Abstract
Background
Although atrial fibrillation (AF) guidelines indicate that pharmacologic blockade of the renin-angiotensin system may be considered for primary AF prevention in hypertensive patients, previous studies have yielded conflicting results. We sought to determine whether randomization to lisinopril reduces incident AF or atrial flutter (AFL) compared to chlorthalidone in a large clinical trial cohort with extended post-trial surveillance.
Methods and Results
We performed a secondary analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), a randomized, double-blind, active-controlled clinical trial that enrolled hypertensive individuals ≥ 55 years of age with at least one other cardiovascular risk factor. Participants were randomly assigned to receive amlodipine, lisinopril, or chlorthalidone. Individuals with elevated fasting LDL-C levels were also randomized to pravastatin versus usual care. The primary outcome was the development of either AF or AFL as diagnosed by serial study ECGs or by Medicare claims data. Among 14,837 participants without prevalent AF or AFL, 2,514 developed AF/AFL during a mean 7.5 ± 3.2 years of follow up. Compared to chlorthalidone, randomization to either lisinopril (HR 1.04, 95% CI 0.94 to 1.15, p = 0.46) or amlodipine (HR 0.93, 95% CI 0.84 to 1.03, p = 0.16) was not associated with a significant reduction in incident AF/AFL.
Conclusions
Compared to chlorthalidone, treatment with lisinopril is not associated with a meaningful reduction in incident AF or AFL among older adults with a history of hypertension.
Clinical Trial Registration
ClinicalTrials.gov; Unique Identifier: NCT00000542
Keywords: atrial fibrillation, renin angiotensin system, primary prevention
Journal Subject Terms: Atrial Fibrillation, ACE/Angiotension Receptors/Renin Angiotensin System, Epidemiology, Primary Prevention
Over three million adults in the United States are living with atrial fibrillation (AF), and this number is expected to grow substantially in future years.1 AF is associated with increased morbidity,2 excess mortality,3 and substantial healthcare costs.4 Although the medical and economic impact of AF has generated considerable interest in the primary prevention of this arrhythmia,5 no efficacious therapies have been definitively identified.
Several classes of medications hold promise for AF risk reduction. Angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARB) have received substantial attention due to their direct antiarrhythmic properties,6 beneficial effects on left atrial pressure,7 and ability to prevent atrial fibrosis.8 Prior investigations, however, have reached conflicting results regarding the efficacy of these treatments with regard to arrhythmia prevention; some studies have shown reductions in incident AF with ACE inhibitor/ARB therapy9,10 while others have failed to identify a significant benefit.11 Current AF management guidelines from the American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) conclude that ACE inhibitor/ARB therapy may be considered for the prevention of incident AF in hypertensive patients (Class IIb, level of evidence B),12 while European Society of Cardiology (ESC) guidelines more emphatically state that ACE inhibitor/ARB treatment should be considered for primary AF prevention in the setting of hypertension (Class IIa, level of evidence B).13 HMG-CoA reductase inhibitors, due to their anti-oxidant and anti-inflammatory effects, have also been proposed as a potential AF primary prevention therapy.14 While this class of medications has been associated with reductions in AF after cardiothoracic surgery,15 other investigations outside of the perioperative setting have not demonstrated significant benefit with this treatment.16,17
Prior investigations examining the benefit of pharmacologic therapy for AF prevention have largely relied upon serial electrocardiograms (ECGs) to identify incident AF, which substantially underestimates AF outcomes compared to hospital discharge coding.18 Furthermore, this technique may introduce bias through the preferential detection of persistent disease. To increase our ability to detect paroxysmal AF and augment statistical power, we performed a secondary analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) that utilized both serial study ECGs and Centers for Medicare Services (CMS) coding to identify episodes of incident AF or AFL. We hypothesized that randomization to lisinopril (relative to chlorthalidone) would reduce incident AF, while treatment with amlodipine (again relative to chlorthalidone) would not result in AF risk reduction. In light of the above potential benefits of statin therapy, we also leveraged the factorial ALLHAT study design to test the hypotheses that randomization to pravastatin would result in 1) reduced AF risk and 2) heightened antiarrhythmic effect when administered in combination with lisinopril.
Methods
The data, analytic methods, and study materials can be requested by contacting the ALLHAT Collaborative Research Group.
Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Study Design
The ALLHAT study was a randomized, double blind clinical trial sponsored by the National Heart, Lung and Blood Institute. Eligibility, enrollment, and follow-up protocols have been previously published.19,20 Briefly, 42,418 hypertensive individuals aged 55 or older with at least one other cardiac risk factor were eligible for enrollment. Participants with a history of heart failure hospitalization, treatment for symptomatic heart failure, or severe systolic dysfunction (ejection fraction ≤ 35%) were excluded. After undergoing a baseline physical exam and ECG, participants were randomized to treatment with chlorthalidone, amlodipine, lisinopril, or doxazosin. In addition to antihypertensive randomization, participants with elevated fasting LDL-C levels (120 to 189 mg/dL or 100 to 129 mg/dL if known atherosclerotic coronary heart disease was present) were also randomized in a non-blinded fashion to either pravastatin or usual care. After randomization, participants in the original trial were followed for the combined primary endpoint of fatal coronary heart disease or nonfatal myocardial infarction. ECGs were obtained every two years.
Study Cohort
The ALLHAT doxazosin treatment arm was prematurely terminated due to evidence of increased stroke and cardiovascular disease events compared to chlorthalidone; participants randomized to doxazosin treatment (n = 9,061) were not included in the present investigation. Participants with prevalent AF or AFL, as identified on the baseline study ECG, were also excluded. To facilitate post-trial surveillance, only those individuals with valid Medicare or Social Security numbers enrolled from non-Veterans Affairs medical centers within the United States were included in the study cohort.
Treatment
Participants were randomly assigned to chlorthalidone, lisinopril, or amlodipine in a ratio of 1.7:1:1. Individuals were treated with escalating doses of antihypertensive medication according to their treatment randomization group to achieve a goal blood pressure of < 140/90 mmHg. Maximal allowable doses were 25 mg/day for chlorthalidone, 10 mg/day for amlodipine, and 40 mg/day for lisinopril. In the lipid-lowering arm, participants randomized to pravastatin were initially treated with 20 mg/day followed by a dosage increase to 40 mg/day as needed to lower LDL-C by at least 25%. This strategy was amended after the first 1,000 participants were enrolled and a standardized pravastatin 40 mg/day dose was used thereafter.
Covariate Assessment
Clinical variables potentially associated with AF/AFL and assessed during ALLHAT enrollment were identified a priori. Race, smoking status, and medical history were recorded using a standardized form upon study enrollment. Definitions of comorbid conditions, including atherosclerotic cardiovascular disease, coronary heart disease, diabetes, and smoking status are described in Supplemental Table 1. Left ventricular hypertrophy was diagnosed by ECG using the Cornell voltage criteria.21 Serum potassium, creatinine, glucose, and lipid profiles were obtained in a fasting state at the baseline visit.
Atrial Fibrillation, Atrial Flutter, and Death Ascertainment
Participants were considered to have fulfilled the primary outcome if they were diagnosed with AF or AFL by either study ECG or by CMS coding. Standard 12-lead ECGs were obtained in the supine position at 0, 24, 48, 72, and 96 months of study follow-up. ECGs were analyzed using the Minnesota Code Classification System for Electrocardiographic Findings and visually inspected for accuracy in a core laboratory at the University of Minnesota (Minneapolis, Minnesota). Minnesota codes 8-3-1 or 8-3-3 were used to diagnose AF; 8-3-2 or 8-3-4 were used to diagnose AFL. After linking participants to CMS databases, AF and AFL diagnoses were identified from inpatient hospitalization and ambulatory healthcare encounters using the International Classification of Diseases Ninth Edition (ICD-9) codes 427.31 and 427.32, respectively. Death was determined by physician report and by searching CMS, National Death Index, and Social Security Index databases.
Statistics
Continuous variables are presented as mean ± standard deviation (SD) and were compared using two-sample t tests. The association between categorical variables was determined using Pearson Chi-squared test. Cox proportional hazard models were used to determine the association between baseline covariates and incident AF/AFL both before and after controlling for a priori identified confounders. The proportional hazards assumption of the adjusted primary analysis was assessed by evaluating for a global time-by-covariate interaction (p = 0.13) and visually verified using a log-minus-log plot. Together, these findings indicated that the proportional hazards assumption was not violated. Sensitivity analyses were performed to examine the clinical predictors of incident AF (omitting AFL from the outcome definition) and to evaluate predictors of incident AF/AFL limited only to diagnoses made during active trial follow-up. To ensure that differential mortality rates between treatment groups did not influence our results, further sensitivity analyses incorporating Fine-Gray models that treated death as a competing risk were also used to determine the association between treatment randomization and AF/AFL outcomes. In all analyses, an intention-to-treat methodology was utilized.
Data were analyzed using Stata 12 (StataCorp, College Station, Texas, USA). A two-tailed p < 0.05 was considered statistically significant. All participants provided written informed consent upon enrollment and all individual ALLHAT centers received institutional review board approval. Certification to use deidentified ALLHAT data was obtained from the University of California, San Francisco Committee on Human Research.
Results
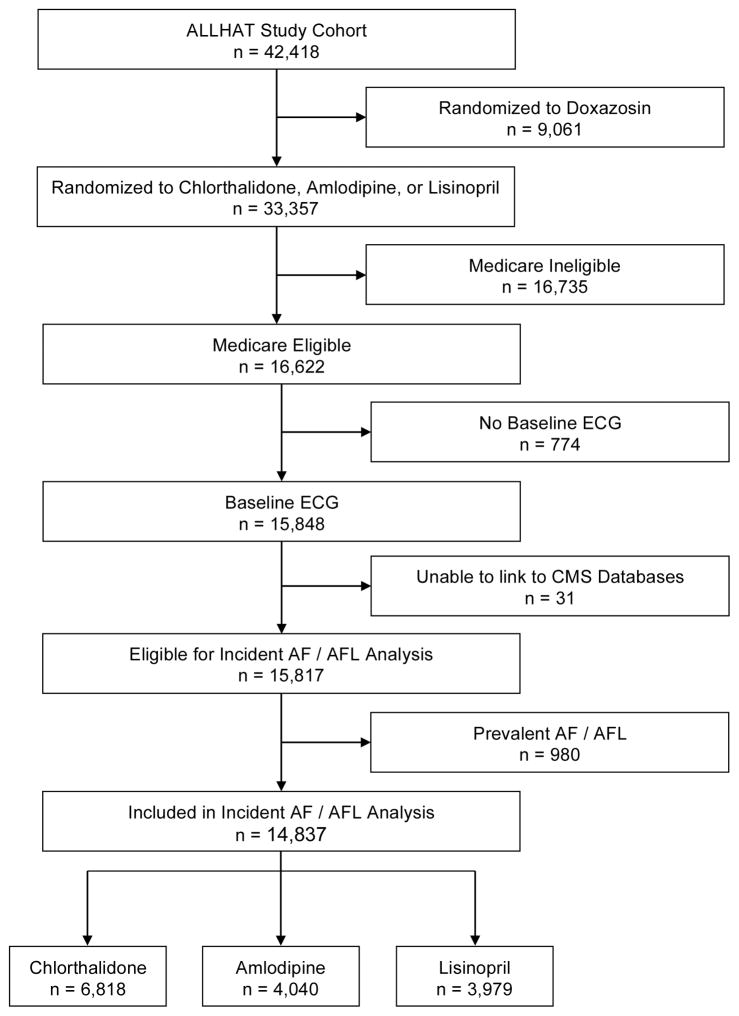
Among the 16,622 Medicare eligible ALLHAT participants, 774 were excluded due to absence of a baseline ECG, 31 were excluded due to an inability to link the individual to CMS databases, and 980 were excluded due to prevalent AF/AF (Figure 1). While there were identifiable differences between included and excluded participants, there was no significant difference in antihypertensive treatment assignment between these groups (Supplemental Table 2). The remaining cohort of 14,837 participants was followed for a mean 7.5 ± 3.2 years. A total of 2,514 (17%) participants developed incident AF or AFL over this time period. The overall incidence of AF or AFL was 22.6 (95% CI 21.7 to 23.5) per 1,000 person years. Participants who developed AF/AFL were on average older, more likely to be men, more likely to be White, and had a higher prevalence of medical comorbidities including coronary heart disease and left ventricular hypertrophy (Table 1). The majority of participants were diagnosed with either AF or AFL by CMS data alone (Table 2).
Figure 1.
Selection of Study Participants from the Overall ALLHAT Cohort
AF, atrial fibrillation; AFL, atrial flutter; CMS, Centers for Medicare Services; ECG, electrocardiogram.
Table 1.
Baseline Characteristics of ALLHAT Participants with and Without Incident Atrial Fibrillation or Atrial Flutter*
| Characteristic | Without AF/AFL (n = 12,323) | Incident AF/AFL (n = 2,514) | P value* |
|---|---|---|---|
| Age, years, mean (SD) | 71.1 (6.5) | 72.9 (6.4) | <0.001 |
| Female Gender, n (%) | 7,043 (57.2) | 1,324 (52.7) | <0.001 |
| Race, n (%) | <0.001 | ||
| White Non-Hispanic | 5,440 (44.2) | 1,610 (64.0) | |
| Black | 4,399 (35.7) | 644 (25.6) | |
| Hispanic | 2,297 (18.6) | 235 (9.4) | |
| Other | 187 (1.5) | 25 (1.0) | |
| Body Mass Index, kg/m2, mean (SD) | 29.1 (5.9) | 29.5 (6.2) | 0.005 |
| Current Smoker, n (%) | 2,076 (16.8) | 390 (15.5) | 0.102 |
| Coronary Heart Disease, n (%) | 3,190 (25.9) | 859 (34.2) | <0.001 |
| Diabetes, n (%) | 4,839 (39.3) | 978 (38.9) | 0.88 |
| Left Ventricular Hypertrophy, n (%) | 593 (4.8) | 164 (6.5) | 0.001 |
| GFR, ml/min/1.73 m2, mean (SD) | 74.3 (19.4) | 71.8 (19.1) | <0.001 |
| Serum potassium, mEq/L, mean (SD) | 4.3 (0.5) | 4.3 (0.5) | 0.78 |
| Cholesterol, mg/dL, mean (SD) | |||
| Total | 217.9 (43.9) | 213.3 (43.2) | <0.001 |
| HDL | 48.5 (14.9) | 46.9 (15.3) | <0.001 |
| LDL | 136.8 (37.2) | 133.3 (36.1) | <0.001 |
P values are for the comparison of the indicated characteristic in participants with versus without incident atrial fibrillation or flutter. Participants with prevalent atrial fibrillation or flutter have been excluded. AF, atrial fibrillation; AFL, atrial flutter; GFR, glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein; SD, standard deviation.
Table 2.
Atrial Fibrillation and Flutter Diagnoses by Ascertainment Methodology
| Atrial Fibrillation | Atrial Flutter | Atrial Fibrillation and Flutter | |
|---|---|---|---|
| ECG Only | 80 | 5 | 0 |
| CMS Only | 1,856 | 67 | 302 |
| Both ECG and CMS | 170 | 0 | 34 |
| Total | 2,106 | 72 | 336 |
Methodology used to diagnose 2,514 cases of incident atrial fibrillation and flutter. Participants were considered to be diagnosed by “Both ECG and CMS” if the diagnoses occurred at the same time period. CMS, Centers for Medicare Services; ECG, electrocardiogram.
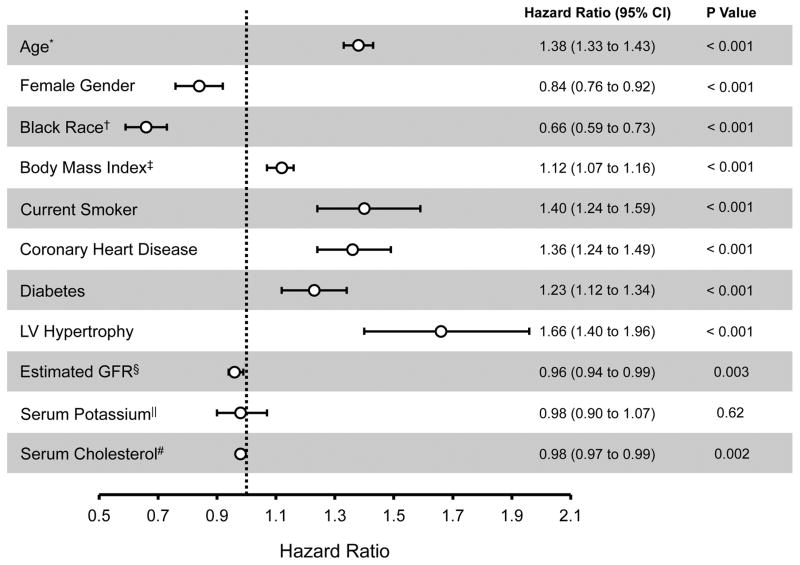
In multivariable models that adjusted for antihypertensive treatment, statin treatment, and the variables described in Table 1, age, BMI, smoking status, coronary heart disease, diabetes, left ventricular hypertrophy, and glomerular filtration rate were each significantly associated with an increased risk of incident AF/AFL (Figure 2). Female and Black participants, on the other hand, demonstrated a significantly reduced adjusted risk of AF/AFL.
Figure 2.
Multivariable Predictors of Incident Atrial Fibrillation or Flutter
*Per 5 year increase in age. †Compared to White non-Hispanics. ‡Per 5 kg/m2 increase. §Per 10 ml/min/1.73 m2 increase. ||Per mEq/L increase. #Per 10mg/dl increase. Error bars denote 95% confidence intervals. Hazard ratios describe the adjusted association between the given variable and AF/AFL after controlling for all clinical variables included in the Figure, antihypertensive treatment assignment, and statin treatment assignment. CI, confidence interval; GFR, glomerular filtration rate; HR, hazard ratio.
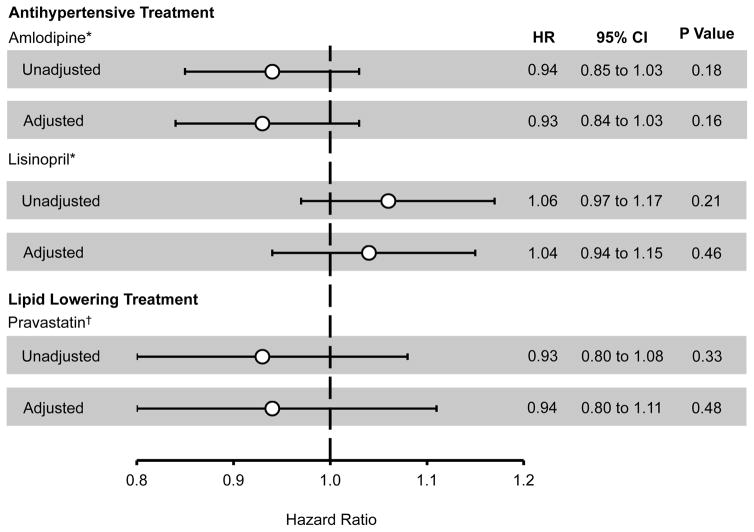
Randomization to lisinopril or amlodipine therapy was not associated with a significant difference in incident AF or AFL (Figure 3, Supplemental Figure 1) compared to chlorthalidone, either before or after multivariable adjustment. Similarly, randomization to pravastatin was not associated with a reduced risk of incident disease. These results were not substantially changed in sensitivity analyses that restricted the outcome to incident AF (AFL was omitted from the outcome definition), when only in-trial outcomes were examined, or when Fine-Gray competing risk models were used (Supplemental Table 3). We did not identify a significant interaction between lisinopril and pravastatin treatment (p value for interaction = 0.72).
Figure 3.
ALLHAT Treatment Randomization and Incident Atrial Fibrillation or Flutter
*Compared to chlorthalidone. †Compared to usual care. Error bars denote 95% confidence intervals. Adjusted hazard ratios describe the adjusted association between treatment randomization and incident atrial fibrillation or flutter after adjustment for all clinical variables included in Table 1. CI, confidence interval; HR, hazard ratio.
Discussion
In a large cohort of hypertensive older adults randomized to antihypertensive and statin therapy, we found that the diagnosis of AF or AFL was common and associated with risk factors such as age, male gender, and White race. We did not observe a significant reduction in incident AF/AFL among participants treated with lisinopril compared to chlorthalidone, nor did we observe a significant reduction in these atrial arrhythmias with pravastatin versus usual medical care.
Previous randomized trials performed among hypertensive individuals have reached divergent conclusions regarding the efficacy of renin angiotensin system inhibition for the primary prevention of AF. While some studies have shown a reduction in incident events with either ACE inhibitor or ARB therapy,9,10 others have not demonstrated clear benefit.11,22,23 Notably, many of these prior investigations relied upon study ECGs performed once every 1–2 years,9–11 only utilized physician report,22,23 or did not explicitly define criteria for AF outcomes.24 AF treatment guidelines currently recommend consideration of ACE inhibitor/ARB treatment for the primary prevention of AF in hypertensive individuals, albeit with slight differences in recommendation strength (AHA/ACC/HRS Class IIb, level of evidence B; ESC Class IIa, level of evidence B).12,13 AF can be difficult to diagnose, especially when it is paroxysmal and asymptomatic. Although ECG remains the gold standard for diagnosis, prior data suggests that screening ECGs are very insensitive and likely to miss the majority of patients ultimately diagnosed with this arrhythmia.18 Furthermore, reliance upon ECG screening has the potential to introduce bias, as this methodology preferentially identifies individuals with persistent AF and AFL.
Our analysis utilized both serial study ECGs and Medicare claims data to diagnose AF and AFL. This combined diagnostic approach has the advantage of identifying AF/AFL outcomes detected during both inpatient and outpatient healthcare encounters with the added benefit of asymptomatic ECG screening. In addition, the use of Medicare data facilitated monitoring for outcomes beyond the trial follow up period. Notably, the association between pharmacologic therapy and incident AF was previously studied using ALLHAT trial data.25 This prior study, which did not identify an association between ACE inhibitor randomization and incident AF/AFL, only considered serial study ECGs for AF detection and utilized logistic regression for risk comparison, potentially limiting statistical power. Our analysis extends these findings by substantially improving arrhythmia detection through the inclusion of CMS data and by applying a time to event analysis. For instance, in the prior ALLHAT analysis, 537 (2%) of participants were found to have incident AF or AFL. In our study, which was restricted to ALLHAT participants enrolled in Medicare, we identified 2,514 AF or AFL diagnoses (17% of participants). Despite this substantial improvement in outcome ascertainment, we were unable to identify a clear benefit of ACE inhibitor therapy for the reduction of incident AF/AFL among older, hypertensive individuals. While small reductions in AF/AFL risk cannot be excluded, the 95% confidence intervals of our point estimates exclude a large, clinically significant treatment effect.
Although prior investigations15,26,27 have suggested a benefit of statin therapy for AF prevention after cardiac surgery, this medication has not been shown to reduce incident arrhythmia in randomized trials performed in ambulatory patient cohorts.16,17 These prior negative trials again relied solely upon ambulatory ECGs for the diagnosis of AF and we again reasoned that our methodology could improve power for detection of a medication benefit, if present. Our results are in agreement with these prior studies and again suggest that statin therapy does not reduce incident AF outside of the perioperative setting.
In addition to examining the individual association between treatment assignment and incident AF, the ALLHAT study design allowed us to examine for an interaction between randomization to both lisinopril and pravastatin. To our knowledge, this is the first study with a factorial design that allowed for such an analysis. We hypothesized that participants randomized to both of these therapies might have more profound reductions in incident AF/AFL given the blockade of multiple potential arrhythmogenic or atrial-substrate related pathways. In light of our primary data, it was not surprising that there was no statistically significant interaction between these two therapies.
Limitations of our investigation should be recognized. AF and AFL were not primary, adjudicated ALLHAT endpoints and the majority of participants in our investigation were diagnosed with AF or AFL using administrative database coding. Although this methodology could have introduced bias, it should be noted that administrative diagnostic codes at a large health maintenance organization exhibited 95% sensitivity and 99% specificity for the diagnosis of AF when compared to record review by trained abstractors.28 In addition, the absence of a substantial or sharp rise in AF/AFL diagnoses during the first few years after treatment randomization with the addition of CMS surveillance (Supplemental Figure 1) suggests that prevalent disease was adequately excluded. While there were differences in characteristics between participants excluded from our analysis due to absence of a baseline ECG and the final included cohort, these differences were generally small and did not confer a consistently increased or decreased AF risk. Importantly, individuals without a baseline ECG were equally distributed by antihypertensive treatment assignment; it is therefore unlikely that excluding these participants resulted in substantial bias in our overall point estimates. Finally, we used CMS data to extend our follow up beyond the active ALLHAT study period to improve outcome ascertainment. It is possible that this methodology could have biased our results towards the null hypothesis if participants discontinued ACE inhibitor or statin treatment at the conclusion of the trial. However, as the results from a sensitivity analysis limited to in-trial events did not substantially differ from the overall results, we do not think this explanation accounts for our findings.
In a randomized trial performed among older adults with a history of hypertension, we found that treatment with either lisinopril or pravastatin was not associated with a meaningful reduction in incident AF or AFL despite efforts to enhance arrhythmia detection and statistical power. Although ACE inhibitor and statin therapies offer benefit beyond arrhythmia suppression, their use for the primary prevention of AF and AFL among hypertensive patients should not be promoted.
Supplementary Material
WHAT IS KNOWN?
Previous randomized trials performed among hypertensive individuals have reached divergent conclusions regarding the efficacy of renin angiotensin system inhibition for the primary prevention of atrial fibrillation (AF).
Notably, many of these prior investigations relied upon study ECGs performed once every 1–2 years, only utilized physician report, or did not explicitly define criteria for AF and atrial flutter (AFL) outcomes.
WHAT THE STUDY ADDS?
Despite substantial improvement in outcome ascertainment through the use of serial study ECGs and Medicare claims data, we found that treatment with lisinopril (compared to chlorthalidone) was not associated with a meaningful reduction in incident AF or AFL among older adults with a history of hypertension.
Although ACE inhibitors offer benefit beyond arrhythmia suppression, these data suggest that their use for the primary prevention of AF and AFL among hypertensive patients should not be promoted.
Acknowledgments
The funding sources played no role in the study design, data collection, data management, data analysis, data interpretation, manuscript preparation, manuscript review, or manuscript approval.
Sources of Funding: This work was made possible by grant number 12POST11810036 (TAD) and 16EIA26410001 (AA) from the American Heart Association and by the Joseph Drown Foundation (GMM). This study was also supported by contracts NO1-HC-35130 and HHSN268201100036C with the National Heart, Lung, and Blood Institute (NHLBI).
Footnotes
Disclosures: The authors have no disclosures to report.
References
- 1.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, D’Agostino RB. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Wolowacz SE, Samuel M, Brennan VK, Jasso-Mosqueda J-G, Van Gelder IC. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace. 2011;13:1375–1385. doi: 10.1093/europace/eur194. [DOI] [PubMed] [Google Scholar]
- 5.Marcus GM. Predicting incident atrial fibrillation: an important step toward primary prevention. Arch Intern Med. 2010;170:1874–1875. doi: 10.1001/archinternmed.2010.426. [DOI] [PubMed] [Google Scholar]
- 6.Sicouri S, Cordeiro JM, Talarico M, Antzelevitch C. Antiarrhythmic effects of losartan and enalapril in canine pulmonary vein sleeve preparations. J Cardiovasc Electrophysiol. 2011;22:698–705. doi: 10.1111/j.1540-8167.2010.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster MW, Fitzpatrick MA, Nicholls MG, Ikram H, Wells JE. Effect of enalapril on ventricular arrhythmias in congestive heart failure. Am J Cardiol. 1985;56:566–569. doi: 10.1016/0002-9149(85)91186-5. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, Nattel S. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–2614. doi: 10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 9.Schmieder RE, Kjeldsen SE, Julius S, McInnes GT, Zanchetti A, Hua TA VALUE Trial Group. Reduced incidence of new-onset atrial fibrillation with angiotensin II receptor blockade: the VALUE trial. J Hypertens. 2008;26:403–411. doi: 10.1097/HJH.0b013e3282f35c67. [DOI] [PubMed] [Google Scholar]
- 10.Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlöf B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Devereux RB. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–719. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 11.Salehian O, Healey J, Stambler B, Alnemer K, Almerri K, Grover J, Bata I, Mann J, Matthew J, Pogue J, Yusuf S, Dagenais G, Lonn E HOPE Investigators. Impact of ramipril on the incidence of atrial fibrillation: results of the Heart Outcomes Prevention Evaluation study. Am Heart J. 2007;154:448–453. doi: 10.1016/j.ahj.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 12.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 13.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 14.Shiroshita-Takeshita A, Schram G, Lavoie J, Nattel S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation. 2004;110:2313–2319. doi: 10.1161/01.CIR.0000145163.56529.D1. [DOI] [PubMed] [Google Scholar]
- 15.Chen WT, Krishnan GM, Sood N, Kluger J, Coleman CI. Effect of statins on atrial fibrillation after cardiac surgery: a duration- and dose-response meta-analysis. J Thorac Cardiovasc Surg. 2010;140:364–372. doi: 10.1016/j.jtcvs.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 16.Macfarlane PW, Murray H, Sattar N, Stott DJ, Ford I, Buckley B, Jukema JW, Westendorp RGJ, Shepherd J. The incidence and risk factors for new onset atrial fibrillation in the PROSPER study. Europace. 2011;13:634–639. doi: 10.1093/europace/eur016. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GG, Chaitman BR, Goldberger JJ, Messig M. High-dose atorvastatin and risk of atrial fibrillation in patients with prior stroke or transient ischemic attack: analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Am Heart J. 2011;161:993–999. doi: 10.1016/j.ahj.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 20.Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT, Cushman WC, Grimm RH, LaRosa J, Whelton PK, Perry HM, Alderman MH, Ford CE, Oparil S, Francis C, Proschan M, Pressel S, Black HR, Hawkins CM. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. Am J Hypertens. 1996;9:342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 21.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75:565–572. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 22.Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, Luomanmäki K, Dahlöf B, de Faire U, Mörlin C, Karlberg BE, Wester PO, Björck JE. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999;353:611–616. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- 23.Hansson L, Lindholm LH, Ekbom T, Dahlöf B, Lanke J, Scherstén B, Wester PO, Hedner T, de Faire U. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354:1751–1756. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- 24.Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators. Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 25.Haywood LJ, Ford CE, Crow RS, Davis BR, Massie BM, Einhorn PT, Williard A ALLHAT Collaborative Research Group. Atrial fibrillation at baseline and during follow-up in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) J Am Coll Cardiol. 2009;54:2023–2031. doi: 10.1016/j.jacc.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Mannacio VA, Iorio D, De Amicis V, Di Lello F, Musumeci F. Effect of rosuvastatin pretreatment on myocardial damage after coronary surgery: a randomized trial. J Thorac Cardiovasc Surg. 2008;136:1541–1548. doi: 10.1016/j.jtcvs.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 27.Patti G, Chello M, Candura D, Pasceri V, D’Ambrosio A, Covino E, Di Sciascio G. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114:1455–1461. doi: 10.1161/CIRCULATIONAHA.106.621763. [DOI] [PubMed] [Google Scholar]
- 28.Glazer NL, Dublin S, Smith NL, French B, Jackson LA, Hrachovec JB, Siscovick DS, Psaty BM, Heckbert SR. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med. 2007;167:246–252. doi: 10.1001/archinte.167.3.246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.