Abstract
The aim of this trial was to examine the efficacy and safety of antihypertensive fixed combination lisinopril plus hydrochlorothiazide (Lopril H, Bosnalijek dd) in the treatment of essential arterial hypertension.
In our trial we included 297 patients, aged 54,65±9,6 years, with treated or untreated hypertension and with high risk of cardiac events, in an opened trial of therapy based on lisinopril plus hydrochlorothiazide. Upon the examination by physicians, patients were divided into three groups in accordance with European Society of Cardiology guidelines for the management of arterial hypertension. Patients from five European countries were followed up for a period of 12 weeks. Duration of treatment was 12 weeks. We adjusted daily doses of lisinopril plus hydrochlorothiazide after every clinical examination and recorded adverse effects of drugs.
After 12 weeks of treatment, 288 patients (96%) were evaluated for efficacy, tolerability and safety. In almost 81,5% patients with mild, moderate and severe hypertension, we recorded a reduction in blood pressure to approximately normal values SBP and DBP (140/90 mmHg). Drug-related side-effects occurred in 11 patients (3,66%). The most commonly reported adverse effects associated with lisinopril plus hydrochlorothiazide were cough (5) and dry mouth (5).
This research has proved good efficacy of fixed combination lisinopril plus hydrochlorothiazide with more than 97% patients. Based on subjective estimation by patients: this drug improved quality of life in all cases.
Keywords: essential hypertension, lisinopril, hydrochlorthiazide
INTRODUCTION
High arterial pressure is probably the most important public health problem in developed countries. That is frequently asymptomatic condition that is easily discovered and it is mostly cured in simple manner, but it might lead to severe and fatal complications often if not treated on time, sufficiently and adequately. It is estimated that around billion people have hypertension, and that this number will be increased by 60% until 2025, i.e. it shall amount to a billion and 560 million at that moment. Essential hypertension is nowadays by far the most important risk factor in appearance of cardiovascular diseases. A range of studies that were conducted in last couple of decades, starting from Framingham study onwards, undoubtedly tell in favour of the fact that efficient treatment of high arterial pressure might prevent complications on target organs, or, if nothing else, to move them from such a life period when patients are at peak of their intellectual and production capabilities, what is of enormous value for social structure of each society.
OBJECTIVE
The aim of this trial was to examine the efficacy and safety of antihypertensive fixed combination lisinopril plus hydrochlorothiazide (Lopril H, Bosnalijek dd) in the treatment of essential arterial hypertension.
PATIENTS AND METHODS
The trial was open, multi-centre, prospective, clinical trial lasting 12 weeks per patient. Totally 297 subjects with essential hypertension were included in the trial, of I-III degree, both genders aged 35 to 65 years. On the basis of average values of three independent measurements of blood pressure (BP) (the same physician, the same measuring apparatus) were divided into three groups First group - Patients with essential hypertension of first degree (Mild hypertension) (BP 140-159/90-99 mmHg). Second group - Patients with essential hypertension of second degree (Moderate hypertension) (BP 160-179/ 100 -109 mmHg) Third group - Patients with essential hypertension of third degree (Severe hypertension) (BP above 180/110 mmHg)
Total duration of the trial per patient was 12 weeks. The following examinations and measurings were performed prior to including into trial:
Blood pressure and pulse
Electrocardiogram (Sokolow - Lyons; Cornell)
Echocardiography (of heart obligatory LVMI - left ventricle mass index)
Ultrasound of carotid arteries - (thickness of the wall and attention directed at arterioslerotic plaques)
X-rays of the chest
Eye fundus examination
Average age of all patients included into trial was 54,64±9,6 years, and 157 women and 140 men were included into trial. On the basis of initial results of blood pressure, the patients were classified in one of the following groups on basis of severity of hypertension, according to European Classification of Hypertension (2003)(1): mild, moderate and severe hypertension. There were no statistically significant differences between the groups by gender and age. Average duration of illness for all patients was around 6,24±5,18 years, and duration of illness was very similar among the groups (Table 1).
TABLE 1.
Baseline characteristics and risk factors of the patients
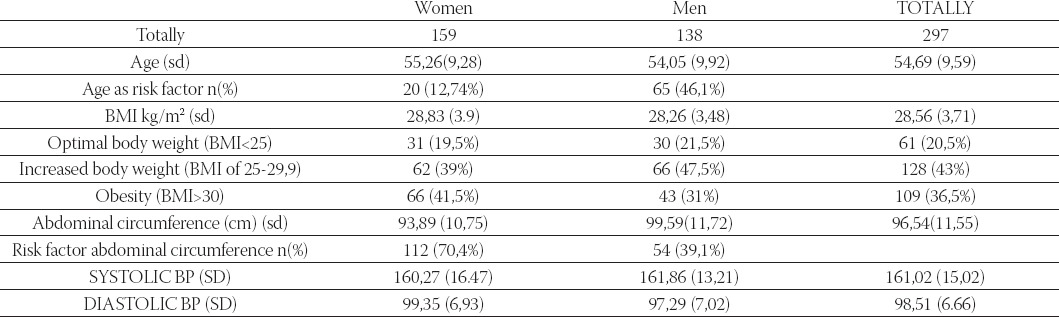
In the beginning of trial, we estimated also risk factors, so that smoking, increased body weight and heredity were equally distributed in the groups and no significant differences were reported between the risk factors (Table 1). In addition to high blood pressure, increased body weight (body mass index 25,0-29,9) was present in 39% women and 47,5% men, and obesity (body mass index above 30) was present in 41,5% women and 31% men, so that completely 19,5% women and 21,5% men had optimal body weight. Statistical processing of results
Statistical analyses were made in statistical programme MedCalc for Windows, version 9.2.1.0 (MedCalc Software, Mariakerke, Belgium). Results are shown through the usual descriptive statistics. As an estimate of significance of differences between the average measuring, significance was taken of p<0,05.
METHODS
The study was done in compliance with Guidelines for Good Clinical Practice and Declaration Helsinki (2002) of the World Medical Association and all patients provided written informed consent before the start of the study. This 12-week opened prospective clinical trial was done at 12 centres in five European countries. After 2 weeks washout period for patients receiving antihypertensive medications, patients entered in study. Upon the examination by physicians, patients were divided into three groups in accordance with European Society of Cardiology guidelines for the management of arterial hypertension. Patients were followed up for period of 12 weeks and duration of treatment was 12 weeks. We adjusted daily doses of lisinopril plus hydrochlorothiazide (Lopril H, Bosnalijek) after every clinical examination and recorded adverse effects of drugs.
RESULTS
Total number of 297 patients (ITT) were included in the trial, 288 patients completely finalised the trial (by trial protocol, PP, 98%), and 9 patients did not finish the study (3%). Reasons for giving up of trial are not related to side-effects of drugs, nor intolerance to medicines, but they are linked to patients’ non-cooperation in the trial (e.g. irregular coming to control check-ups and irregular administration of therapy) (Table 2). In Table 2 differences in values of blood pressure are shown in relation to baseline values. Biggest improvements in lowering blood pressure in relation to initial values were reported in a group of patients with severe hypertension (n=43) so that the accomplished lowering for sys/dia BP was at the average by 48,12mmHg/23,79mmHg or by 26,57%/22,28% in relation to initial values. In group of patients with moderate hypertension, the largest number of patients was included (n=153), lowering of sys/dia were accomplished at the average by 35,34mmHg/20,9mmHg, or by 21,54%/20,39% in relation to initial values (Figure 1, 2). In a group suffering from mild form of hypertension (n=92), lowering was accomplished at the average by 22,74mmHg/14,55mmHg, or by 15,36%/15,66% in relation to initial values. We made analysis for all included patients (n=288) in the trial, and average lowering was accomplished for sys/dia BP 33,22mmHg/19,17mmHg, or by 20,56%/19,43% (Figure 3).
TABLE 2.
Estimating efficacy on the basis of administered therapy in relation to basic values
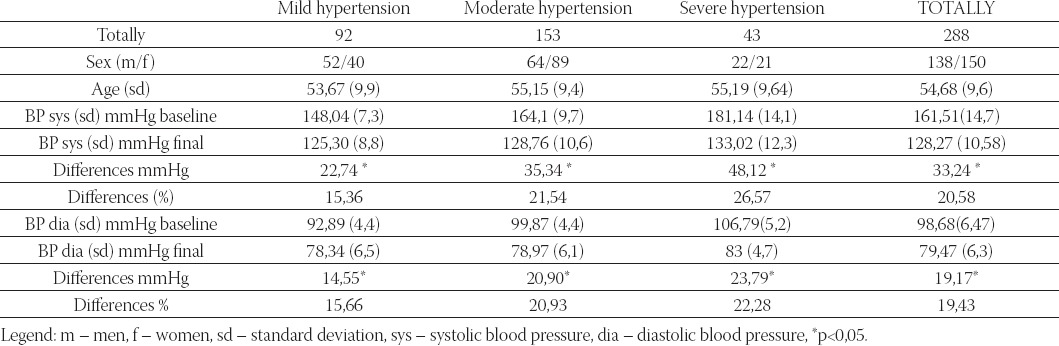
FIGURE 1.

Effect of treatment and decreased from baseline in mean sitting systolic blood pressure (96)
FIGURE 2.
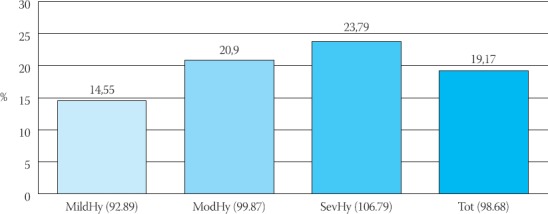
Effect of treatment and decreased from baseline in mean sitting diastolic blood pressure (%)
FIGURE 3.
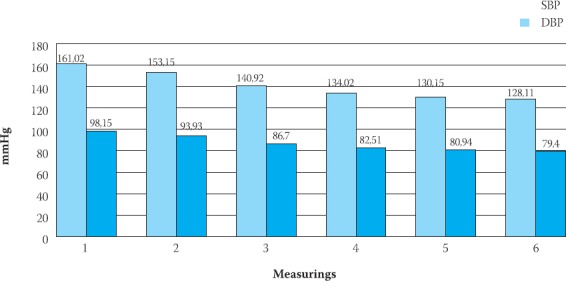
Changes of mean sitting systolic blood pressure (SBP) and diastolic blood pressure (DBP) during of trial (mmHg)
Analysis of results has shown that we obtained the best results in medication’s effect on the systolic blood pressure, where lowering was accomplished in all the groups from 15,36% to 26,57%, while differences between baseline and final values of diastolic blood pressure by groups were from 15,66% to 22,28% (Figure 1,2,3).
DISCUSSION
Despite the availability of over 75 different antihyper-tensive agents in 9 different classes, an estimated 45% of treated patients in the United States remain uncontrolled in 2004.(2,3,4) The lack of control of hypertension is of particular concern in patients with coexisting cardiovascular disorders. To reduce this unacceptably high rate of prior control, most patients will require combination therapies, as noted in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7)(3). In our trial we used fixed combination of two anti-hypertensives lisinopril and hydrohlorthiazid (Lopril H, Bosnalijek). The efficacy can be seen in the reduction of systolic and diastolic pressure, and in the great number of patients (81,5%), the control of blood pressure under 140/90 mmHg has been achieved. Many older studies utilized diastolic blood pressure to guide therapy, systolic blood pressure control is even more important (3,5,6,7). For example, among patients with stage 1 hypertension, a sustained 12 mm Hg decrease in systolic blood pressure for 10 years will prevent 1 death for every 12 treated patients who have diabetes mellitus or cardiovascular disease. Furthermore, even small reductions in systolic blood pressure (3 mm Hg to 5 mm Hg) produce dramatic reductions in events such as heart failure and stroke (8,9). Controlling the systolic blood pressure is critical, as demonstrated in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) (10). Despite a design that should have led to similar control in all groups (chlorthalidone, amlodipine, or lisinopril) over 5 to 6 years, systolic blood pressure remained significantly higher in the lisinopril group than in the diuretic control group throughout the study. The higher rate of stroke in the lisinopril group compared with the chlorthalidone group was most likely a manifestation of this poor control. The importance of controlling the systolic blood pressure early was demonstrated in the Valsartan Antihyper-tensive Long-term Use Evaluation (VALUE) trial (11), which hypothesized that angiotensin blockade would be superior to calcium antagonism. Although both treatments reduced blood pressure, the effects of the amlodipine regimen on systolic blood pressure were more pronounced, especially in the first year of the study. The 4-year results showed no difference in the primary endpoint of time to a first cardiac event, but there was a significant increase in fatal and nonfatal myocardial infarction in the valsartan group, particularly in the first year of the study when systolic blood pressure control favored amlodipine. Of interest is that more patients developed heart failure on amlodipine compared with valsartan, despite better blood pressure control with amlodipine (11). We recorded excellent efficacy in the reduction of systolic and diastolic blood pressure in 95,14% of patients for systolic blood pressure (reduction for 10-15 mmHg and higher) and 97,57% for diastolic blood pressure (reduction for 5-10 mm Hg and higher).
CONCLUSION
This research has proved good efficacy and tolerability fixed combination of lisinopril plus hydrochlorothiazide with more than 97% patients. Based on subjective estimation by patients: this drug improved quality of life in all.
SEPARATE
This trial supported by pharmaceutical company “Bosnalijek”, Sarajevo, Bosnia and Herzegovina.
REFERENCES
- 1.European Society of Hypertension-European Society of Cardiology Guidelines Committee: 2003. European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association Heart Disease and Stroke Statistics — 2004 Update 2003. Dallas, Texas: American Heart Association; [Google Scholar]
- 3.Chobanian A.V, Bakris G.E, Black H.R. National High Blood Pressure Education Program Coordinating Committee The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Hyman D.J, Pavlik V.N. Characteristics of patients with uncontrolled hypertension in the United States Erratum in. N Engl J Med. 2002:346–544. doi: 10.1056/NEJMoa010273. N Engl J Med; 2001: 345; 479-486. [DOI] [PubMed] [Google Scholar]
- 5.Izzo J.L, Jr, Levy D, Black H.R. Clinical Advisory Statement Importance of systolic blood pressure in older Americans. pertension. 2000;35:1021–1024. doi: 10.1161/01.hyp.35.5.1021. [DOI] [PubMed] [Google Scholar]
- 6.Guidelines Committee 2003. European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J. Hypertens. 2003;21:1011–1053. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization International. Society of Hypertension Writing Group 2003 World Health Organization (WHO)/ International Society of Hypertension (ISH) statement on management of hypertension. J. Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Staessen J.A, Wang J.G, Thijs L. Cardiovascular prevention and blood pressure reductiona quantitative overview updated until 1 March 2003. J. Hypertens. 2003;21:1055–1076. doi: 10.1097/00004872-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 10.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diureticThe Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 11.Julius S, Kjeldsen S.E, Weber M. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine the VALUE randomized trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]


