pacific Working Group on Colorectal Cancer. Asia Pacific consensus recommendations for colorectal cancer screening. Gut. 2008;57:1166–1176. [DOI] [PubMed] [Google Scholar]
- 6.Levin B, Lieberman DA, McFarland B, et al. American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. [DOI] [PubMed] [Google Scholar]
- 7.Touzin NT, Bush KN, Williams CD, Harrison SA.Prevalence of colonic adenomas in patients with nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2011;4:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleiner DE, Brunt EM.Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13. [DOI] [PubMed] [Google Scholar]
- 9.Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozakova M, Palombo C, Eng MP, et al. RISC Investigators. Fatty liver index, gamma-glutamyltransferase, and early carotid plaques. Hepatology. 2012;55:1406–1415. [DOI] [PubMed] [Google Scholar]
- 11.Calori G, Lattuada G, Ragogna F, et al. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54:145–152. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao PJ, Kuo KK, Shin SJ, et al. Significant correlations between severe fatty liver and risk factors for metabolic syndrome. J Gastroenterol Hepatol. 2007;22:2118–2123. [DOI] [PubMed] [Google Scholar]
- 13.Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60:829–836. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Lipka S, Kumar A, Mustacchia P.Association between nonalcoholic fatty liver disease and colorectal adenoma: a systemic review and meta-analysis. J Gastrointest Oncol. 2014;5:440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willett WC, Dietz WH, Colditz GA.Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. [DOI] [PubMed] [Google Scholar]
- 16.Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S.Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38:954–961. [DOI] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. Third National Health and Nutrition Examination Survey: hepatic steatosis assessment procedure manual. Available at: http://www.cdc.gov/nchs/data/datalinkage/nh3_file_layout_public_2010.pdf Accessed June 1, 2016.
- 18.Liu CS, Hsu HS, Li CI, et al. Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. BMC Gastroenterol. 2010;10:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Targher G, Bertolini L, Scala L, Zoppini G, Zenari L, Falezza G.Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men. Role of visceral adipose tissue. Diabet Med. 2005;22:1354–1358. [DOI] [PubMed] [Google Scholar]
- 20.Sung MK, Yeon JY, Park SY, Park JH, Choi MS.Obesity-induced metabolic stresses in breast and colon cancer. Ann N Y Acad Sci. 2011;1229:61–68. [DOI] [PubMed] [Google Scholar]
- 21.Giovannucci E.Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–s842. [DOI] [PubMed] [Google Scholar]
- 22.Tran TT, Naigamwalla D, Oprescu AI, et al. Hyperinsulinemia, but not other factors associated with insulin resistance, acutely enhances colorectal epithelial proliferation in vivo. Endocrinology. 2006;147:1830–1837. [DOI] [PubMed] [Google Scholar]
- 23.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M.Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. [DOI] [PubMed] [Google Scholar]
- 24.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ.C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–590. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Keku TO, Martin C, et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaji T, Iwasaki M, Sasazuki S, Tsugane S.Interaction between adiponectin and leptin influences the risk of colorectal adenoma. Cancer Res. 2010;70:5430–5437. [DOI] [PubMed] [Google Scholar]
- 27.Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW.Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010;60:99–119. [DOI] [PubMed] [Google Scholar]
- 28.Lee BI, Hong SP, Kim SE, et al. Multi-Society Task Force for Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management. [Korean guidelines for colorectal cancer screening and polyp detection]. Korean J Gastroenterol. 2012;59:65–84. [DOI] [PubMed] [Google Scholar]
- 29.Kahi CJ, Rex DK, Imperiale TF.Screening, surveillance, and primary prevention for colorectal cancer: a review of the recent literature. Gastroenterology. 2008;135:380–399. [DOI] [PubMed] [Google Scholar]
- 30.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JMAmerican College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739–750. [DOI] [PubMed] [Google Scholar]


