Abstract
Background:
The involvement of inflammatory components in the pathophysiology of low back pain (LBP) is poorly understood. It has been suggested that spinal manipulative therapy (SMT) may exert anti-inflammatory effects.
Purpose:
The purpose of this study was to determine the involvement of inflammation-associated chemokines (CC series) in the pathogenesis of nonspecific LBP and to evaluate the effect of SMT on that process.
Methods:
Patients presenting with nonradicular, nonspecific LBP (minimum pain score 3 on 10-point visual analog scale) were recruited according to stringent inclusion criteria. They were evaluated for appropriateness to treat using a high velocity low amplitude manipulative thrust in the lumbar-lumbosacral region. Blood samples were obtained at baseline and following the administration of a series of 6 high velocity low amplitude manipulative thrusts on alternate days over the period of 2 weeks. The in vitro levels of CC chemokine ligands (CCL2, CCL3, and CCL4) production and plasma levels of an inflammatory biomarker, soluble E-selectin (sE-selectin), were determined at baseline and at the termination of treatments 2 weeks later.
Results:
Compared with asymptomatic controls baseline production of all chemokines was significantly elevated in acute (P=0.004 to <0.0001), and that of CCL2 and CCL4 in chronic LBP patients (P<0.0001). Furthermore, CCL4 production was significantly higher (P<0.0001) in the acute versus chronic LBP group. sE-selectin levels were significantly higher (P=0.003) in chronic but not in acute LBP patients. Following SMT, patient-reported outcomes showed significant (P<0.0001) improvements in visual analog scale and Oswestry Disability Index scores. This was accompanied by a significant decline in CCL3 production (P<0.0001) in both groups of patients. Change scores for CCL4 production differed significantly (P<0.0001) only for the acute LBP cohort, and no effect on the production of CCL2 or plasma sE-selectin levels was noted in either group.
Conclusions:
The production of chemotactic cytokines is significantly and protractedly elevated in LBP patients. Changes in chemokine production levels, which might be related to SMT, differ in the acute and chronic LBP patient cohorts.
Key Words: low back pain, CC chemokines, sE-selectin, spinal manipulation, inflammation
The etiology of nonspecific (mechanical) low back pain (LBP) is multifaceted.1,2 Acute LBP is generally considered to be associated with enhanced pain sensitivity of the spinal/paraspinal structures.3 The persistence of spinal pain >12 weeks, normally sufficient for completion of connective tissue healing, suggests that acute spinal pain has become chronic.4
Among nonpharmacological treatments for LBP, the use of spinal manipulative therapy (SMT) has been widely practiced and its relative effectiveness both for chronic and acute LBP has been reviewed.5,6 While correcting segmental restrictions and biomechanical aberrations may provide a feasible justification for efficacy of spinal manipulation, biological mechanisms associated with this form of therapy remain unclear.7 Recent investigations, suggest that SMT may exert anti-inflammatory effect(s)8 and thereby may impact the integrated network of inflammatory and immunoregulatory mediators inherent in spinal pain.9
Tissue injury-associated inflammatory responses are accompanied by increased neuronal excitability and migration of leukocytes to the affected sites.10,11 This process is mediated by a gradient of a subfamily of chemotactic cytokines (chemokines) including macrophage chemotactic protein (CC chemokine ligand [CCL2]), macrophage inflammatory proteins 1α (CCL3), and 1β (CCL4), generated on the surface of endothelial cells. Inflammatory cytokine-activated endothelial cells12,13 upregulate the production of chemokines and vascular adhesion proteins including E-selectin, a potent mediator of leukocyte movement into tissues.14
Chemokines are inducers of inflammation,15 play a role in communication between inflammatory cells and neurons,15–17 and contribute to pain transmission.18 Studies of the relationship between clinical pain and the production of CC chemokines are limited.19–22 Levels of inducible CCL2 and CCL3 production have been shown to be significantly increased, alongside the heightened production of inflammatory cytokines, in patients with chronic and recurrent cervical neck pain.23 However, potential involvement of chemokines in the pathophysiology of nonspecific LBP has not been examined. Also, to our knowledge, no studies have explored potential association of endothelial adhesion molecules with inflammatory response in spinal pain. Nonetheless, a possible role of E-selectin as a marker of lumbar spine disease has been suggested.24 Elevated levels of soluble E-selectin (sE-selectin) production are considered a reliable biomarker of local or systemic inflammatory response.25 Secretion of sE-selectin and its accumulation in herniated disk specimens and in human intervertebral disk cultures have been also recently reported.26 Thus, elevation of systemic (plasma) levels of sE-selectin could be symptomatic of inflammatory condition(s) involving paraspinal tissues and/or lumbar disk disease.
As part of an ongoing study of the role of inflammation in nonspecific LBP, the present investigation was undertaken to assess the production of migratory/nociceptive chemokines, CCL2, CCL3, and CCL4, and that of sE-selectin in patients with acute and chronic LBP before and after SMT.
METHODS
Patients
Patients of both sexes aged between 22 and 60 years, experiencing acute (<4 wk in duration) or chronic (≥12 wk in duration) nonspecific LBP (L1-L5, with or without sacroiliac joint involvement) were enrolled into the study through the Canadian Memorial Chiropractic College (CMCC) outpatient clinics (Fig. 1). A small group of patients was referred from field practitioners. This study was approved by the Research Ethics Board of the CMCC and was registered with Clinical Trials.gov (#NCT01766141).
FIGURE 1.
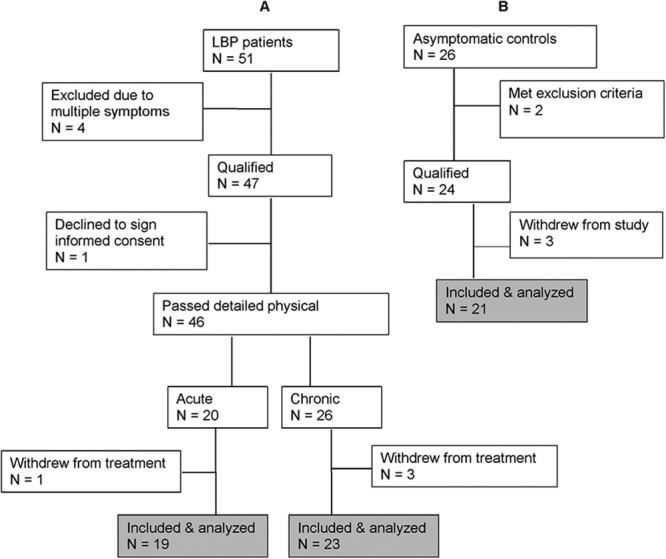
Flow chart of participant enrolment showing process of exclusion and inclusion in the low back pain (A) and control (B) groups.
Participants were screened for eligibility, according to strict inclusion and exclusion criteria (see below), by the primary investigator (S.H.I.). If accepted, they were asked to sign the study-informed consent form and complete the CMCC clinic intake forms including an Oswestry Disability Index (ODI) form and a 10-point visual analog scale (VAS) for pain. The utility and validity of these assessment tools has been established.27,28 A final decision whether to accept or reject a potential patient was on the basis of the detailed history and physical findings of the intern/clinician to whom patient care was assigned. Patients were excluded if they fell outside the age limits of 22 to 60 years, had a pain level below 3/10 on VAS, had received any form of manual treatment in the preceding 15 days, had taken anti-inflammatory medications in the preceding 48 hours, had any type of unresolved known inflammatory condition (including systemic or musculoskeletal other than the presenting LBP condition), autoimmune conditions, coagulopathies, infections and neoplastic diseases, were pregnant, were unwilling to sign the study consent form, or were unwilling/unable to adhere to study schedule. Finally, patients were instructed to abstain from anti-inflammatory medications throughout the study period. This request was enforced at each treatment visit.
A cohort of age-matched and sex-matched healthy asymptomatic participants was recruited from the general population to serve as control group. The same exclusion criteria, including the presence of LBP, applied in this recruitment process (Fig. 1). Importantly, these participants did not receive any form of treatment. The purpose of this cohort was to control for possible changes in outcomes that might occur naturally in the span of the observation period (2 weeks).
SMT
SMT consisted of a single high velocity low amplitude manipulative thrust to the involved segment in the lumbosacral region. This could be in the form of a spinal push or spinal pull type adjustment to the lumbar spine, or an SI adjustment.29 Six SMT treatments were carried out by the attending clinicians on alternate days in the span of 2 weeks. The participating clinicians delivered the treatments according to their findings of segmental restriction in the lumbosacral region on a given day. Although the assessment might indicate involvement of >1 spinal segment, clinicians were to apply a manipulative thrust to 1 segment only as indicated by pain or restricted motion upon palpation. No other treatment modalities were utilized for the duration of the study. The treatment intervention was designed to explore the effect of a series of single manipulative thrusts rather than chiropractic treatment as might occur in a typical chiropractic-patient encounter, where treatment dose and duration are typically longer.30
At the termination of the treatment period all patients were asked to complete the VAS and ODI forms again.
Asymptomatic participants in the study received no treatments. Upon their return 2 weeks later, for a second blood withdrawal, participants were screened to rule out possible development of excluding factors that would mitigate their participation in the study.
LABORATORY STUDIES
Blood Collection
Heparinized samples of peripheral blood (7 mL each) from patients with LBP and controls were collected twice over the study period by venipuncture from the antecubital fossa area of the arm. The first sample was obtained before any manipulative intervention, the second at their seventh visit (within 48 h of the last treatment) alongside the second VAS and ODI questionnaires. The second blood collection from asymptomatic participants was carried out 2 weeks after the initial one.
All blood samples were coded, transferred to the laboratory and processed within 60 minutes of collection. Two milliliters of each blood sample was centrifuged for 10 minutes at 4°C for preparation of plasma, whereas the remainder was used to set up cultures. Aliquoted plasma samples were frozen at −80°C for later studies.
Culture System
Whole blood (WB) culture system, similar to that described by Yaqoob et al,31 was used. Briefly, blood samples were diluted 10-fold with RPMI 1640 (Gibco, Invitrogen, Grand Island, NY) supplemented with 5×10−5 mol/L of 2-mercaptoethanol and a commercial solution of l-glutamin-penicillin-streptomycin (Gibco, Life Technologies, Burlington, ON).
The production of the studied chemokines was investigated in inducer-activated preparations. Spontaneous (constitutive) secretion of these mediators could not be assessed because of the limitation of biological material. To induce the production of CCL2 and CCL4, WB cultures were cultivated for 48 hours at 37°C, in a humidified 5% CO2 incubator, in the presence of lipopolysaccharide (Sigma-Aldrich, St. Louis, MO) at a concentration of 1 μg/mL and 10 μg/mL of phytohemagglutinin (Sigma-Aldrich). The production of CCL3 was examined in cultures activated for 24 hours with lipopolysaccharide alone. At the conclusion of the incubation period, culture supernatants from each subject were pooled, centrifuged to remove any contaminating cellular material, aliquoted and frozen at −80°C until further analysis.
Determination of CC Chemokine and sE-Selectin Levels
The levels of in vitro production of the CC chemokines were determined by specific enzyme-linked immunosorbant assays (ELISA) using DuoSet ELISA development system for natural and recombinant human cytokines, and Quantikine ELISA (R&D Systems, Minneapolis, MN) was used to determine plasma sE-selectin levels. All quantitative determinations were performed according to the manufacturer’s recommendations. Each of the studied culture supernatants was tested a minimum of 3 times at 2 to 4 different dilutions. The absorbance of the color developed following the enzymatic reaction in the studied samples was measured at λ=540 nm using multichannel spectrophotometer (Epoch; Bio-Tech, Winooski, VT). Concentrations of the tested mediators were then determined using Gen5 Data Analysis Software (Bio-Tech).
Detection limits for CCL2 and CCL4 were 15 pg/mL, 10 pg/mL for CCL3 and 0.25 ng/mL for human sE-selectin.
Statistics
Sample Size
Data published for TNF α levels in chronic neck pain patients versus asymptomatic controls23 were used to calculate a sample size estimate for this study. From the Cohen table,32 on the basis of a power of 0.8, a 2-tailed test with a P<0.05, the sample size was estimated to be no less than 17 per group (Fig. 1).
Data Analysis
The primary outcomes for this study were established as differences in the production of mediators (1) between patient groups, that is, between acute and chronic LBP and asymptomatic controls measured at the time of admission into the study (baseline, Time 1); and (2) within group differences between chemokine production at baseline and after completion of SMT treatments for the acute versus chronic LBP groups and the control group (Time 2).
One-way analysis of variance (ANOVA) was completed comparing the baseline levels of CCL2, CCL3, CCL4, and sE-selectin production for the asymptomatic control participants against patients in the acute and chronic pain groups. All baseline data were tested for normality. Where non-normal distributions were found, data were transformed and analysis repeated. Where tests for equal variances failed, Kruskal-Wallis tests were used to confirm results. If statistically significant F-values were found using ANOVA, contrasts between groups were assessed using Scheffe tests.
Differences in scores were then calculated between the baseline and postintervention or the second assessment values (Time 2) for the acute and chronic LBP patient groups and asymptomatic controls. Tests for normality and the Barlett test for equal variances were used to assess assumptions. As the equal variances assumptions were not met, Kruskal-Wallis tests were used for all tests of difference in scores and subsequent contrasts between groups.
A total of 4 one-way ANOVAs were used, and 4 Kruskal-Wallis analyses were performed (1 for each of the outcome measures). Thus, an adjusted value of P<0.00625 was accepted as significant.
Pre-SMT and post-SMT VAS and ODI values were obtained for observational purposes and for hypothesis generation and analyzed using a paired t test.
RESULTS
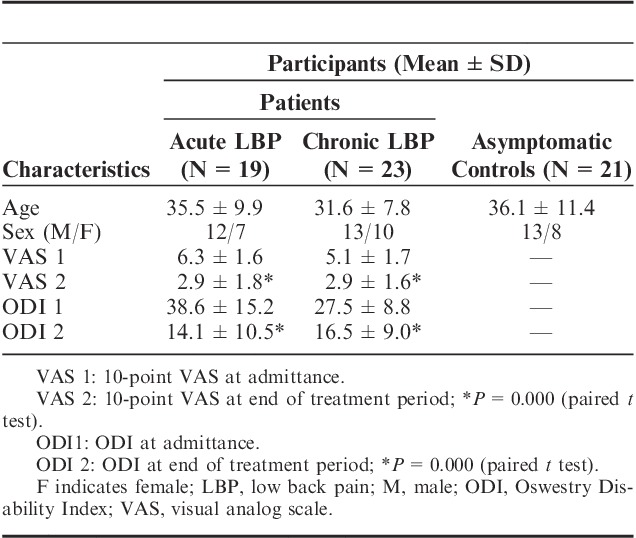
The studies were completed by 19 patients with acute LBP, 23 with chronic LBP, and 21 asymptomatic volunteers (Fig. 1). Demographic profiles of LBP patients and asymptomatic control participants were comparable in terms of age and sex (Table 1). Admission time VAS scores were not significantly different between the patient groups and decreased significantly (P<0.0001) post-SMT interventions in both acute and chronic LBP patients (6.3±1.6 vs. 2.9±1.8 and 5.1±1.7 vs. 2.9±1.6, respectively) (Table 1). At admission, mean ODI scores for the acute and chronic LBP cohorts were 38.6±15.2 and 27.5±8.8, respectively. Following the 2-week treatment period the scores had declined significantly (P<0.0001) to 14.1±10.5 and 16.5±9.0, respectively.
TABLE 1.
Demographic Characteristics and Measures of Pain and Disability of Participants Enrolled in the Study
Baseline Levels of CC Chemokine and sE-Selectin Production
Baseline levels of the studied chemokines were assessed in supernatants from WB cultures prepared from the LBP patients and asymptomatic controls at the time of their admission into the study (Time 1). Figures 2,3">,4 (open circles) illustrate the ranges and means of CC-chemokine production in all study patients. Post-ANOVA contrasts indicated that, at baseline (Time 1), significant differences existed in levels of the studied chemokines between LBP patients and asymptomatic controls. Relative to controls the production of CCL2, CCL3, and CCL4 were significantly augmented (P=0.004, <0.0001, and <0.0001, respectively) in acute LBP patients. In patients with chronic LBP, the production of CCL2 and CCL4 was also significantly elevated (P<0.0001 and <0.0001), whereas that of CCL3 trended higher (P=0.008) (Figs. 2,3">4).
FIGURE 2.
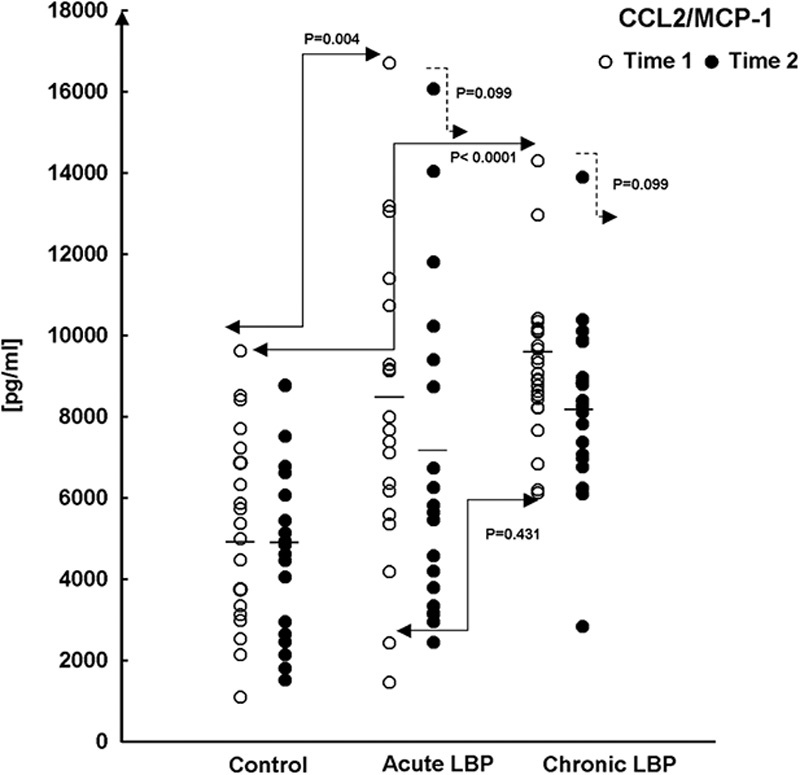
Production of CCL2/MCP-1 in WB cultures from acute and chronic LBP patients and asymptomatic participants (Control) at baseline (Time 1) and after 2 weeks (Time 2) during which LBP patients received 6 SMT treatments. WB preparations were activated at initiation with the combination of LPS/PHA, and culture supernatants were collected 24 hours later. At baseline (Time 1, →), statistical significance of differences exists between control and acute, and control and chronic LBP patients (P=0.004 and <0.0001, respectively; Scheffe’s test). The means of CCL2 production (—) are shown for each study group. CCL indicates CC chemokine ligand; LBP, low back pain; LPS, lipopolysaccharide; MCP, monocyte chemoattractant protein; PHA, phytohemagglutinin; SMT, spinal manipulative therapy; WB, whole blood.
FIGURE 3.
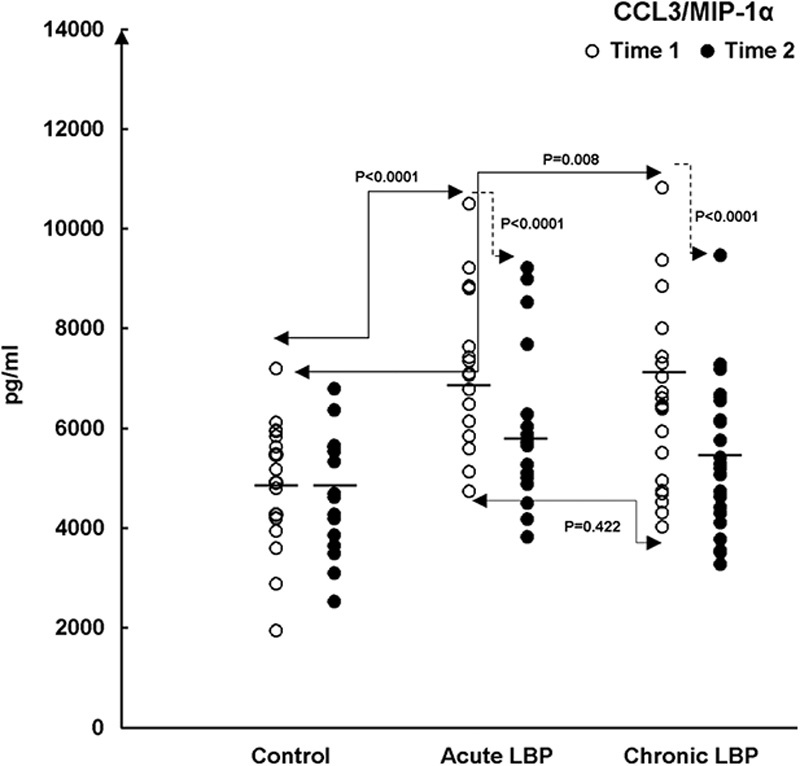
Production of CCL3/MIP-1α in LPS-stimulated WB cultures from the studied LBP patients and asymptomatic patients (Control). Patient cultures were established before the initiation of SMT treatments (baseline, Time 1) and following their completion (Time 2). Control participants were studied within the span of 2 weeks between Time 1 and Time 2. The figure depicts statistical significance of differences in: (A) the baseline levels (Time 1, →) of CCL3 production between control versus acute LBP patients (P<0.0001, Scheffe’s test). At baseline, CCL3 levels trend higher (P=0.008, Scheffe’s test) in patients with chronic LBP (B) SMT-related changes in acute and chronic LBP patient groups compared with changes in asymptomatic patients at Time 2 (→) (P<0.0001 and <0.0001, respectively; Kruskal-Wallis test). The means of chemokine production (—) are shown for each study group. CCL indicates CC chemokine ligand; LBP, low back pain; LPS, lipopolysaccharide; MIP, macrophage inflammatory protein; PHA, phytohemagglutinin; SMT, spinal manipulative therapy; WB, whole blood.
FIGURE 4.
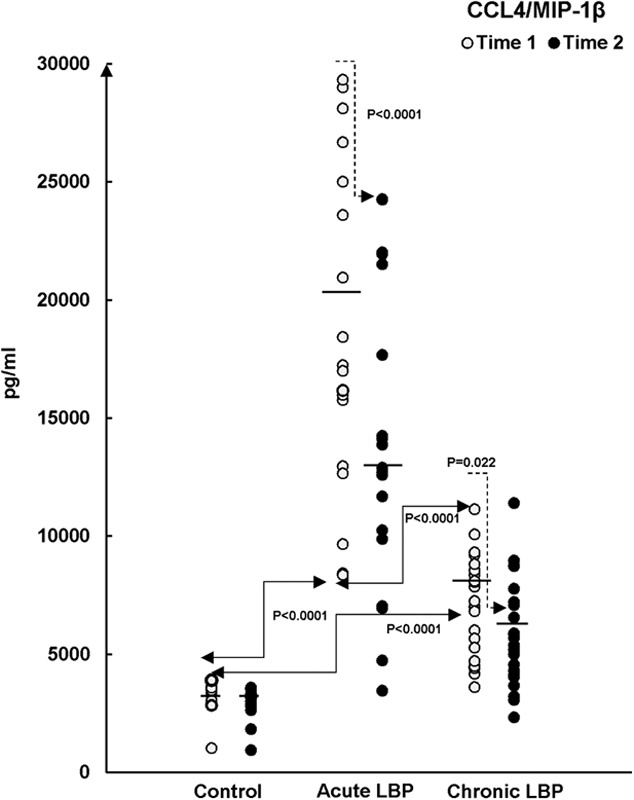
Production of CCL4/MIP-1β in LPS/PHA-activated WB cultures from patients with acute and chronic LBP and asymptomatic participants (Control) at baseline (Time 1) and after 2 weeks during which the patients received SMT treatments (Time 2). The statistical significance of differences is apparent in (A) the baseline levels (Time 1, →) of CCL4 production between all study groups (P<0.0001, Scheffe’s test) and (B) SMT-related changes (Time 2, →) in acute LPB group versus time-related changes in the asymptomatic control group (P<0.0001, Kruskal-Wallis test). The means of chemokine production in all study groups are also shown (—). CCL indicates CC chemokine ligand; LBP, low back pain; LPS, lipopolysaccharide; MIP, macrophage inflammatory protein; PHA, phytohemagglutinin; SMT, spinal manipulative therapy; WB, whole blood.
The capacity of CC chemokine production was compared between acute and chronic LBP cohorts at baseline. The production of CCL4 was significantly higher (P<0.0001) in the acute LBP group (Fig. 4), whereas both acute and chronic LBP groups did not differ significantly in baseline levels of CCL2 (P=0.431) and CCL3 (P=0.422) production.
The plasma content of sE-selectin varied somewhat between the study groups (Fig. 5). Compared with controls, sE-selectin levels were not significantly different in patients with acute LBP (P=0.04) but were significantly elevated (P=0.003) in the chronic LBP group. Analysis of the data excluding the outlier in the chronic LBP group did not affect the significance of these results (P=0.005).
FIGURE 5.
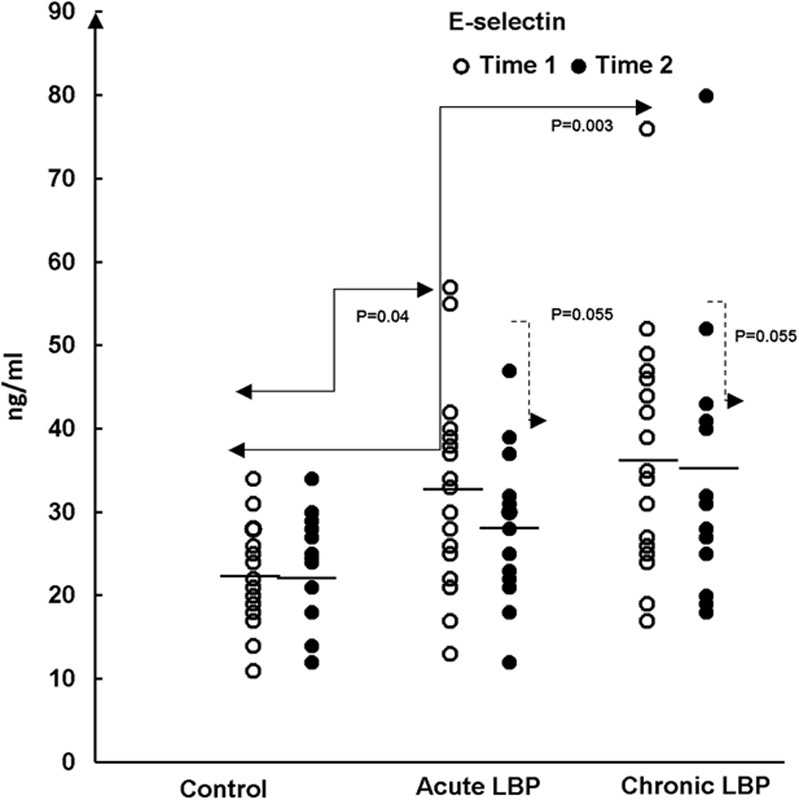
Plasma levels of soluble E-selectin (sE-selectin) in samples from the studied LBP patients and asymptomatic patients (Control). Samples were collected before (baseline, Time 1) and after spinal manipulative therapy treatments (Time 2). Control participants were studied 2 weeks apart, between Time 1 and Time 2. A statistically significant difference (P=0.003; Kruskal-Wallis test) in the level of sE-selectin was observed between the control and patients with chronic LBP at baseline (Time 1) and following SMT (Time 2).The means of sE-selectin level are shown for each study group (—). LBP indicates low back pain; SMT, spinal manipulative therapy.
Effect of SMT on the Production of the Studied Mediators
Posttreatment levels of the studied mediators were assessed in supernatants from WB cultures prepared from LBP patients at the termination of the SMT treatment period and from asymptomatic controls retested 2 weeks after Time 1 (ie, at Time 2). Mean chemokine production declined across the board in both groups of LBP patients while remaining essentially unchanged in asymptomatic participants (Figs. 2,3">,4, closed circles). However, the effect of SMT varied in relation to the controls and also between the acute and chronic LBP groups. Kruskal-Wallis analysis indicated treatment-related changes were statistically significant with regard to CCL3 (χ2=14.65; P=0.001) and CCL4 (χ2=31.2; P<0.0001) production. Contrast analysis for changes in CCL3 production indicated a significantly greater change (P<0.0001) for both acute and chronic LBP patients versus the asymptomatic controls (Fig. 3). Analysis of contrasts for changes in CCL4 production showed a statistically significant difference (P<0.0001) existed between the acute pain and the control groups but not between the control and chronic pain group (P=0.022) (Fig. 4). Furthermore, SMT-related change scores in CCL4, but not in CCL3, production differed significantly (P<0.0001) between patients with acute and chronic LBP groups.
Although the mean levels of post-SMT production of CCL2 were reduced markedly (up to 1400 pg/mL) in both groups of patients, the effect of intervention-related change did not reach statistical significance (χ2=4.63; P=0.099; Fig. 2).
SMT had no significant effect (χ2=5.78; P=0.055) on the systemic levels of sE-selectin production, which remained significantly elevated in chronic LBP patients and unchanged in the acute pain group (Fig. 5).
DISCUSSION
To our knowledge, the results of the present study are the first to demonstrate that, compared with asymptomatic controls, the capacity for the inducible (in vitro) synthesis of the CC-subfamily chemokines is significantly enhanced in patients presenting with nonspecific acute and chronic LBP (Figs. 2,3">,4). Furthermore, we have demonstrated that in vitro production of these mediators differs between the acute and chronic LBP groups. The baseline production of CCL4 is significantly higher in the acute compared with the chronic pain group (Fig. 4). In contrast, systemic release of sE-selectin, significantly elevated in patients with chronic LBP, only trends higher in the acute pain group (Fig. 5).
The underlying physiological mechanisms of the aforementioned variability in chemokine production vis a vis the acute and chronic LBP are certainly complex and cannot be elucidated within the scope of the present study. It is known, however, that the inducible production of CC chemokines is differentially regulated by proinflammatory and immunoregulatory cytokines33–35 and inflammatory profiles may differ in patients with acute and chronic LBP, a possibility that is currently under study in our laboratory.36 Such differences may, at least partially, contribute to divergence in chemokine production observed between the groups presenting with acute or chronic LB pain.
SMT-associated changes in the production of chemokines also differed between the studied groups of LBP patients. The overall decline in the production of the investigated chemokines was observed in both groups. However, while significantly relevant differences (decline) in the production of both CCL3 and CCL4 were found for the acute pain patients, SMT exerted a statistically significant effect only with respect to CCL3 production in patients with chronic LB pain.
Despite a decline in the post-SMT production of the aforementioned chemokines their levels did not revert to that observed in asymptomatic patients (Figs. 2,3">,4). Correspondingly, systemic levels of sE-selectin release were essentially unchanged remaining markedly or significantly higher than physiological (Fig. 5). These findings seem consistent with the lack of complete resolution of symptoms indicated by the VAS and ODI scores at the termination of the treatment period (Table 1). The sustained levels of these mediators may suggest that leukocyte inflammatory pathways in SMT-receiving LBP patients remain activated despite significant decreases in VAS and ODI scores reported by patients in both study groups. Clearly, the 6 single SMT regime utilized in this study was insufficient to resolve any tissue irritation that might be associated with LBP. In contrast, as suggested by Bialosky et al,37 the molecular mechanism(s) implicated in pain perception might have been altered by neurophysiological responses triggered within the peripheral and central nervous system by the force applied through spinal manipulation. Such responses might include modification of sensitivity or expression of nociceptive receptors within the affected spinal tissues or throughout the central nervous system at the level of spinal cord.38
Chemokine receptor expression is regulated by a combined action of various inflammatory stimuli including cytokines.12,18 The production of inflammatory mediators has been indeed shown to be upregulated in patients with cervical spine pain.23 Thus, attenuation of their production following spinal manipulation8 may alter expression and/or sensitivity of chemokine receptors in the treated patients.
A correlational study of changes in clinical outcomes (VAS and ODI) in relation to changes in chemokine levels was beyond the scope of this study. Furthermore, it may be argued that changes in clinical outcomes reported by patients following SMT treatments might be because of the placebo effect. Changes in pain perception related to the placebo effect have been reported.39 It is possible, however, that the reduction in pain and disability in SMT-treated patients might be related to a chemokine controlled shift in the migration of leukocytes releasing pain-inhibiting rather than pain-inducing mediators. Selectins and chemotactic cytokines mediate transendothelial migration of leukocytes producing both hyperalgesic and proalgesic mediators not only in the circulation but also at the site of inflammation.40 Leukocyte production of opioid peptides and anti-inflammatory cytokines increases during the late phase of inflammatory response.41 In contrast to pleiotropic activity of cytokines, chemokines can act in a targeted manner on a specific subpopulation of cells. Quantitative changes within the population of circulating leukocytes in patients reporting with acute or chronic LBP, if found, might be reflective of differences in the recruitment of pain-inducing macrophages or T lymphocytes to the affected spinal tissues.41–43 Accordingly, phenotypic analyses of peripheral blood mononuclear cells from patients with acute and chronic LBP, before and after SMT treatments are currently underway in our laboratory. Consistent with this approach are the recent findings by Li et al44 of significantly increased levels of CD14/16+ (proinflammatory) peripheral blood monocytes as well as attenuated secretion of β-endorphin in patients with chronic LBP.
To our knowledge, no previous reports exist to evidence the involvement of proinflammatory and nociceptive chemokines as well as endothelial cell activation in the etiology of LBP. Following a short course of SMT, reduction in pain intensity was observed in patients with both acute and chronic LBP despite reduced, albeit sustained elevation in the production of mediators of pain and inflammation. It has been accepted that mechanical LBP resolves to a large extent spontaneously within 6 weeks.45 In contrast, persistence or recurrence of LBP are common in many patients46 even after initial improvement in response to SMT.5,6 Protracted activation of chemokine-mediated inflammatory responses beyond improvements reported in pain and disability, as observed in the present study, may contribute to the pathophysiology of this syndrome. The present investigation was limited to 2 weeks and the results gleaned from the study are sufficient only to suggest an association between SMT treatments and the observed reduction in the production of inflammatory chemokines. Further studies with long-term follow-up periods utilizing a parallel LBP control group receiving standard treatment other than SMT should help explore these observations further.
ACKNOWLEDGMENTS
The authors are grateful to Maricelle Dinulos, BIS for her assistance in identifying potential participants for the study. The authors wish to express their appreciation and gratitude to Drs C. DeGraauw, DC, R. Gringmuth, DC, A. Pulinec, DC, and L. Wiltshire, DC, for performing the spinal manipulations, and to Dr A. Teitelbaum, MD, for performing phlebotomy. The excellent technical assistance of Amber Corless, BSc, is deeply appreciated.
Footnotes
Supported by funds from Canadian Memorial Chiropractic College, Toronto, ON, Canada.
The authors declare no conflict of interest.
REFERENCES
- 1.Manchikanti L, Singh V, Datta S, et al. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12:E35–E70. [PubMed] [Google Scholar]
- 2.Starkweather AR, Ramesh D, Lyon DE, et al. Acute low back pain: differential somatosensory function and gene expression compared with healthy no-pain controls. Clin J Pain. 2016;32:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borenstein D. Mechanical low back pain-a rheumatologist’s view. Nat Rev Rheumatol. 2013;9:643–653. [DOI] [PubMed] [Google Scholar]
- 4.Kalin S, Rausch–Osthoff AK, Bauer CM. What is the effect of sensory discrimination training on chronic low back pain? A systematic review. BMC Musculoskelet Disord. 2016;17:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubinstein SM, van Middelkoop M, Assendelft WJJ, et al. Spinal manipulative therapy for chronic low-back pain. Cochrane Database Syst Rev. 2011;2 doi: 10.1002/14651858.CD008112.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Rubinstein SM, Terwee CB, Assendelft WJJ, et al. Spinal manipulative therapy for acute low-back pain. Cochrane Database Syst Rev. 2012;9 doi: 10.1002/14651858.CD008880.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickar JG. Neurophysiological effects of spinal manipulation. Spine J. 2002;5:357–371. [DOI] [PubMed] [Google Scholar]
- 8.Teodorczyk-Injeyan J, Injeyan HS, Ruegg R. Spinal manipulative therapy reduces inflammatory cytokines but not substance P production in normal subjects. J Manipulative Physiol Ther. 2006;29:14–21. [DOI] [PubMed] [Google Scholar]
- 9.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luster AD, Alon R, von Adrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. [DOI] [PubMed] [Google Scholar]
- 11.Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82. [DOI] [PubMed] [Google Scholar]
- 12.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Ann Rev Immunol. 2014;32:659–702. [DOI] [PubMed] [Google Scholar]
- 13.Turner MD, Nedjai B, Hurst H, et al. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563–2582. [DOI] [PubMed] [Google Scholar]
- 14.Westra J, Kułdo JM, van Rijswijk MH, et al. Chemokine production and E-selectin expression in activated endothelial cells are inhibited by p38 MAPK (mitogen activated protein kinase) inhibitor RWJ 67657. Int Immunopharmacology. 2005;5:1259–1269. [DOI] [PubMed] [Google Scholar]
- 15.Baggiolini M, Loetscher P. Chemokines in inflammation and immunity. Immunol Today. 2000;21:418–420. [DOI] [PubMed] [Google Scholar]
- 16.Oh SB, Tran PB, Gillard SE, et al. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005;4:834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbadie C. Chemokines, chemokine receptors and pain. Trends Immunol. 2005;26:529–534. [DOI] [PubMed] [Google Scholar]
- 19.Burke JG, Watson RWG, McCormack D, et al. Spontaneous production of monocyte chemoattractant protein-1 and interleukin-8 by the human lumbar intervertebral disc. Spine. 2002;27:1402–1407. [DOI] [PubMed] [Google Scholar]
- 20.Desireddi NV, Phillip L, Campbell PL, et al. Monocyte chemoattractant protein-1 (MCP) and macrophage inflammatory protein-1α (MIP) as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008;179:1857–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn SH, Cho YW, Ahn MW, et al. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911–917. [DOI] [PubMed] [Google Scholar]
- 22.Codullo V, Baldwin H, Singh MD, et al. An investigation of the inflammatory cytokine and chemokine network in systemic sclerosis. Ann Rheum Dis. 2011;70:1115–1121. [DOI] [PubMed] [Google Scholar]
- 23.Teodorczyk-Injeyan JA, Triano JJ, McGregor M, et al. Elevated production of inflammatory mediators including nociceptive chemokines in patients with neck pain: a cross sectional evaluation. J Manipulative Physiol Ther. 2011;34:498–505. [DOI] [PubMed] [Google Scholar]
- 24.Sen O, Aydin MV, Bagdatoglu C, et al. Can E-selectin be a reliable marker of inflammation in lumbar disc disease? Neurosurg Rev. 2005;28:214–217. [DOI] [PubMed] [Google Scholar]
- 25.Kuenz B, Lutterotti A, Khalil M, et al. Plasma levels of soluble adhesion molecules sPECAM-1, sP-selectin and sE-selectin are associated with relapsing-remitting disease course of multiple sclerosis. J Neuroimmunol. 2005;167:143–149. [DOI] [PubMed] [Google Scholar]
- 26.Tufan K, Sen O, Cekinmez M, et al. Comparison of E-selectin and other inflammatory markers in lumbar disc herniation: a new promising therapeutical window for radicular pain. J Spinal Disord Tech. 2012;25:443–446. [DOI] [PubMed] [Google Scholar]
- 27.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2952. [DOI] [PubMed] [Google Scholar]
- 28.Howker GA, Mian S, Kendzierska T, et al. Measures of adult pain. Arthritis Care Res. 2011;63:S240–S252. [DOI] [PubMed] [Google Scholar]
- 29.Bergmann TF, Peterson DH. Chiropractic Technique. Principles and Procedures, 3rd ed St Louis: Elsevier/Mosby; 2011. [Google Scholar]
- 30.Haas M, Vavrek D, Peterson D, et al. Dose response and efficacy of spinal manipulation for care of chronic low back pain: a randomized controlled trial. Spine J. 2014;14:1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yacoob P, Newsholme D, Calder PC. Comparison of cytokine production in cultures of whole human blood cells and purified mononuclear cells. Cytokine. 1999;11:600–605. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 33.Yamashiro S, Komohara H, Yoshimura T. Alteration in the responsiveness to tumour necrosis factor-α is crucial for maximal expression of monocyte chemoattractant protein-1 in human neutrophils. Immunology. 2000;101:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherry B, Espinoza M, Manogue KR, et al. Induction of the chemokine beta peptides, MIP-1 alpha and MIP-1 beta, by lipopolysaccharide is differentially regulated by immunoregulatory cytokines gamma-IFN, IL-10, IL-4, and TGF-beta. Mol Med. 1998;4:648–657. [PMC free article] [PubMed] [Google Scholar]
- 35.Gruber HE, Hoelscher GL, Ingram JA, et al. Proinflammatory cytokines modulate the chemokine CCL2 (MCP-1) in human annulus cells in vitro: CCL2 expression and production. Exp Mol Pathol. 2015;98:102–105. [DOI] [PubMed] [Google Scholar]
- 36.Teodorczyk-Injeyan JA, Injeyan HS, McGregor M, et al. Divergent inflammatory profiles in acute and chronic low back pain patients: a single blind pilot clinical trial. WFC 13th Biennial Congress, Athens, Greece; May 14-16, 2015.
- 37.Bialosky JE, Bishop MD, Price DD, et al. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller RJ, Jung H. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;194:417–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. [DOI] [PubMed] [Google Scholar]
- 40.Mousa SA, Machelska H, Schafer M, et al. Immunohistological localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. J Neuroimmunol. 2002;126:5–15. [DOI] [PubMed] [Google Scholar]
- 41.Brack A, Rittner HL, Michalski H, et al. Control of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cells. Pain. 2004;112:229–238. [DOI] [PubMed] [Google Scholar]
- 42.Rittner HL, Brack A, Machelska H, et al. Opioid peptide-expressing leukocytes; identification, recruitment, and simultaneously increasing inhibition of inflammatory pain. Anesthesiology. 2001;95:500–508. [DOI] [PubMed] [Google Scholar]
- 43.Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Liu J, Liu Z, et al. Inflammation in low back pain may be detected from the peripheral blood: suggestions for biomarker. Biosci Rep. 2016;36, e00361. doi: 10.1042/BSR20160187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menezes-Costa LC, Maher CG, Hancock MJ, et al. The prognosis of acute and persistent low-back pain: a meta-analysis. CMAJ. 2012;184:E613–E624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starkweather AR, Lyon DE, Kinser P, et al. Comparison of low back pain recovery and persistence: a descriptive study of characteristics of pain onset. Bio Res Nurs. 2016;18:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]


