Abstract
Morinda citrifolia, commonly known as the noni berry, is a tropical fruit that has been used for more than 2000 years as a Polynesian herbal remedy (1). Since 1996, it has been sold widely in the United States as a general remedy for a wide array of health problems including cancer, diabetes, HIV/AIDS, gastric ulcers, hypertension, infections, depression, and chronic fatigue (2,3). We report a case of acute hepatotoxicity after ingestion of an energy drink containing noni berries in a previously healthy 14-year-old boy.
CASE REPORT
A previously healthy 14-year-old boy presented with a 1-week history of fatigue and a 2-day history of scleral icterus. Initial presentation at the patient’s pediatrician’s office demonstrated that the patient’s alanine aminotransferase (ALT) was elevated to 3000 U/L with a direct bilirubin of 4.4 mg/dL and international normalized ratio of 1.6. Further questioning revealed that the patient had 2 episodes of nonbilious, nonbloody emesis and 2 days of diarrhea without hematochezia or melena. No change in mental status, acute bleeding, recent travel, sick contacts, or fever was reported. Medications within the last 6 months included ibuprofen taken approximately 2 times weekly for headaches. The patient had no history of acetaminophen, alcohol, tobacco, or any illicit drug use. Home, education, activities, drug use and abuse, sexual behavior, suicidality, and depression examination was negative. In regard to animal exposure, the patient resided on a farm and reported exposure to sheep, chickens, snakes, and mice. The only dietary change was ingestion of 10 two-oz bottles of an over-the-counter antioxidant drink during the course of 2 months (~600 mL total) for improving energy during the current track and field season. The last ingestion of the antioxidant drink occurred on the day of admission to the hospital. Ingredients of the antioxidant drink were aloe vera, acai berry, muscadine grape, mangosteen, noni berry, gogi berry, pomegranate, blueberry, green tea extract, and plant-derived vitamins and minerals. The ingested dose of noni berry was approximately 91 mg/kg. His family history was significant for Hashimoto thyroiditis and migraines. There was no previous report of liver disease in the family.
On presentation, the patient had significant scleral icterus and a palpable liver edge approximately 1 to 2 cm beyond the costal margin. The patient was lucid and answered all questions appropriately. Body mass index for sex, height, and weight was at the 30th percentile. Laboratory analysis revealed aspartate aminotransferase 1584 U/L; ALT 2860 U/L; γ-glutamyltransferase 141 U/L; total and direct bilirubin of 4.4 mg/dL and 3.4 mg/dL, respectively; normal ammonia of 22 umol/L; and elevated prothrombin time at 19.4 seconds and international normalized ratio of 1.7. A workup for etiology of acute hepatitis, inclusive of viral hepatitis panel, cytomegalovirus/Epstein-Barr virus polymerase chain reaction, serum ceruloplasmin and copper, serum iron, α-1-antitrypsin genotype, HIV, herpes simplex virus, anti-nuclear antibody, anti-smooth muscle antibody, and anti-liver-kidney-microsome antibody, was negative. An abdominal ultrasound with Doppler was consistent with hepatitis and did not show any signs of biliary tree obstruction.
A liver biopsy revealed acute hepatitis with portal inflammation and periportal necrosis, hepatocellular cholestasis with focal ductular proliferation, and presence of numerous eosinophils (Figs 1 and 2). No plasma cells were found. Qualitative copper and iron stains were negative.
FIGURE 1.
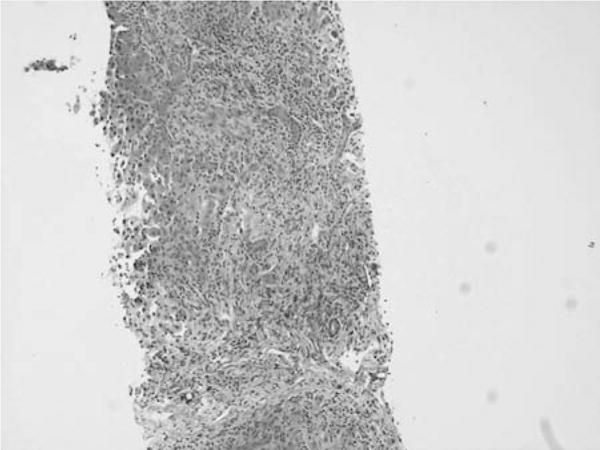
Core biopsy of hepatic parenchyma with bridging fibrosis, ductular proliferation, and mixed portal inflammatory infiltrates with a background of single-cell necrosis and hepatocytes with mild anisonucleosis. (Trichrome, ×10 objective.)
FIGURE 2.
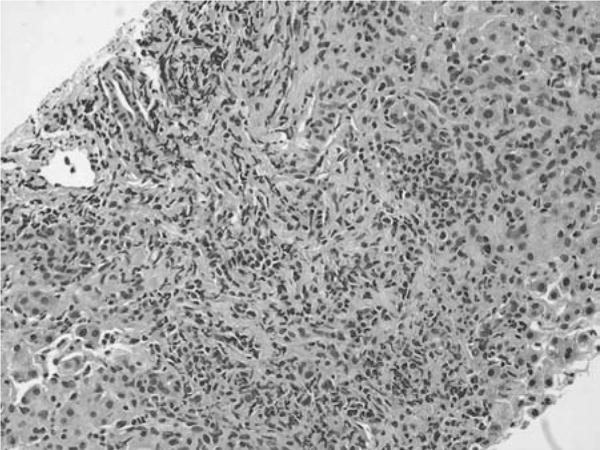
Core biopsy of hepatic parenchyma highlighting expanded portal tract with increased mixed portal inflammation comprising lymphocytes, histiocytes, plasma cells, and numerous eosinophils, with interface activity and a background of pseudocholangiolar transformation and hepatocytes with mild anisonucleosis. (Hematoxylin & eosin, ×20 objective.)
The classification of hepatotoxic agents may be divided in general into those drugs that cause a known histological response and those that produce an idiosyncratic response, either because the drug produces the toxic response in a small percentage of the population or its hepatotoxic effects are poorly described. The histologic patterns of drug injury may be divided into 6 categories: necroinflammatory injury such as zonal necrosis, an acute or chronic hepatitis-like picture, or granulomatous inflammation; cholestatic disease, including biliary sclerosis-like or hepatocellular and/or cholestatic injury; steatosis, either micro- or macrovesicular type; vascular injury, including lesions that may recapitulate veno-occlusive disease or Budd-Chiari syndrome; fibrosis and cirrhosis; and neoplastic change, including hepatocellular adenomas and carcinomas, cholangiocarcinoma, and angiosarcoma (4). In this case, the reaction would be considered idiosyncratic, but categorization may change as more cases are documented. Given the clinical scenario and corroborating histology, the patient’s acute hepatotoxicity was attributed to noni berry ingestion.
CASE RESOLUTION
The patient did well overall, with a peak ALT of 3407 U/L and peak direct bilirubin of 12.3 mg/dL, which normalized 2 months after cessation of noni berry juice consumption. The patient has recovered completely and continues to do well without any additional signs or symptoms of liver disease.
DISCUSSION
Hepatotoxicity in this case was attributed to noni berry ingestion. Although it is possible that other components of the energy drink may have contributed to the hepatotoxicity, acai, mangosteen, gogi berries, pomegranate, and plant-derived vitamins and minerals have not previously been associated with hepatotoxicity.
Active compounds in noni berries include flavonoids, glycosides, vitamins, anthraquinones, and polyunsaturated fatty acids. Hepatotoxicity is thought to be secondary to anthraquinones, which create oxygen-derived free radicals and lead to oxidative stress. Oxygen-derived free radical production causes depletion of intra-cellular reduced glutathione and mitochondrial membrane potential, which initiates lipid peroxidation and, ultimately, cell death (2,3).
To date, 6 cases of acute hepatotoxicity resulting from noni berry consumption have been reported in the literature (5). These prior cases and the present case are summarized in Table 1 (5–9). To the best of our knowledge, we present the youngest patient with noni berry–induced hepatotoxicity.
TABLE 1.
Summary of previous case reports of hepatotoxicity from noni berry ingestion
| Case | Age/sex | Amount of ingestion | Time to onset of symptoms | Presentation | Labs (peaks) | Histology | Concomitant meds | Outcome | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 43, M | 0.56 L | 2 wk | Routine checkup | AST 192 U/L, ALT 516 U/L, bilirubin 0.6 mg/dL | — | Levitiracetam | Spontaneous recovery | (5) |
| 2 | 45, M | 1 glass daily × a few weeks | Few wk | Malaise, thoracic discomfort | AST 604 U/L, ALT 1995 U/L, bilirubin 0.82 mg/dL | Acute hepatitis: mixed inflammatory reaction; numerous eosinophils in portal tracts, hepatocellular cholestasis in zone 3 | None | Spontaneous recovery | (6) |
| 3 | 29, M | 1.5 L | 3 wk | Acute liver failure | AST 1557 U/L, ALT 1626 U/L, bilirubin 45.3 mg/dL | Acute hepatitis: confluent hepatocyte necrosis, ductular reaction and metaplasia with inflammatory infiltrates | Chinese herbs | Liver transplant | (8) |
| 4 | 62, F | 2 L | 4 wk | Diarrhea | AST 2020 U/L, ALT 3570 U/L, bilirubin 3.9 mg/dL | Acute hepatitis: centrolobular areas with hepatocyte necrosis, ballooned hepatocytes and mild inflammatory infiltrate | None | Spontaneous recovery | (8) |
| 5 | 24, F | 1–1.5 L | 3 wk | Subacute liver failure | AST 2818 U/L, ALT 348 U/L, bilirubin 43.5 mg/dL | Periportal and intralobular necrosis, mixed inflammatory reaction and prominent canalicular cholestasis | IFN-β-1 | Spontaneous recovery | (7) |
| 6 | 33, F | Unknown | 1 wk | Abdominal pain | AST 3382 U/L, ALT 2740 U/L, bilirubin 8.1 mg/dL | — | None | Spontaneous recovery | (9) |
| 7 | 14, M | 0.6 L | 8 wk | Scleral icterus | AST 2280 U/L, ALT 3407 U/L, bilirubin 13.7 mg/dL | Acute hepatitis: portal inflammation and periportal necrosis, hepatocellular cholestasis with focal ductular proliferation; numerous eosinophils | None | Spontaneous recovery | This case |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; IFN = interferon.
Although 1 patient required liver transplantation after noni berry–induced hepatotoxicity, most (6/7) patients have recovered spontaneously without intervention other than cessation of noni berry consumption. A broad and varied time span (4 weeks–4 months) exists between time of noni berry ingestion and onset of symptoms. In this present report, time of onset was approximately 2 months.
Literature does exist refuting the hepatotoxicity of noni berries; however, most of these articles have been written by Tahitian noni industry–sponsored researchers (10). It is thus important to recognize the potential toxicity of noni berries. We recommend that people at high risk for liver injury (ie, patients with chronic hepatitis or other forms of liver injury) avoid ingestion of products containing noni berries.
SUMMARY
We present a case of a 14-year-old previously healthy boy with acute hepatotoxicity after noni berry juice consumption. As the popularity of noni berry consumption continues to increase, heightened awareness of the relation between noni berry consumption and acute hepatotoxicity is important.
Footnotes
The authors report no conflicts of interest.
References
- 1.West BJ, Su CX, Jensen CJ. Hepatotoxicity and subchronic toxicity tests of Morinda citrifolia (noni) fruit. J Toxicol Sci. 2009;34:581–5. doi: 10.2131/jts.34.581. [DOI] [PubMed] [Google Scholar]
- 2.Potterat O, Hamburger M. Morinda citrifolia (Noni) fruit—phytochemistry, pharmacology, safety. Planta Med. 2007;73:191–9. doi: 10.1055/s-2007-967115. [DOI] [PubMed] [Google Scholar]
- 3.Mian-Ying W, West BJ, Jensen CJ, et al. Morinda citrifolia (Noni): a literature review and recent advances in Noni research. Acta Pharacol Sin. 2002;23:1127–41. [PubMed] [Google Scholar]
- 4.Burt AD, Portmann BC, Ferrell LD, editors. MacSween’s Pathology of the Liver. Edinburgh: Elsvier/Churchill Livingstone; 2007. [Google Scholar]
- 5.Stadlbauer V, Weiss S, Payer F, et al. Herbal does not all mean innocuous: the sixth case of hepatotoxicity associated with Morinda citrofolia (noni) Am J Gastroenterol. 2008;103:2406–07. doi: 10.1111/j.1572-0241.2008.02010_8.x. [DOI] [PubMed] [Google Scholar]
- 6.Millonig G, Stadlmann S, Vogel W. Herbal hepatotoxicity: acute hepatitis caused by a Noni preparation (Morinda citrifolia) Eur J Gastroenterol Hepatol. 2005;17:445–7. doi: 10.1097/00042737-200504000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Yuce B, Gulberg V, Diebold J, et al. Hepatitis induced by Noni juice from Morinda citrifolia: a rare cause of hepatotoxicity or the tip of the iceberg? Digestion. 2006;73:167–70. doi: 10.1159/000094524. [DOI] [PubMed] [Google Scholar]
- 8.Stadlbauer V, Fickert P, Lackner C, et al. Hepatotoxicity of NONI juice: report of two cases. World J Gastroenterol. 2005;11:4758–60. doi: 10.3748/wjg.v11.i30.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Cepero Andrada JM, Lerma Castilla S, Fernandez Olvera, et al. Hepatotoxicity caused by a Noni (Morinda Citrifolia) preparation. Rev Esp Enferm Dig. 2007;99:179–81. doi: 10.4321/s1130-01082007000300017. [DOI] [PubMed] [Google Scholar]
- 10.West BJ, Jensen CJ, Westendorg J. Noni juice is not hepatotoxic. World J Gastroenterol. 2006;12:3616–9. doi: 10.3748/wjg.v12.i22.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]


