Abstract
Objectives:
PainDETECT is a self-report questionnaire that can be used to identify features of neuropathic pain. A proportion of patients with knee osteoarthritis (OA) score highly on the PainDETECT questionnaire. This study aimed to determine whether those with a higher “positive neuropathic” score on the PainDETECT questionnaire also had greater pain, hypersensitivity, and reduced function compared with individuals with knee OA with lower PainDETECT scores.
Materials and Methods:
In total, 130 participants with knee OA completed the PainDETECT, Western Ontario and McMaster Universities Arthritis Index (WOMAC), and Pain Quality Assessment Scale questionnaires. Quantitative sensory testing was carried out at 3 sites (both knees and elbow) using standard methods. Cold and heat pain thresholds were tested using a Peltier thermode and pressure pain thresholds using a digital algometer. Physical function was assessed using 3 timed locomotor function tests.
Results:
In total, 22.3% of participants scored in the “positive neuropathic” category with a further 35.4% in the unclear category. Participants in the “positive neuropathic” category reported higher levels of pain and more impaired function based on the WOMAC questionnaire (P<0.0001). They also exhibited increased levels of hyperalgesia at the knee and upper limb sites for all stimulation modalities except heat pain thresholds at the OA knee. They were also slower to complete 2 of the locomotion tasks.
Discussion:
This study identified a specific subgroup of people with knee OA who exhibited PainDETECT scores in the “positive neuropathic” category. These individuals experienced increased levels of pain, widespread, multimodality hyperalgesia, and greater functional impairment than the remaining cohort. Identification of OA patients with this pain phenotype may permit more targeted and effective pain management.
Key Words: PainDETECT, neuropathic pain, knee osteoarthritis, multimodality hyperalgesia, functional impairment
Osteoarthritis (OA) is a common arthritic disorder,1,2 often associated with pain and local tenderness or pressure hyperalgesia around the affected joint(s).3,4 Although knee OA has been considered the archetypal model of inflammatory or nociceptive pain,5 it is increasingly apparent that people with knee OA may present with different pain phenotypes. It is now recognized that some individuals with knee OA exhibit features of neuropathic pain6 and it has been suggested that neuropathic pain in OA may be the result of damage to sensory neurons in subcortical bone as a result of the degenerative pathology.7–9 This relates to the concept of neuropathic pain being, “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system.”10
One approach to evaluating the presence of neuropathic pain has been to use self-report questionnaires such as PainDETECT, Doleur Neuropathic 4 (DN4), and the Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS). These questionnaires predominantly evaluate the degree to which the individual reports phenomena such as burning pain, shooting or lancinating pain, tactile allodynia, and other features that are normally associated with neuropathic pain states.
The PainDETECT questionnaire uses a combination of visual analog scale, body diagram, and Likert-type questions to ask about everyday frequency of symptoms such as “electric shocks” or “painful light touch.” A total score is calculated, with participants scoring ≤12 classified as “negative neuropathic” and those scoring ≥19 as “positive neuropathic.” The group with intermediate scores (13 to 18) is classified as unclear or possible neuropathic.11
A number of studies have evaluated people with knee OA using the PainDETECT questionnaire and demonstrated that some individuals score in the “positive neuropathic” range. The percentage of people with increased PainDETECT scores (≥19) in the “positive neuropathic” category seems to vary between OA cohorts, ranging from 5.4% to 32% although the majority of studies suggest a percentage at the higher end of this range.6,7,12 Similar percentages have also been identified using the DN4 questionnaire (29.4%)13 and the S-LANSS questionnaire (30%).14
Previous research also suggests that increased PainDETECT scores in individuals with knee OA are associated with changes in quantitative sensory testing (QST) measures suggestive of increased pain sensitivity and with higher scores on the Western Ontario and McMaster Universities Arthritis Index (WOMAC).14,15 However, these studies have not clearly differentiated between PainDETECT categories in terms of QST measures and functional capacity.
The current study sought to explore the relationship between self-report of neuropathic pain (based on PainDETECT scores) and pain report, sensory impairment, multimodality hyperalgesia, and impaired physical function in individuals with knee OA.
The primary aim of the study was to determine if there were differences in measures of pain, hyperalgesia, sensation, and function between 3 subgroups of participants categorized by PainDETECT scores (“negative neuropathic” [≤12], “unclear neuropathic” (13 to 18) and “positive neuropathic” (≥19).
MATERIALS AND METHODS
Participants
In total, 130 participants with painful knee OA were recruited from the Perth community. Participants were assessed for suitability by a Rheumatologist, using the American College of Rheumatology (ACR) classification system.16 People who were diagnosed as having knee OA based on the ACR criteria and who reported pain ≥4/10 were included in the study. Exclusion criteria included: history of systemic inflammatory conditions; neurological disorders affecting sensory or motor function; recent (<6 mo) lower limb injury or surgery; or history of other chronic pain disorders (eg, fibromyalgia).
All participants provided written informed consent before participating in the study. Ethical approval was provided by Royal Perth Hospital Medical Research Ethics Committee (EC2009/100 and REG 13-005) and by Curtin University Human Research Ethics Committee (HR26/2010 and 79/2013).
Study Design and Procedure
The study used a cross-sectional design, with participants attending for 1 test session. Participants underwent a washout period equal to 5 half lives of their analgesic or nonsteroidal anti-inflammatory drugs medication before testing. They were able to use paracetamol (acetaminophen) for analgesia if required during this washout period but were asked to refrain from its use for 12 hours before testing. All participants initially completed the WOMAC Osteoarthritis Index for the Knee,17 the PainDETECT questionnaire,11 and the Pain Quality Assessment Scale (PQAS).18 They then completed a series of QST measures and a series of tests of physical function.
Self-report Questionnaires
PainDETECT is a validated self-report tool with good internal consistency and high sensitivity and specificity that has been used to identify neuropathic pain features in a range of conditions.11 The maximum score is 30 with scores ≥19 being designated as “positive neuropathic.”
PQAS was also used to provide data regarding the type of spontaneous pain experienced.18 The questionnaire includes 17 questions about the type of pain plus additional numerical rating scales for unpleasantness and surface versus deep pain. Three pain subscores are then calculated18: paroxysmal, surface, and deep. The questionnaire has demonstrated good reliability and excellent internal consistency for all of the subscales.18 It has been suggested that differences between the deep and surface or paroxysmal subscale scores may differentiate nociceptive-type and neuropathic-type pain.18
WOMAC was used to evaluate subjective pain, stiffness, and functional limitation. This OA-specific self-report scale has been widely used to measure pain and disability from knee OA, demonstrating good internal validity and test-retest reliability.17 A higher score denotes greater functional limitation.
Physical Function Tests
The aggregated locomotor function (ALF) test19 was used as a measure of observed locomotor function. The score was calculated by summing the time (seconds) taken to complete 3 locomotor tasks: walk 2-meter to a chair, sit, stand, and walk back 2-meter; 8-meter return walk; ascend/descend 10 stairs. All instructions were standardized, with participants asked to complete each task “as briskly as possible.” The score has good interrater reliability and is moderately well correlated with both WOMAC and SF-36 function indices, and is reported to be responsive to change following intervention over a short time period.19
QSTs
All QSTs were applied using standardized instructions at standardized sites: at the OA knee and the contralateral knee (medial joint line) and at the ipsilateral elbow over the extensor carpi radialis brevis (ECRB) muscle.20 Triplicate measures were obtained. Order of testing was randomized between QST modalities and between test sites.
Pressure pain threshold (PPT) was assessed using an electronic digital pressure algometer (Somedic AB, Sweden), a device with good test-retest reliability.21 A 1 cm2 algometer probe was applied at 90 degrees to the skin at a rate of 40 kPa/s. Participants were instructed to press the hand-held switch as soon as the sensation of pressure became one of painful pressure.22 Lower PPT values indicate increased sensitivity.
Cold detection threshold (CDT) and cold pain threshold (CPT) were measured using a Peltier thermode (Medoc, Israel) and standard method of limits.23 The probe was attached to the test site with a Velcro strap. The temperature reduced at a rate of 1°C/s from a baseline temperature of 32°C to a minimum of 0°C. CDT was always measured first. Participants were instructed to press the hand-held switch as soon as they perceived any cooling change from baseline. For CPT, participants were instructed to press the switch as soon as the cooling sensation changed to one of painful cold. Some participants failed to indicate cold pain before the thermode reached the minimum temperature of 0°C. These participants were assigned a CPT of 0°C. Higher CPT values indicate increased cold pain sensitivity. Warm detection threshold (WDT) and heat pain threshold (HPT) were measured with the Medoc Peltier thermode using similar methodology to cold testing (baseline 32°C, 1°C/s ascending ramp), with maximum temperature set at 50°C. WDT was defined as the temperature (°C) at which participants first perceived an increase in warmth from baseline, whereas HPT was defined as the temperature (°C) at which participants perceived that the heating sensation had become one of painful heat. Some participants failed to indicate heat pain before the thermode reached the maximum temperature of 50°C. These participants were assigned a HPT of 50°C. Lower HPT values indicate increased heat pain sensitivity.
Statistical Analyses
Data were analyzed using SPSS version 22 (IBM Corp.) with alpha set at P<0.05. Participants were divided post hoc into 3 groups based on PainDETECT score (≤12, 13 to 18, ≥19). Data were evaluated to determine if they met the assumption of normality using the Shapiro-Wilk test. Those measures that were normally distributed were analyzed using 1-way analysis of variance (ANOVA) with Dunnett t post hoc tests using the high PainDETECT group (≥19) as control. Data that were not normally distributed were analyzed using the nonparametric independent samples Kruskal-Wallis test and the Mann-Witney U test.
On the basis of previous research it was predicted that 15% to 25% of participants would score in the “positive neuropathic” category on PainDETECT.6,7,12 With an estimated sample size of n=20 for the high PainDETECT group, it was calculated that the study would have 80% power to detect a between-groups mean difference of 38 kPa (SD, 57 kPa) in PPT, a 5.4°C (SD, 2.3°C) difference in CPT and a 7.8 mm (SD, 16 mm) difference in total WOMAC score.24 These values equate to a 15% to 20% between-group difference.24 On the basis of the high PainDETECT group constituting 15% of the overall cohort a sample of 130 participants with knee OA was recruited for the study.
RESULTS
Participant Demographics
The 130 participants (62 male:68 female) had a mean age of 66 years (range, 50 to 88 y). They reported moderate pain (WOMAC pain, 18.5/50) and functional disability (WOMAC function, 60.6/250).
On the basis of PainDETECT score 29 participants (22.3%) were classified as “positive neuropathic” (score ≥19), 46 as “unclear neuropathic” (35.4%) (score, 13 to 18) and 55 as “negative neuropathic” (42.3%) (score ≤12).
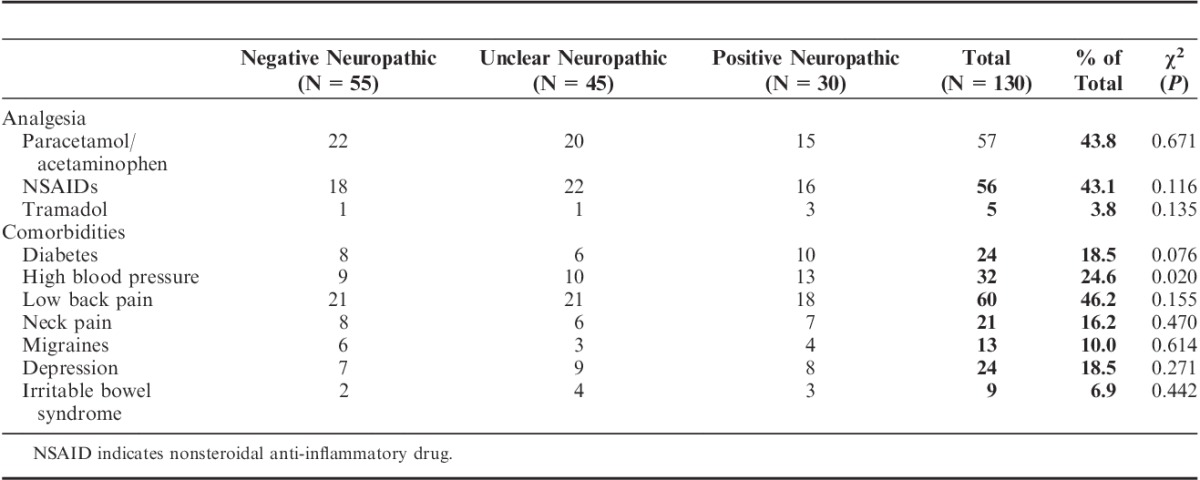
Participants predominantly used paracetamol/acetaminophen or nonsteroidal anti-inflammatory drugs for pain management (Table 1). They reported a number of comorbidities with diabetes and high blood pressure reported by a higher proportion of the “positive neuropathic” group (Table 1).
TABLE 1.
Comparison of Medication Use and Self-reported Comorbidities for Each of the 3 PainDETECT Groups
Self-report Questionnaires
There were significant differences between PainDETECT categories for WOMAC pain scores (F2,127 =18.23, P<0.0001), function scores (F2,127 =18.30, P<0.0001), stiffness scores (F2,127=10.38, P<0.0001), and total scores (F2,127=22.28, P<0.0001). Post hoc tests (Dunnett’s t) showed significant differences between the “positive neuropathic” group and each of the other groups for pain, function, stiffness, and total score (Fig. 1).
FIGURE 1.
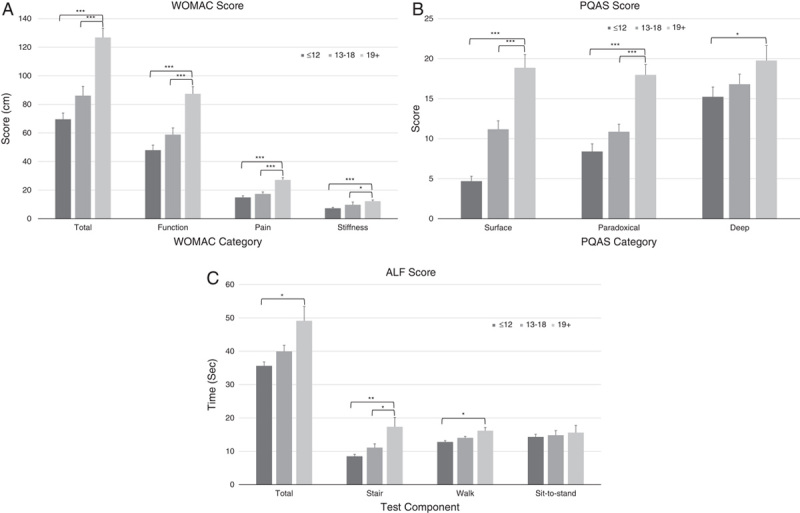
Comparison between the “negative neuropathic” (≤12), unclear (13 to 18) and “positive neuropathic” (19+) PainDETECT categories for scores obtained in the subcategories of the WOMAC questionnaire (A), the PQAS questionnaire (B), and the ALF test (C). ALF indicates aggregated locomotor function; PQAS, Pain Quality Assessment Scale; WOMAC, Western Ontario and McMaster Universities Arthritis Index. *P<0.05, **P<0.001, ***P<0.0001.
PQAS scores also showed differences between the PainDETECT groups for the paradoxical (F2,127=18.66, P<0.0001) and surface (F2,127=43.44, P<0.0001) pain categories but there was no significant difference for the deep pain category (F2,127=2.33, P=0.10). Post hoc tests showed significant differences (P<0.0001) between the “positive neuropathic” group and each of the other PainDETECT groups for paradoxical pain and surface pain (Fig. 1). There was a significant difference between the “positive neuropathic” and “negative neuropathic” groups (P=0.029) for deep pain but no difference between the “positive neuropathic” group and the unclear group (P=0.141) (Fig. 1).
Physical Function Tests
There was a significant difference between PainDETECT groups for the stair climb (P=0.001) and walk (P=0.004) components of the ALF, and the total score (P=0.007) but no significant difference for the sit-to-stand (P=0.676) component (Fig. 1). Comparisons between the “positive neuropathic” and “negative neuropathic” groups followed the same pattern (stair, P<0.001; walk, P=0.002; sit-to-stand, P=0.369; total, P=0.003). There was also a significant difference between the “positive neuropathic” group and the unclear group for the stair component of the test (P=0.024).
QSTs
Pain Thresholds
There were significant differences in PPTs at the index knee (F2,127=24.56, P<0.0001), contralateral knee (F2,127=27.69, P<0.0001), and ECRB sites (F2,127=10.22, P<0.0001). Post hoc tests showed significantly (P<0.0001) lower PPTs (sensitized) for the “positive neuropathic” group relative to the “negative neuropathic” group at all test sites but no significant difference between the “positive neuropathic” group and the unclear group (Fig. 2).
FIGURE 2.
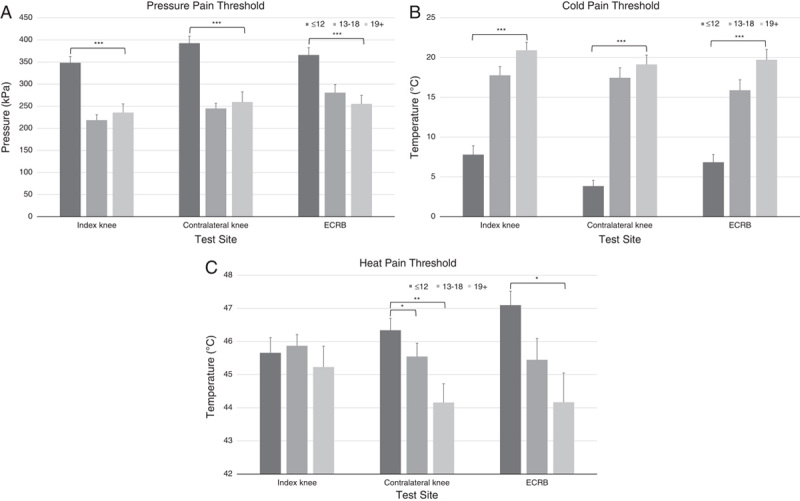
Comparison between the “negative neuropathic” (≤12), unclear (13 to 18), and “positive neuropathic” (19+) PainDETECT categories for pressure pain thresholds (A), cold pain thresholds (B), and heat pain thresholds (C) at each of 3 test sites. ECRB indicates extensor carpi radialis brevis. *P<0.05, **P<0.001, ***P<0.0001.
CPTs were also significantly different between PainDETECT groups at all sites (Kruskal-Wallis test: index knee, P<0.001; contralateral knee, P<0.001; ECRB, P<0.001). CPTs for the “positive neuropathic” group were significantly (P<0.001) higher (sensitized) than the “negative neuropathic” group at all sites but there was no difference between the “positive neuropathic” group and the unclear group at any site (Fig. 2).
Similarly, there were significant differences in HPTs at the contralateral knee (P=0.004) and the ECRB site (P=0.02) but not at the index knee (P=0.72). HPTs for the “positive neuropathic” group were significantly lower (sensitized) than the “negative neuropathic” group at the contralateral knee (P=0.001) and ECRB sites (P=0.007) but not at the index knee (P=0.472). There was a significant difference between the “positive neuropathic” group and the unclear group at the contralateral knee (P=0.041) but no difference at the other sites (index knee, P=0.466; ECRB, P=0.212) (Fig. 2).
Sensory Thresholds
CDTs were not significantly different at any site (index knee, P=0.935; contralateral knee, P=0.455; ECRB, P=0.118) (Fig. 3). There was a significant difference in WDTs at the contralateral knee (P=0.005) but not at the other sites (index knee, P=0.069; ECRB, P=0.453) (Fig. 3). At the index knee (P=0.033) and the contralateral knee (P=0.018) there was a significant difference between the “positive neuropathic” group and the “negative neuropathic” group indicating some degree of sensory impairment but there was no difference between the “positive neuropathic” group and the unclear group at any site.
FIGURE 3.
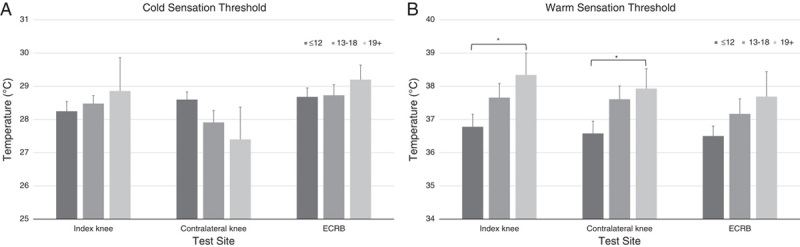
Comparison between the “negative neuropathic” (≤12), unclear (13 to 18), and “positive neuropathic” (19+) PainDETECT categories for cold sensation thresholds (A) and warm detection thresholds (B) at each of 3 test sites. ECRB indicates extensor carpi radialis brevis. *P≤0.01.
DISCUSSION
This study investigated levels of pain, hyperalgesia, and physical function in participants with knee OA, grouped according to PainDETECT score. Those scoring in the “positive neuropathic” category reported the greatest pain and disability, and demonstrated widespread hyperalgesia and greater functional limitations.
Participants with knee OA in this study demonstrated a range of PainDETECT scores from 0 to 30 (of a maximum score 30), reflecting very heterogenous pain experiences. In total, 22.3% of the participants scored in the “positive neuropathic” category, suggesting that they may be experiencing features of neuropathic pain. In previous studies the percentage of participants scoring in the “positive neuropathic” pain category has ranged from 5.4% to 32%.6,7,12 The findings from this study are therefore a little less than some previous studies but nevertheless within the previously published range.
When tested across a range of other self-report measures, participants in the “positive neuropathic” pain category reported increased pain and decreased function relative to the remaining patient cohort. WOMAC pain, stiffness, and function subscores were elevated for this group. There are no previous studies that have evaluated minimum clinically important differences (MCID) between patient cohorts, but the 32% reduction in WOMAC total score between the “positive neuropathic” group and the intermediate group is considerably >16% MCID for reduction in total WOMAC score following drug treatments.25
The “positive neuropathic” pain group also reported significantly higher scores than the remaining cohort for the surface and paradoxical pain quality subscales of PQAS, both of which are thought to reflect features of neuropathic pain.18 It therefore seems that this group experiences not just increased pain severity but also distinctive pain qualities that are often associated with neuropathic pain.
In addition to WOMAC self-report of reduced functional capacity, participants in the “positive neuropathic” group were slower to complete physical tasks. They exhibited slower times for the stair climb and walk components of the ALF test and had significantly increased total times. This further emphasizes that they were experiencing greater functional limitation associated with their pain.
Participants in the “positive neuropathic” category also exhibited widespread, multimodality hyperalgesia, or increased pain sensitivity relative to those in the “negative neuropathic” category. In addition to increased pain sensitivity at the OA knee, these participants were also more sensitive to measures of PPT, CPT, and HPT at the distant ECRB test site in the upper limb. Differences in PPT between groups exceeded the reported MCID of 114 kPa26 at both knees but not at the ECRB site. Interestingly, there was no significant difference in HPT at the index knee. This was a somewhat surprising finding given the clear differences that were present at the other test sites and the other test modalities. In a recent publication we have demonstrated that a subgroup of patients with increased CPTs also present with widespread multimodality hyperalgesia and increased PainDETECT scores.27
However, a “positive neuropathic” score on the PainDETECT questionnaire alone is not diagnostic of pain that is neuropathic in origin. Treede et al10 have proposed a grading system with categories of possible, probable, and definite neuropathic pain. Inclusion in the probable neuropathic pain category requires the presence of a measured sensory deficit in an area clearly related to the area of neuropathic pain report. Definite neuropathic pain also requires the existence of imaging or other findings showing a clear causative neuropathology.10
The current study’s findings suggest that while some individuals with knee OA may score highly on the PainDETECT questionnaire there is limited evidence of associated sensory impairment. There were no marked changes in sensory thresholds for cold although there were differences in WDTs suggesting some impaired sensation in the “positive neuropathic” grouping. However, these findings are inconclusive. It should be noted that sensory testing was only carried out at one knee location (medial joint line). As the area around the knee is innervated by multiple peripheral nerves,28 a single test site is unlikely to adequately evaluate sensory deficits. It is also important to acknowledge a limitation of the study in that comprehensive testing of light touch, pinprick, and vibration sensations was not carried out. Further research is therefore warranted to explore more closely the relationship between pain and neurological deficits in patients with knee OA. In addition to sensory deficits the concurrent presence of proprioceptive deficits might also be explored. It is notable that deficits in proprioceptive function have previously been identified in patients with knee OA.29 Future studies would also benefit from including data from a control cohort to account for normal variations in sensation among an older cohort.
It may also be the case that an increased PainDETECT score in association with widespread multimodality hyperalgesia may simply reflect a centrally augmented pain state14,15 rather than the presence of neuropathic pain. This may reflect enhanced central sensitization and possibly also impaired pain modulation.4 Further research is required to evaluate the development of widespread pain sensitivity and impaired pain modulation to determine if these findings are also present in individuals who do not present with increased PainDETECT scores.
Although this study evaluated a relatively small cohort, the findings clearly suggest that scores on the PainDETECT questionnaire may be a useful indicator of those with a more severe pain state. These findings add further support to the concept that people with knee OA present with different pain phenotypes and so may have significantly different experiences of OA pain.9 In particular, they suggest that a subcohort of patients with knee OA experience more severe “neuropathic-type” pain that has a greater impact on physical function than other individuals with the same condition. A previous study showed that patients with ongoing pain >1 year postjoint replacement surgery showed that this group had higher PainDETECT scores and more functional impairment than patients with minimal pain following surgery.30 This suggests that a standardized approach to pain management might result in some patients with knee OA receiving inadequate treatment and highlights the need for further research to develop clear criteria to diagnose neuropathic pain in knee OA and to optimize the management of pain in this patient group. In particular, it may be appropriate to consider the use of neuropathic pain medications in a subgroup of people with knee OA. Further research is warranted to evaluate this grouping in larger patient cohorts and clinical trials evaluating the efficacy of drugs used to manage neuropathic pain in this subgroup of OA sufferers.
CONCLUSIONS
Individuals with knee OA may report markedly different scores on the PainDETECT questionnaire. Those who score highly on the questionnaire tend to report increased pain, different pain qualities, more functional impairment and more widespread, multimodality hyperalgesia, and pain sensitivity than other people with a diagnosis of knee OA. Further research is needed to determine whether these individuals can be clearly classified as having neuropathic pain if they would benefit from more targeted pain management.
ACKNOWLEDGMENTS
The authors gratefully acknowledge technical assistance from Lisa Webster, BSc and Evelyn Webb, BSc, Research Officers, School of Physiotherapy and Exercise Science, Curtin University, Perth, Australia.
Footnotes
Supported by an Investigator Initiated Studies Grant from Merck Inc., Kenilworth, NJ. The authors declare no conflict of interest.
REFERENCES
- 1.Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9:883–891. [DOI] [PubMed] [Google Scholar]
- 2.Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- 3.Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain. 2000;4:229–238. [DOI] [PubMed] [Google Scholar]
- 4.Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581. [DOI] [PubMed] [Google Scholar]
- 5.Harden RN, Wallach G, Gagnon CM, et al. The osteoarthritis knee model: psychophysical characteristics and putative outcomes. J Pain. 2013;14:281–289. [DOI] [PubMed] [Google Scholar]
- 6.Hochman JR, Gagliese L, Davis AM, et al. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19:647–654. [DOI] [PubMed] [Google Scholar]
- 7.Ohtori S, Orita S, Yamashita M, et al. Existence of a neuropathic pain component in patients with osteoarthritis of the knee. Yonsei Med J. 2012;53:801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivanavicius SP, Ball AD, Heapy CG, et al. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterisation. Pain. 2007;128:272–282. [DOI] [PubMed] [Google Scholar]
- 9.Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol. 2014;10:374–380. [DOI] [PubMed] [Google Scholar]
- 10.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. [DOI] [PubMed] [Google Scholar]
- 11.Freynhagen R, Baron R, Gockel U, et al. PainDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. [DOI] [PubMed] [Google Scholar]
- 12.Valdes AM, Suokas AK, Doherty SA, et al. History of knee surgery is associated with higher prevalence of neuropathic pain-like symptoms in patients with severe osteoarthritis of the knee. Semin Arthritis Rheum. 2014;43:588–592. [DOI] [PubMed] [Google Scholar]
- 13.Oteo-Alvaro A, Ruiz-Iban MA, Miguens X, et al. High prevalence of neuropathic pain features in patients with knee osteoarthritis: a cross-sectional study. Pain Pract. 2015;15:618–626. [DOI] [PubMed] [Google Scholar]
- 14.Moreton BJ, Tew V, das Nair R, et al. Pain phenotype in people with knee osteoarthritis; classification and measurement properties of painDETECT and S-LANSS in a cross-sectional study. Arthritis Care Res. 2015;67:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochman JR, Davis AM, Elkayam J, et al. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1236–1242. [DOI] [PubMed] [Google Scholar]
- 16.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43:1905–1915. [DOI] [PubMed] [Google Scholar]
- 17.Jinks C, Jordan K, Croft P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Pain. 2002;100:55–64. [DOI] [PubMed] [Google Scholar]
- 18.Victor TW, Jensen MP, Gammaitoni AR, et al. The dimensions of pain quality: factor analysis of the Pain Quality Assessment Scale. Clin J Pain. 2008;24:550–555. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy CJ, Oldham JA. The reliability, validity and responsiveness of an aggregated locomotor function (ALF) score in patients with osteoarthritis of the knee. Rheumatology. 2004;43:514–517. [DOI] [PubMed] [Google Scholar]
- 20.Riek S, Carson RG, Wright A. A new technique for the selective recording of extensor carpi radialis longus and brevis EMG. J Electromyogr Kinesiol. 2000;10:249–253. [DOI] [PubMed] [Google Scholar]
- 21.Jones DH, Kilgour RD, Comtois AS. Test-retest reliability of pressure pain threshold measurements of the upper limb and torso in young healthy women. J Pain. 2007;8:650–656. [DOI] [PubMed] [Google Scholar]
- 22.Moss P, Sluka K, Wright A. The initial effects of knee joint mobilization on osteoarthritic hyperalgesia. Man Ther. 2007;12:109–118. [DOI] [PubMed] [Google Scholar]
- 23.Fruhstorfer H, Lindblom U, Schmidt WC. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976;39:1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss P, Knight E, Wright A. Subjects with knee osteoarthritis exhibit widespread hyperalgesia to pressure and cold. PloS One. 2016;11:e0147526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hmamouchi I, Allali F, Tahiri L, et al. Clinically important improvement in the WOMAC and predictor factors for response to non-specific non-steroidal anti-inflammatory drugs in osteoarthritic patients: a prospective study. BMC Res Notes. 2012;5:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walton DM, Levesque L, Payne M, et al. Clinical pressure pain threshold testing in neck pain: comparing protocols, responsiveness, and association with psychological variables. Phys Ther. 2014;94:827–837.24557645 [Google Scholar]
- 27.Wright A, Benson HAE, Will R, et al. Cold pain threshold identifies a sub-group of patients with knee osteoarthritis that present with multi-modality hyperalgesia and elevated pain levels. Clin J Pain. 2017;33:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horner G, Dellon AL. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;301:221–226. [PubMed] [Google Scholar]
- 29.Garsden LR, Bullock-Saxton JE. Joint reposition sense in subjects with unilateral osteoarthritis of the knee. Clin Rehabil. 1999;13:148–155. [DOI] [PubMed] [Google Scholar]
- 30.Wright A, Moss P, Sloan K, et al. Abnormal quantitative sensory testing is associated with persistent pain one year after TKA. Clin Orthop Relat Res. 2015;473:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]


