Abstract
The aim of this study was to investigate the antioxidant capacity (AC) in the lipophilic fraction of postmortem motorcortex (MC), nucleus caudatus (NC) and gyrus temporalis (GT) from controls (C) and Alzheimer’s disease (AD) patients. The initial samples consisted of 50 human brain tissues of AD and C. AC of the different region of human brain were measured by using the fluorescent method of the oxygen radical absorbance capacity (ORAC). Peroxyl and hydroxyl radical generators were used in the analysis. All ORAC analysis were carried out on the Perkin-Elmer spectrofluorometer LS 55 with fluorescent filters, Ex: 485 nm; Em 520 nm. Final results were calculated using the differences between area under the quenching curve of fluorescein (FL), blank and analyzed biological samples. AC against peroxyl radicals (ORAC-ROO°) of lipophilic fraction in MC of AD was statistically significantly lower in comparison with MC of C (p < 0,008). No changes in the AC against hydroxyl radicals (ORAC-°OH) of lipophilic fraction of AD were found in comparison with C. Reduction of total protein in GT of AD (p < 0,03) was found. The results showed that in the MC of AD brain the balance between production of free radicals and the neutralization by a complex antioxidant system is disturbed. The manual fluorescent method for AC measurements proved to be sufficiently appropriate and sensitive for the AC measurements of lipophilic fraction of postmortem brain tissues from different patologica! conditions.
Keywords: antioxidant capacity, oxygen radical absorbance capacity (ORAC) assay, peroxyl radical, hydroxyl radical, oxidative stress, Alzheimer’s disease
INTRODUCTION
Damage and progressive cell death that occurs in neurodegenerative disorders such as Alzheimer’s disease (AD) has been associated with activities of different free radicals (FR). However, there is growing evidence for a cascade of multiple deleterious factors, including oxidative stress, lipid peroxidation and altered iron metabolism leading to excess FR production, mitochondrial dysfunction and disturbed calcium homeostasis, which in turn result in cytosceletal damage and cell death in AD. Oxidative stressors induce an imbalance between oxidants and antioxidants in favor of the former leading to the oxidative damage of the molecules like deoxyribonucleic acid, lipids and proteins (1, 2). Furthermore, brain aging in AD patients is accompanied by the alteration of several neurotransmitter systems with a pronounced deficit in the cholinergic system and abnormal accumulation of amyloid-β peptides and hyperphosphorylated tau. Nucleus basalis Maynert, hypocampus and cortex are mostly affected area in AD brain. The present study was aimed to investigate the AC in the lipophilic fraction of different brain regions, by means of ORAC method involving two different FR generators (2, 3, 4, 5, 6, 7, 8), modified by Sofic et al. (9). ORAC method has an advantage over other assays, because this method utilizes an area-under- curve technique and thus combines both inhibition time and inhibition degree of FR action by an antioxidant into a single quantity. Fluorescein (FL) was used as a target of FR attack, with 2,2-azobis (2-amidino-propane) dihydrochloride (AAPH) as a peroxyl radical (ROO°) generator, and hydrogen peroxide with cupric sulfate penta hydrate as an hydroxyl radical (OH°) generator. ROO° is a common FR found in the body and used in the antioxidant activity assays (3,4,5). It is slightly less reactive than OH° and thus possesses an “extended” half-life of seconds instead of nanoseconds. In original developed automated method beta - phycoerythrin (β-PE), a protein from Porphyridium cruentum, was used as a target of FR attack (2,3,4,5,6,7,8). An improved ORAC method has been developed and validated using FL as the photo sensor. Ou et al., (10) demonstrated that FL is superior to β-PE. In clinical studies where analysis of antioxidant status is important, ORAC method have been used to evaluate the hydrophilic and lipophilic antioxidants in postmortem brain tissue.
MATERIALS AND METHODS
Chemicals
Fluorescein, Standard Fluka for fluoresceine - free acid was obtained from Fluka Chemie GmbH, Steinheim, Germany. 2,2’-azobis (2-amidino-propane) dihydrochloride, and 6-hydroxy-2,5,7,8-tetramethyl- chroman-2-carboxylic acid (Trolox) were purchased from Sigma Aldrich Chemie GmbG Germany. Nhexane was obtained from Merck (Germany). Cupric sulphate penta hydrate and hydrogen peroxide were obtained from Kemika, Zagreb, Croatia.
Patients and Controls
The initial sample of total 50 subjects consisted of 26 controls (C) brains and 24 Alzheimer’s disease (AD) brains. Motorcortex (MC), gyrus temporalis (GT) and nucleus caudatus (NC) were collected and stored at - 80°C until analyzed. Brains were matched for age (C 76,6 ± 10,1 years; AD 84,7 ± 7,7) and postmortem time (C 27 ± 18 hours; AD 36,3 ± 24,6). Control brains did not show any abnormal histopathological changes. The death of control subjects was mainly caused by cardiac and pulmonary arrest and by different tumours. Neuropathological diagnosis was based on histological examination of characteristic Alzheimer’s degeneration, the number of senile plaques and neurofibrillary tangles were determined according to the graduation of Khachaturian (11). AD patients died from cardiac and pulmonary deficits. All patients, before death, were underwent psychopharmaceutic therapy and received antibiotics.
Sample preparation
The crude tissue extracts from postmortem brain were prepared by homogenizing the tissues in a 75 mM phosphate buffer pH = 7,3 (10 ml buffer per gram of tissue). 100μ¡ of brain tissue homogenate was transferred to a glass tube, 200μ! of ethanol and 100μ! of water was added and mixed, and then 400μ! of hexane was added, followed by mixing. The mixture was left to sit for 1-2 min or until two layers appeared, followed by centrifugation for 5 min at 14000 rpm. The hexane layer was removed and added to a separate amber tube. An additional 400μ! of hexane was added to the original tube, mixed, left to settle for 2min, and then centrifuged for 5 min at 14000 rpm. The hexane layer was removed and combined with first extract. 400μ! of 0,5 M perchloric acid was added in the combined hexane extract to precipitate the protein. The sample was then centrifuged for 5 min at 14000 rpm. From the supernatant, 200 μ was added to 800μ! of phosphate buffer, mixed and used for the further measurement.
The manual antioxidant radical absorbance capacity (ORAC) assay
Manual ORAC analysis were performed on a Perkin Elmer spectrofluorometer LS 55 with a fluorescent filters (Ex: 485 nm; Em: 520 nm). In the final assay mixture (2 ml total volume) FL (10,5 nM) was used as a target of FR attack, with AAPH (32 mM) as a peroxyl radical generator (ORAC-ROO° assay), and H2O2-Cu2+ (H2O2 0,3 %; CuSO4 x 5H2O 0,9 mM) as mainly a hydroxyl radical generator (ORAC-OH° assay). The spectrofluorometer was programmed to record the fluorescence of FL every 10 min after AAPH, or H2O2 -Cu2+ were added for as long as 180 min and the samples were thermostated at 37°C (KP 20-D Lauda, Lauda Koenigshofen). All fluorescence measurements were expressed relative to the initial reading. Final results were calculated using the differences of areas under the FL decay curves between the blank and a sample and expressed as a μσ^ per g of fresh brain tissue.
Biochemical analysis
Colorimetric determination of total protein was based on the principle of the Biuret reaction using bovine serum albumin as a standard. Cholesterol and triglycerides were measured using comercially available enzymatic kits, which are based on the spectrophotometric determination.
Statistical analysis
For statistical comparisons Student’s test was performed.
RESULTS
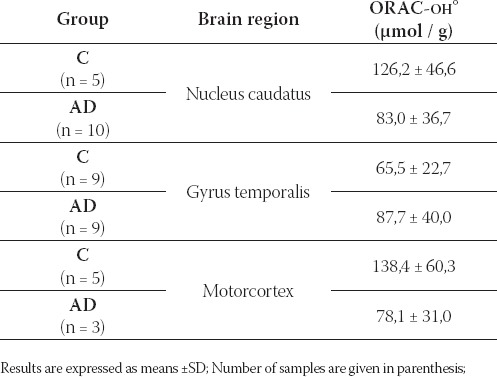
The ORAC-ROO ‘ and ORAC-OH. values from lipophilic NC, GT and MC fractions of C and AD are shown in Table 1 and Table 2.
TABLE 1.
Antioxidant capacity against peroxyl radical, ORAC-ROO (μmol/g) in different brain regions of C and AD
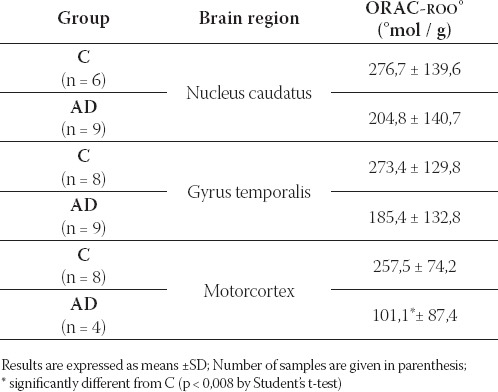
TABLE 2.
Antioxidant capacity against hydroxyl radical, ORAC-OH. (μmol/g) in different brain regions of C and AD
In the MC of AD patients antioxidant capacity against peroxyl radical was significantly lower than in the MC of C, p < 0,008 by Student’s t-test (Table 1).
No changes in the antioxidant capacity were found in brain tissues of AD in comparison with C, when hydroxyl radical generator was used in the assay (Table 2).
Total protein, cholesterol and triglycerides in different brain regions of C and AD are presented in Table 3.
TABLE 3.
Total protein, cholesterol and triglycerides in different brain regions of C and AD
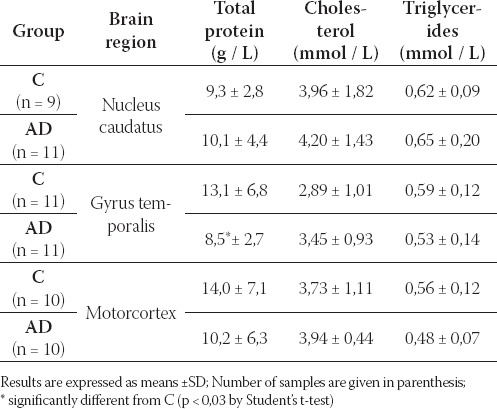
Reduction of total protein in GT of AD, p < 0,03 was found. No changes of cholesterol and triglycerides were found in brain tissues of AD in comparison with C (Table 3).
DISCUSSION
FR and oxidative stress involvement in the pathophysiology of AD has been in the focus of AD research (12, 13). Multiple biochemical deficits have been observed in the postmortem brain tissue of AD patients (14, 15, 16) with altered neurotransmission (17, 18) and pronounced deficit in the presynaptic cholinergic system (19), reduced protein content (14, 20) and altered lipid composition (21) which may all be inducing factors of sequalae for cell death. Discovery of isoprostanes (22, 23) and data from the recent studies performed in living patients (24, 25), make important contribution to better understanding and defining the role which oxygen radicals might play role in AD-athogenesis. From the point of view AC is dependent of chemical composition of complex biological sample and upon FR generator used in assay. AC measured with different FR generators have different numerical values. Because of this fact it is necessary for estimation of antioxidant status of some sample to determine antioxidant score, which is a sum of ORAC-R00° and ORAC-OH° in hydrophilic fraction and in lipophilic fraction. In this study, AC of lipophilic brain antioxidants was measured. Extracting the lipophilic components with hexane before analysis of the hydrophilic antioxidant fraction did not significantly alter the AC of the hydrophilic fraction (7). Thus, it appeared that there was no carry over of the lipophilic components into the aqueous compartment, and one could obtain the same hydrophilic ORAC value, whether the samples were first extracted with hexane or not. Regarding our previously published data of decreased AC in hydrophilic fraction of different AD brain regions (9), AC in lipophilic fraction was about 20 - 30 % of AC measured in hydrophilic fraction. Protein content, which may be regarded an index of cell loss or atrophy (26) was reduced in GT of AD brains in comparison with C. However, no differences between AD and C were found with respect to the cholesterol and triglycerides investigated. Our results suggested that lipophilic antioxidant defense system in brain is region-dependently disturbed.
CONCLUSION
-Lipophilic fractions ORAC-ROO° in MC of AD was statistically significantly lower in comparison with MC of C (p < 0,008).
-No changes in the AD lipophilic fraction ORAC-°OH were found in comparison with C.
-Reduction of total protein in GT of AD (p < 0,03) in comparison with C was found.
-No changes of cholesterol and triglycerides were found in brain tissues of AD in comparison with C.
-The results showed that in the MC of AD brain the balance between production of FR and the neutralization by a complex lipophilic antioxidant system is disturbed.
-The manual fluorescent method for AC measurements proved to be sufficiently appropriate and sensitive for the AC measurements of lipophilic fraction of postmortem brain tissues.
LIST OF ABBREVIATIONS
AC - antioxidant capacity
MC - motorcortex
NC - nucleus caudatus
GT - gyrus temporalis
C - controls
AD - Alzheimer’s disease
ORAC - oxygen radical absorbance capacity
ORAC-ROO° - antioxidant capacity against peroxyl radicals
ORAC- °OH - antioxidant capacity against hydroxyl radicals
FR - free radicals
AAPH - 2, 2’ - azobis (2 - amidino - propane) dihydrochloride
R00° - peroxyl radical
°OH - hydroxyl radical
FL - fluorescein
β-PE - β- phycoerythrin
Acknowledgment
Research was supported by the DAAD - German Academic Exchange Service, Stability Pact for East Europe (Referat 324 - 25.11.2003). The author expresses gratitude to Prof. Dr P. Riederer, Clinical Neurochemistry, Clinic and Policlinic for Psychiatry, University of Wuerzburg, Germany, for providing human brain tissue.
REFERENCES
- 1.Altan N, Dinçel A.S, Koca C. Diabetes mellitus and oxidative stress. Turk. J. Biochem. 2006;31:51–56. [Google Scholar]
- 2.Sofic E, Rustembegovic A, Kroyer G, Cao G. Serum antioxidant capacity in neurological, psychiatric, renal diseases and cardiomyopathy. J. Neural. Transm. 2002;109:711–719. doi: 10.1007/s007020200059. [DOI] [PubMed] [Google Scholar]
- 3.Cao G, Alessio H.M, Cutler R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free. Rad. Biol. Med. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- 4.Cao G, Verdon C, Wu A.H.B, Wang H, Prior R.L. Automated oxygen radical absorbance capacity assay using the COBAS FARA II. Clin. Chem. 1995;41:1738–1744. [PubMed] [Google Scholar]
- 5.Cao G, Sofic E, Prior R. Antioxidant capacity of tea and common vegetables. J. Agric. Food. Chem. 1996;44:3426–3431. [Google Scholar]
- 6.Cao G, Sofic E, Prior R. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free. Rad. Biol. Med. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 7.Prior R.L, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, Hampsch-Woodill M, Huang D, Ou B, Jacob R. Assays for hydrophilic and lipophilic antioxidant capacity [oxygen radical absorbance capacity (ORAC-FL)] of plasma and other biological and food samples. J. Agric. Food. Chem. 2003;21(11):3273–9. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- 8.Wang C.C, Chu C.Y, Chy K.O, Choy K.W, Khaw K.S, Rogers M.S, Pang C.P. Trolox equivalent antioxidant capacity assay versus oxygen radical absorbance capacity assay in plasma. Clin. Chem. 2004;50:952–954. doi: 10.1373/clinchem.2004.031526. [DOI] [PubMed] [Google Scholar]
- 9.Sofic E, Sapcanin A, Tahirovic I, Jellinger K, Reynolds G.P, Tatschner T, Riederer P. Antioxidant capacity in the postmortem brain tissues of Parkinson’s disease and Alzheimer’s disease. J. Neural. Transm. Suppl. 2006;71:39–43. doi: 10.1007/978-3-211-33328-0_5. [DOI] [PubMed] [Google Scholar]
- 10.Ou B, Hampsch-Woodill M, Prior R. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food. Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 11.Khachaturian Z.S. Diagnosis of Alzheimer’s disease. Arch. Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 12.Götz M, Freyberger A, Hauer E, Burger R, Heckers S, Sofic E, Jellinger K, Hebenstreit G, Beckmann H, Riederer P. Susceptibility to brains from patients with Alzheimer’s disease to oxygen-stimulated lipid peroxidation and differential scanning calorimetry. Dementia. 1992;3:213–222. [Google Scholar]
- 13.Mariani E, Polidori M.C, Cherubini A, Mefcoci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Sofic E, Froelich L, Riederer P, Jellinger K, Heckers S, Beckmann H, Deinzer E, Pantucek F, Hebenstreit G, Ransmayr G. Biochemical membrane constituents and activity of alkaline and acid phosphatase and cathepsin in cortical and subcortical brain areas of Alzheimer Type. Dementia. 1991;2:39–44. [Google Scholar]
- 15.Pratico D. Alzheimer’s disease and oxygen radicals: new insights. Biochemical Pharmacology. 2002;63:563–567. doi: 10.1016/s0006-2952(01)00919-4. [DOI] [PubMed] [Google Scholar]
- 16.Giasson B, Ischiropoulos H, Lee V, Trojanowski J. The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer’s and Parkinson’s diseases. Free Radical Biology and Medicine. 2002;32(12):1264–1275. doi: 10.1016/s0891-5849(02)00804-3. [DOI] [PubMed] [Google Scholar]
- 17.Hardy J, Adolfsson R, Alafuzoff I, Bucht G, Marcusson J, Pedahl E, Wester P, Winblad B. Transmitter deficits in Alzheimer’s disease. Neurochem. Int. 1985;7:545–563. doi: 10.1016/0197-0186(85)90050-6. [DOI] [PubMed] [Google Scholar]
- 18.Sofic E, Moll G, Riederer P, Jellinger K, Gabriel E. Monoaminerge Läsionen bei seniler Demenz vom Alzheimer Typ (SDAT): vorläufige Befunde. In: Beckmann H, Laux G, editors. Biologische Psychiatrie. Synopsis 1986/1987. Springer: Berlin etc; 1988. pp. 151–157. [Google Scholar]
- 19.Coyle J.T, Price D.L, DeLong M. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 20.Gottfries C.G, Bartfai T, Carlsson A, Eckernas A, Svennerholm L. Multiple biochemical deficits in both gray and white matter of Alzheimer brains. Prog. Neuro-Psychopharmacol. Biol. Psychiat. 1986;10:405–413. doi: 10.1016/0278-5846(86)90014-x. [DOI] [PubMed] [Google Scholar]
- 21.Crino P.B, Ullman D, Vogt B.A, Bird E.D, Volicer L. Brain gangliosides in dementia of the Alzheimer type. Arch. Neurol. 1989;46:398–401. doi: 10.1001/archneur.1989.00520400054019. [DOI] [PubMed] [Google Scholar]
- 22.Montine T.J, Quinn J.F, Montine K.S, Kaye J.A, Breitner J.C. Quantitative in vivo biomarkers of oxidative damage and their application to the diagnosis and management of Alzheimer’s disease. J. Alzheimers. Dis. 2005;8(4):359–67. doi: 10.3233/jad-2005-8405. [DOI] [PubMed] [Google Scholar]
- 23.Flirski M, Sobow T. Biochemical markers and risk factors of Alzheimer’s disease. Curr. Alzheimer. Res. 2005;2(1):47–64. doi: 10.2174/1567205052772704. [DOI] [PubMed] [Google Scholar]
- 24.Pratico D, Sung S. Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer’s disease. J. Alzheimers. Dis. 2003;6(2):171–175. doi: 10.3233/jad-2004-6209. [DOI] [PubMed] [Google Scholar]
- 25.Calabrese V, Scapagnini G, Colombrita C, Ravagna A, Pennisi G, Giuffrida Stella A.M, Galli F, Butterfield D.A. Redox regulation of heat shock protein expression in aging and neurodegenerative disorders associated with oxidative stress: a nutritional approach. mino Acids. 2003;25:437–444. doi: 10.1007/s00726-003-0048-2. [DOI] [PubMed] [Google Scholar]
- 26.Bowen D.M, Smith C.B, White P, Goodhardt M.J, Spillane J.A, Flack R.H.A, Davson A.N. Chemical pathology of organic dementias. II. Quantitative estimation of cellular changes in postmortem brains. Brain. 1977;100:427–453. doi: 10.1093/brain/100.3.427. [DOI] [PubMed] [Google Scholar]


