Abstract
Introduction
131I therapy is a choice for Graves’ hyperthyroidism. Several factors that affect the success of 131I treatment in Graves’ disease (GD) patients have been put forward. The aim of this retrospective study was to evaluate the factors influencing the success of 131I therapy and the occurrence of hypothyroidism after 131I therapy.
Patients and methods
We reviewed 325 GD patients, who were well documented out of 779 cases, treated with 131I in the First Affiliated Hospital of Xi’an Jiaotong University between 2010 and 2016. We collected the potential influencing factors, including demographic data (age, sex, family history), iodine intake state, antithyroid drugs (ATD) taking, thyroid texture, complications of hyperthyroidism, physical and laboratory examinations [thyroid weight, effective 131I half-life time (Teff), 24-h iodine uptake rate, tri-iodothyronine, thyroxine, free tri-iodothyronine (FT3), free thyroxine, thyroid-stimulating hormone, thyroglobulin antibody, thyroid microsome antibody, thyrotropin receptor antibody], and final administered dosages according to Quimby formula. The correlations between the prognosis of GD patients and these factors were analyzed by logistic regression analysis.
Results
Out of 325 patients, 247 (76.00%) were treated successfully with radioiodine. GD patients who were cured by 131I therapy were more likely to have smaller thyroid [odds ratio (OR)=0.988, 95% confidence interval (CI)=0.980–0.996, P=0.002], lower FT4 levels (OR=0.993, 95% CI=0.988–0.997, P=0.002), and shorter time of ATD withdrawal before 131I treatment (OR=0.985, 95% CI=0.975–0.996, P=0.002). Hypothyroidism occurred in 132 (41.00%) out of 325 patients. There was an increased risk of early hypothyroidism in patients with lower 24-h iodine uptake (OR=0.964, 95% CI=0.941–0.988, P=0.004), and treated with a lower total dose of iodine (OR=0.892, 95% CI=0.824–0.965, P=0.005) and a higher iodine dose per garm of thyroid tissue (OR=5.414E+14, 95% CI=45.495–6.444E+27, P=0.027).
Conclusion
Our results showed that 131I treatment was more successful in patients with lower weight of the thyroid, lower free thyroxine level, and shorter ATD taking period. Furthermore, early hypothyroidism after radioiodine treatment was more likely to occur in patients with lower 24-h iodine uptake, lower total dose of iodine, and higher iodine dose per garm of thyroid tissue.
Keywords: Graves’ disease, hypothyroidism, 131I therapy, logistic regression analysis
Introduction
Graves’ hyperthyroidism is an organ-specific autoimmune disease characterized by abnormal increased thyroid hormone secretion, which is the result of genetic and environmental factors 1,2. The incidence rate of Graves’ disease (GD) in China is about 1.2% and the majority of patients are in the age range of 20–50 years 3. There are three main methods of treatment for Graves’ hyperthyroidism: antithyroid drugs (ATD), radioactive iodine (131I) (RAI), and surgery 4,5. Surgery is the most successful definitive treatment 6, but it is associated with the risk of recurrent laryngeal nerve injury or hypoparathyroidism 7. 131I therapy has been used successfully for the treatment of hyperthyroidism since 1940 8. It is an effective, practical, and inexpensive agent to permanently control hyperthyroidism. The objective of 131I therapy is to cure Graves’ hyperthyroidism by destroying enough thyroid tissue with a single 131I dose and it is considered successful if euthyroidism or hypothyroidism is achieved after 131I therapy. However, so far, there is no general consensus on the determination of 131I dose. Some doctors suggested that 131I should be administered at a fixed dose 9 and others proposed that the dose of 131I can be calculated according to the formula 10. The national guidelines in China for the treatment of GD proposed that the doctors should follow the principle of individual treatment when choosing a treatment regimen 11. Antithyroid drugs are the first-line treatment for the first episode of GD in China. However, many patients cannot adhere to long-term medication, which may lead to a poor clinical outcome. China’s endocrinologists have a relatively conservative approach toward 131I treatment of hyperthyroidism 11.
Several factors that affect the success of 131I treatment in GD patients have been put forward, such as the dose of 131I administration, thyroid volume, age, thyroid uptake of 131I, and the use of antithyroid drugs 12. Some researchers have proven that thyroid volume has a significant influence on the success of treatment and the inefficiency of 131I therapy is closely related to thyroid volume 13,14. The effect of age on the outcome of 131I treatment is still a matter of debate. Some studies did not find any significant association 15, whereas other studies suggested that older age is a risk factor for the poor outcome of 131I therapy 16. However, the impact of these factors on the success of 131I treatment on GD patients remains largely unknown.
In the present study, we investigated the treatment condition of Graves’ hyperthyroidism within our clinical practice to explore the clinical factors that may affect the outcome of 131I treatment. The correlations between the prognosis of GD patients and these factors were analyzed to further optimize radioiodine treatment for individual patients with hyperthyroidism.
Patients and methods
Ethics statement
This is a retrospective clinical study. It presents a summary and analysis of a large number of clinical data. The study was approved by the Ethics Committee for Medical Research, Xi’an Jiaotong University and was carried out in accordance with the Good Clinical Practice. Informed consent was provided by all patients participating in this study.
Patients
A total of 325 GD patients were enrolled, including 12 patients with hyperthyroidism heart disease, 12 patients with periodic paralysis, and 13 patients with abnormal liver function, and treated with 131I at the First Affiliated Hospital of Xi’an Jiaotong University between 2010 and 2016. Among these 325 patients, 239 were females and 86 were males. The average age of the female and male patients was 41.31±12.42 and 41.73±11.98 years, respectively.
Data collection
Before the administration of therapeutic 131I, the patients had undergone routine eligibility examinations, including the assessment of standard clinical symptoms of GD, effective 131I half-life in thyroid gland (Teff), tri-iodothyronine (T3), thyroxine (T4), free tri-iodothyronine (FT3), free thyroxine (FT4), thyroid-stimulating hormone (TSH), thyroglobulin antibody (TGAb), thyroid microsome antibody (TMAb), thyrotropin receptor antibody (TRAb) as well as iodine uptake tests: 24-h (T24). The therapeutic 131I dose was calculated according to the Marinelli–Quimby formula 17: 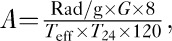
where, A is the 131I dose, G is thyroid weight, Rad/g is iodine dose per garm of thyroid tissue, Teff is effective 131I half-life in the thyroid gland, T24 is 24-h iodine uptake, W is thyroid weight), and 131I was administered once according to the calculated dose and the patient’s condition. Follow-up was performed at 3 months, 6 months, and 1 year after 131I treatment. Most studies reported that 6 months of RAI administration was sufficient to stabilize thyroid functions 18–20. Therefore, RAI therapy in the 325 patients with Graves’ hyperthyroidism in our study was also administered after 6 months. The efficacy of treatment was presented as the percentage of patients with euthyroidism, hypothyroidism, or persistent hyperthyroidism within 6 months since radioiodine administration. We defined complete euthyroidism and hypothyroidism as ‘cured’ (cured group) and persistent hyperthyroidism as ‘uncured’ (uncured group). Similarly, we defined hypothyroidism as the hypothyroidism group, and euthyroidism and persistent hyperthyroidism as the nonhypothyroidism group.
Demographic and related clinical data were recorded, including age (named X1), duration of Graves’ hyperthyroidism (X2), thyroid weight (X3), Teff (X4), 24-h iodine uptake (X5), the total dose of iodine in patients (X6), T3 (X7), T4 (X8), FT3 (X9), FT4 (X10), TGAb (X11), TMAb (X12), TSH (X13), the duration of ATD treatment before iodine administration (X14), the number of weeks before 131I administration antithyroid drugs should be withdrawn (X15), iodine dose absorbed per gram of thyroid tissue (X16), administration of iodine dose per gram of thyroid tissue (X17), T4/T3 (X18), FT4/FT3 (X19), sex (X20), family history of thyroid disease (X21), iodine intake peak forward (X22), the number of times of taking iodine (X24), nodules (X25), thyroid texture (X26), hyperthyroidism heart (X27), periodic paralysis (X28), abnormal liver function (X29), and hematological abnormalities (X30). The cured group and the uncured group were named as Y1. Y1=1 implies the uncured group, whereas Y1=2 indicates the cured group. The hypothyroidism group and the nonhypothyroidism group were named as Y2. Y2=1 implies the nonhypothyroidism group, whereas Y2=2 indicates the hypothyroidism group.
Statistical analysis
Statistical analysis was carried out using SPSS for windows, version 23.0 (SPSS Inc., Chicago, Illinois, USA). Independent-samples t-test was used to investigate the influence of measurement data (X1–X19). The χ2-test was applied to investigate the influence of count data (X20–X30). Moreover, logistic regression analysis was used to evaluate the impact that particular parameters had on the success of treatment with 131I (uncured and cured). In addition, logistic regression analysis was also used to evaluate the impact of particular parameters on the occurrence of hypothyroidism after 131I treatment (nonhypothyroidism and hypothyroidism). The logistic regression analysis took into consideration those parameters that were statistically significant for the outcome (P<0.1). Simultaneously, the factors (X3, X4, X5, X17) in the Marinelli–Quimby formula were also incorporated into the regression equation. P values less than 0.05 in regression analysis were considered to be statistically significant. All P values presented were two tailed.
Results
The clinical characteristics of the patients in this study
The age range of the patients in our study was 13–76 years, and the median age was 41 years. The age range of the female and male patients was 13–76 and 21–67 years, respectively, and the median age of the female and male patients was 41 and 40.5 years, respectively. Overall, as shown in Fig. 1, the 131I therapy was ineffective in 30 patients (9.3% – out of 325 patients). One hundred and fifteen (35.40%) patients achieved euthyroidism, 48 (14.80%) patients showed improvements, and early hypothyroidism occurred in 132 (40.60%) patients. Furthermore, the effective rate of iodine treatment of GD (including euthyroid patients, patients who showed improvement, and early hypothyroid patients) was 90.7%. The cure rate of iodine treatment of GD (including euthyroid patients and early hypothyroid patients) was 76.00%.
Fig. 1.
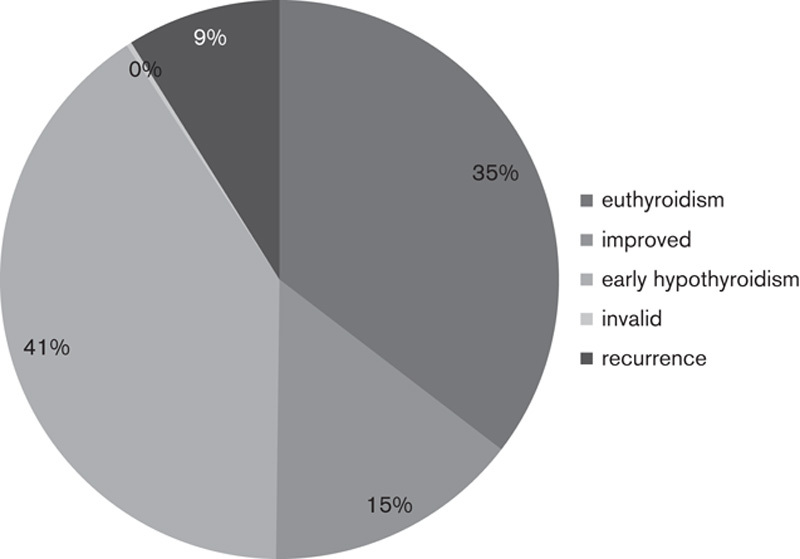
The outcome of treatment with 131I.
Analysis of factors affecting the successful treatment of hyperthyroidism with 131I
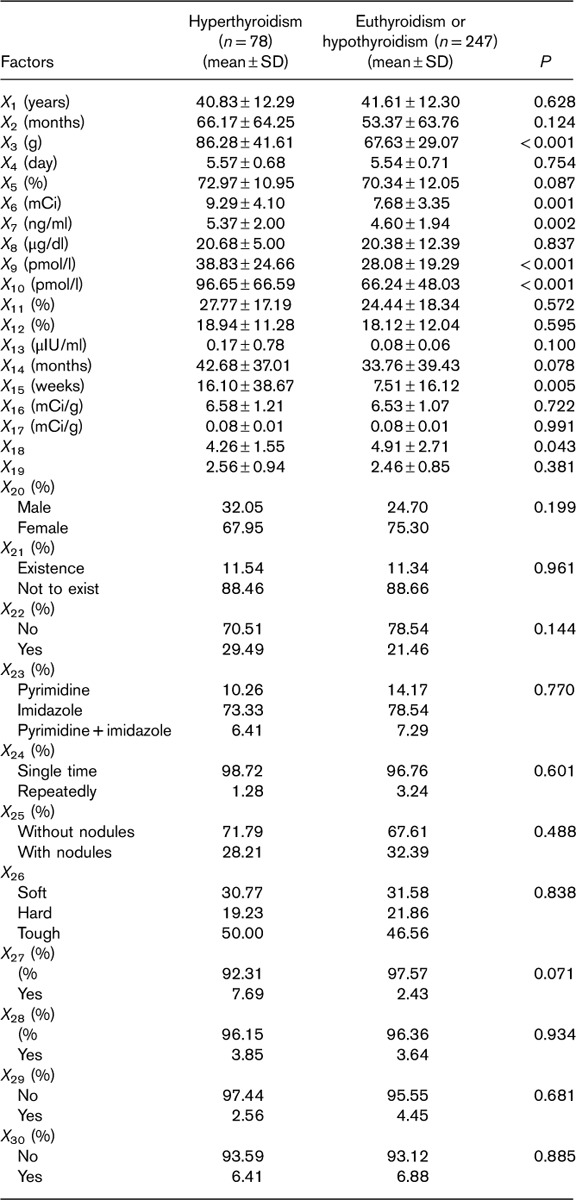
The characteristics of the GD patients in this study, including clinical and physiological parameters and the results of 131I treatment (hyperthyroidism, euthyroidism, or hypothyroidism) that they had undergone, are shown in Table 1. Independent-samples t-test was used to compare the measurement data (X1–X19) between the uncured and the cured groups. The χ2-test was used to compare the count data (X20–X30) between the two groups. The results in Table 1 show that the 131I therapy outcomes were influenced by X3, X5, X6, X7, X9, X10, X13, X14, X15, X18, X27 (P<0.1).
Table 1.
Characteristics of patients under study with respect to clinical and physical parameters and 131I therapy outcomes: hyperthyroidism, euthyroidism, or hypothyroidism
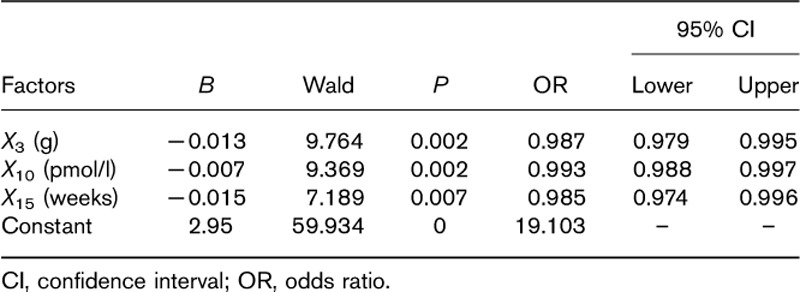
Moreover, the above factors (P<0.1) and factors in the Marinelli–Quimby formula were incorporated into the logistic regression model. The results (Table 2) showed that X3 (g), X10 (pmol/l), and X15 (weeks) were more likely to be associated with 131I therapy outcomes. The regression equation is Y1=2.95−0.013X3−0.007X10−0.015X15. This equation is tested by the likelihood ratio: X2=37.014, P<0.01. Thus, the equation has obvious significance and the model of the degree of fit is better. This equation shows that GD patients who were cured by 131I therapy were more likely to have smaller weight of the thyroid, lower FT4 levels, and shorter time of ATD withdrawal before 131I treatment.
Table 2.
Variables and constants of the regression equation: the chances of 131I cure Graves’ disease
Analysis of factors affecting the occurrence of early hypothyroidism after radioiodine treatment for Graves’ hyperthyroidism
The characteristics of the GD patients in this study, including clinical and physiological parameters and the results of the treatment with 131I (euthyroidism or hyperthyroidism, hypothyroidism) that they had undergone, are shown in Table 3. Independent-samples t-test was used to compare the measurement data (X1–X19) between the two groups (group 3: euthyroidism and hyperthyroidism, group 4: hypothyroidism). The χ2-test was used to compare the count data (X20–X30) between the two groups. The results in Table 3 show that the 131I therapy outcomes was influenced by X3, X5, X6, X10, X14, X22 (P<0.1).
Table 3.
Characteristics of patients under study with respect to clinical and physical parameters and 131I therapy outcomes: euthyroidism or hyperthyroidism, hypothyroidism

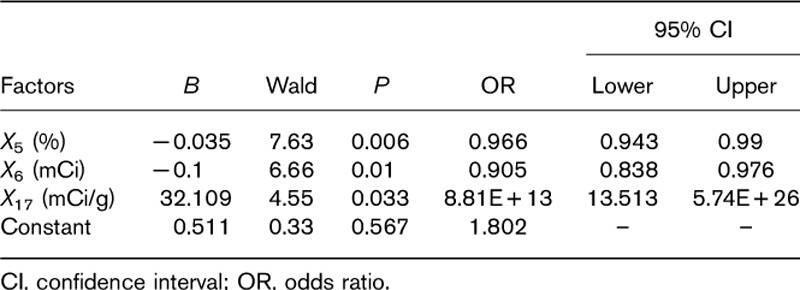
Moreover, the above factors (P<0.1) and factors in the Marinelli–Quimby formula were incorporated into the logistic regression model. The results (Table 4) showed that X5 (%), X6 (mCi), and X17 (mCi/g) were more likely to be associated with the occurrence of hypothyroidism. The regression equation is Y=0.51−0.035X5−0.1X6+32.11X17. This equation is tested by the likelihood ratio: χ2=17.26, P=0.002. Therefore, the equation has obvious significance and the model of the degree of fit is better. This equation shows that there was an increased risk of early hypothyroidism in patients with lower 24-h iodine uptake, and treated with a lower total dose of iodine and a higher iodine dose per garm of thyroid tissue.
Table 4.
Variables and constants of the regression equation: the chance of early hypothyroidism
Discussion
The hyperthyroidism treatment program preferred to use high-dose 131I one time and hypothyroidism is considered acceptable in some countries. However, in China, it is strongly suggested that doctors should use an acceptable minimum dose 131I to curing hyperthyroidism while the hypothyroidism is not occurred. Calculated 131I doses according to the formula are still the main method used. These clinical experiences emphasize the importance of evaluating the corresponding influence factors during 131I treatment. The aim of this study was to analyze the factors that could have a potential influence on the effects of therapy with 131I.
The above results showed that the cure rate of 131I therapy in GD patients depends on the thyroid weight, FT4, and the time of ATD withdrawal before 131I treatment. Markovic and colleagues reported that the chances of recovery are much greater for the patients with thyroids smaller than 62 g (9.6% of unsuccessful attempts) as opposed to patients with thyroids larger than 62 g (44% of cases of persistent hyperthyroidism) 13. Szumowski et al. 14 have proven that the volume of the thyroid gland has a significant (P<0.002) effect on the success of treatment, and the larger the thyroid volume, the worse the treatment efficiency. Similar to the results of other studies, our study showed that the lower weight of the thyroid led to greater success of treatment (P=0.002). Our study showed that a lower FT4 level and greater chances of 131I cured incidence. This was supported by some studies in which FT4 levels had a negative impact on the 131I therapy success rate 16,21–23. The serum levels of FT4 at the onset of GD can reflect the severity of hyperthyroidism. Lower FT4 serum levels, suggesting a less severe GD condition, may lead to a better treatment result. However, some studies found that FT4 levels had no such influence on the 131I therapy success rate 24–26. We found that the shorter the ATD withdrawal time before 131I treatment, the greater the chances of 131I curing GD. It is frequently discussed in the medical literature that how many days anti-thyroid drugs should be stopped before 131I administration 27. However, so far, the answers to this question are varied. Most of the results show that at least 2 weeks of antithyroid drugs withdrawal are suggested. In this study, withdrawal of less than 2 weeks in some patients was because of special conditions, such as severe complications, including heart disease, liver damage, etc. The therapeutic goal in these patients is to relieve symptoms as soon as possible or induce hypothyroidism as a final treatment result. The disease situation of these patients may result in differences between our study and other studies. Other factors included, such as sex, age, and duration of the antithyroid treatment before 131I treatment, did not affect the cure rate of 131I in GD in our study. This result is similar to the reports of other authors 15. We found that there was no impact of the FT4/FT3 ratio on the success of 131I treatment 16. The FT4/FT3 ratio is an indicator of the severity of hyperthyroidism, but it is also affected by antithyroid drugs. Our observation is that the rate of iodine absorption did not significantly affect the outcome of 131I treatment, which is consistent with some other studies 16,24,28. However, another study showed that higher thyroid uptake may be the cause of 131I treatment failure 26,29, possibly owing to a higher iodine turnover.
In general, the greater the total dose of iodine in patients, the higher the incidence of hypothyroidism 21. Ogunjobi and colleagues found that on using a small total dose of iodine in patients, the incidence of hypothyroidism increased, but no statistically significant difference was observed 30. The chances of hypothyroidism are much greater in the patients receiving low RAI doses than those receiving higher RAI doses in patients with multinodular goiter or adenoma in the study of Saara Metso. However, they also found that the RAI doses did not have any effect on the development of hypothyroidism in patients with GD 31. Our study used a smaller total dose of iodine in patients and observed a greater likelihood of early hypothyroidism, and logistic regression analysis showed that the low total dose of iodine in patients was a contributing factor toward the development of hypothyroidism (odds ratio=0.966, 95% confidence interval: 0.943–0.990, P=0.006<0.05). Moreover, we found that the higher iodine dose per gram of thyroid tissue administered to patients increased the risk of early hypothyroidism (odds ratio=8.808E+13, 95% confidence interval: 13.513–5.741E+26, P=0.033<0.05). The reason may be that when using the individual dose method to calculate the 131I dose in clinic, the thyroid weight is more likely to be overestimated and a dose larger than the actual required dose was administered, resulting in hypothyroidism. However, some studies have shown that individual radiosensitivity is regulated by specific genes such as Bc1-2 and Egr-1, which is an important factor affecting the efficacy of 131I treatment 32,33 and also affects the therapeutic outcome. Meanwhile, our study showed that with lower 24-h iodine uptake, a higher chance of early hypothyroidism was observed. The reason may be that the calculated dose increases with the decrease in 24-h iodine uptake, and the iodine absorption rate varies during the therapy period 34.
131I therapy costs less compared with other treatments for GD in China. Patients need to receive 131I treatment only once or twice, and euthyroidism or hypothyroidism can be achieved. At the same time, they do not have to be followed up regularly for thyroid function. If the patients are treated with long-term antithyroid drugs, they need to be followed up regularly for thyroid function and there may be some side effects such as leukopenia, liver damage, and drug rash, which can sometimes lead to severe consequences. From this point of view, more money and time can be saved if patients are treated with 131I treatment compared with antithyroid drugs.
Conclusion
The results of our retrospective study indicated that the cure rate of 131I treatment in GD was higher in patients with smaller weight of the thyroid, lower FT4 levels, and shorter time of ATD withdrawal before 131I treatment, and early hypothyroidism after radioiodine treatment for Graves’ hyperthyroidism is more likely to occur in patients with lower 24-h iodine uptake, lower total dose of iodine, and higher iodine dose per gram of thyroid tissue.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
Footnotes
*Danrong Yanga and Jianjun Xue contributed equally to the writing of this article.
References
- 1.Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev 2003; 24:802–835. [DOI] [PubMed] [Google Scholar]
- 2.Weetman AP. Graves’ disease. N Engl J Med 2000; 343:1236–1248. [DOI] [PubMed] [Google Scholar]
- 3.Zaiying L, Nanshan Z. Internal science, 7th ed Beijing: People’s Health Publishing House; 2008. 591–600. [Google Scholar]
- 4.Bahn Chair RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid 2011; 21:593–646. [DOI] [PubMed] [Google Scholar]
- 5.Jiang N, Lin Y, Guan H, Tan J, Li L, Gao Z, et al. The guide of 131I therapy for Graves’ hyperthyroidism (2013 edition). Chin J Nucl Med 2013; 33:83–94. [Google Scholar]
- 6.Genovese BM, Noureldine SI, Gleeson EM, Tufano RP, Kandil E. What is the best definitive treatment for Graves’ disease? A systematic review of the existing literature. Ann Surg Oncol 2013; 20:660–670. [DOI] [PubMed] [Google Scholar]
- 7.Bartalena L. Diagnosis and management of Graves disease: a global overview. Nat Rev Endocrinol 2013; 9:724–734. [DOI] [PubMed] [Google Scholar]
- 8.Ross DS. Radioiodine therapy for hyperthyroidism. N Engl J Med 2011; 364:542–550. [DOI] [PubMed] [Google Scholar]
- 9.Jarløv AE, Hegedüs L, Kristensen LO, Nygaard B, Hansen JM. Is calculation of the dose in radioiodine therapy of hyperthyroidism worth while? Clin Endocrinol (Oxf) 1995; 43:325–329. [DOI] [PubMed] [Google Scholar]
- 10.Lind P. Strategies of radioiodine therapy for Graves’ disease. Eur J Nucl Med 2002; 29:453–457. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Jiang N. 131I treatment of Graves hyperthyroidism guide (2013 version). Label Immunoassays Clin Med 2014; 21:92–104. [Google Scholar]
- 12.Bonnema SJ, Hegedüs L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev 2012; 33:920–980. [DOI] [PubMed] [Google Scholar]
- 13.Moura-Neto A, Mosci C, Santos AO, Amorim BJ, de Lima MC, Etchebehere EC, et al. Predictive factors of failure in a fixed 15 m Ci 131I iodide therapy for Graves’ disease. Clin Nucl Med 2012; 37:550–554. [DOI] [PubMed] [Google Scholar]
- 14.Szumowski P, Abdelrazek S, Kociura Sawicka A, Mojsak M, Kostecki J, Sykała M, et al. Radioiodine therapy for Graves’ disease – retrospective analysis of efficacy factors. Endokrynol Pol 2015; 66:126–131. [DOI] [PubMed] [Google Scholar]
- 15.Knapska-Kucharska M, Oszukowska L, Lewiński A. Analysis of demographic and clinical factors affecting the outcome of radioiodine therapy in patients with hyperthyroidism. Arch Med Sci 2010; 6:611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Šfiligoj D, Gaberšček S, Mekjavičb PJ, Pirnat E, Zaletel K. Factors influencing the success of radioiodine therapy in patients with Graves’ disease. Nucl Med Commun 2015; 36:560–565. [DOI] [PubMed] [Google Scholar]
- 17.Marinelli LD, Quimby EH, Hine GJ. Dosage determination with radioactive isotopes; practical considerations in therapy and protection. Am J Roentgenol Radium Ther 1948; 59:260–281. [PubMed] [Google Scholar]
- 18.Guhlmann CA, Rendl J, Börner W. Radioiodine therapy of autonomously functioning thyroid nodules and Graves’ disease. Nucl Med 1995; 34:20–23. [PubMed] [Google Scholar]
- 19.Seeger T, Emrich D, Sandrock D. Radioiodine therapy of funcitonal autonomy using the funcitonal autonomous volume. Nucl Med 1995; 34:135–140. [PubMed] [Google Scholar]
- 20.Sabri O, Zimny M, Schulz G, Schreckenberger M, Reinartz P, Willmes K, et al. Success rate of radioiodine therapy in Graves’ disease: the influence of antithyroid drug medication. J Clin Endocrinol Metab 1999; 84:1229–1233. [DOI] [PubMed] [Google Scholar]
- 21.Allahabadia A, Daykin J, Sheppard MC, Gough SC, Franklyn JA. Radio iodine treatment of hyperthyroidism prognostic factors for outcome. J Clin Endocrinol Metab 2001; 86:3611–3617. [DOI] [PubMed] [Google Scholar]
- 22.Alexander EK, Larsen PR. High dose 131I therapy for the treatment of hyperthyroidism caused by Graves’ disease. J Clin Endocrinol Metab 2002; 87:1073–1077. [DOI] [PubMed] [Google Scholar]
- 23.Boelaert K, Syed AA, Manji N, Sheppard MC, Holder RL, Gough SC, et al. Prediction of cure and risk of hypothyroidism in patients receiving 131I for hyperthyroidism. Clin Endocrinol (Oxf) 2009; 70:129–138. [DOI] [PubMed] [Google Scholar]
- 24.Walter MA, Christ-Crain M, Schindler C, Müller-Brand J, Müller B, Walter MA, et al. Outcome of radioiodine therapy without, on or 3 days off carbimazole: a prospective interventional three-group comparison. Eur J Nucl Med Mol Imaging 2006; 33:730–737. [DOI] [PubMed] [Google Scholar]
- 25.Dora JM, Machado WE, Andrade VA, Scheffel RS, Maia AL. Increasing the radioiodine dose does not improve cure rates in severe Graves’ hyperthyroidism: a clinical trial with historical control. J Thyroid Res 2013; 2013:958276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catargi B, Leprat F, Guyot M, Valli N, Ducassou D, Tabarin A. Optimized radioiodine therapy of Graves’ disease: analysis of the delivered dose and of other possible factors affecting outcome. Eur J Endocrinol 1999; 141:117–121. [DOI] [PubMed] [Google Scholar]
- 27.Oszukowska L, Knapska-Kucharska M, Lewiński A. Effects of drugs on the efficacy of radioiodine (131I) therapy in hyperthyroid patients. Arch Med Sci 2010; 6:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esfahani AF, Kakhki VR, Fallahi B, Eftekhari M, Beiki D, Saghari M, et al. Comparative evaluation of two fixed doses of 185 and 370 MBq 131I, for the treatment of Graves’ disease resistant to antithyroid drugs. Hell J Nucl Med 2005; 8:158–161. [PubMed] [Google Scholar]
- 29.DeJong JA, Verkooijen HM, Valk GD, Zelissen PM, de Keizer B. High failure rates after 131I therapy in Graves hyperthyroidism patients with large thyroid volumes, high iodine uptake, and high iodine turnover. Clin Nucl Med 2013; 38:401–406. [DOI] [PubMed] [Google Scholar]
- 30.Enyi Ejeh MJ, Omotayo Ogunjobi K, Enyi Ejeh J, Solomon Adedapo K, F Eniojukan J. Effectiveness of fixed dose radioactive iodine (RAI) for the treatment of hyperthyroidism: experience of a teaching hospital in South West Nigeria. Mol Imaging Radionucl Ther 2013; 22:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metso S, Jaatinen P, Huhtala H, Luukkaala T, Oksala H, Salmi J. Long-term follow-up study of radioiodine treatment of hyperthyroidism. Clin Endocrinol 2004; 61:641–648. [DOI] [PubMed] [Google Scholar]
- 32.Guo K, Gao R, Yu Y, Zhang W, Yang Y, Yang A. Quantitative mRNA expression analysis of selected genes in patients with early-stage hypothyroidism induced by treatment with iodine-131. Mol Med Rep 2015; 12:7673–7680. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Gao R, Yu Y, Guo K, Hou P, Yu M, et al. Iodine-131 induces apoptosis in HTori-3 human thyrocyte cell line and G2/M phase arrest in a p53-independent pathway. Mol Med Rep 2015; 11:3148–3154. [DOI] [PubMed] [Google Scholar]
- 34.Dang Y, Meng X, Xiao J, Deng H. Effects of the iodine absorption rate changes in short term in patients with Graves’ hyperthyroidism on the calculated therapeutic dose. Chin J Nucl Med 2001; 1:374. [Google Scholar]


