Abstract
Background
Women with DCIS are increasingly choosing bilateral mastectomy. We sought to quantify rates of contralateral breast cancer (CBC) and ipsilateral breast tumor recurrence (IBTR) after breast-conserving surgery (BCS) for DCIS, and to compare risk factors for CBC and IBTR.
Methods
From 1978–2011, DCIS patients undergoing BCS with a contralateral breast at risk were identified from a prospectively maintained database. Association of clinicopathologic and treatment factors with CBC and IBTR were evaluated using Kaplan-Meier analysis and competing risk regression (CRR).
Results
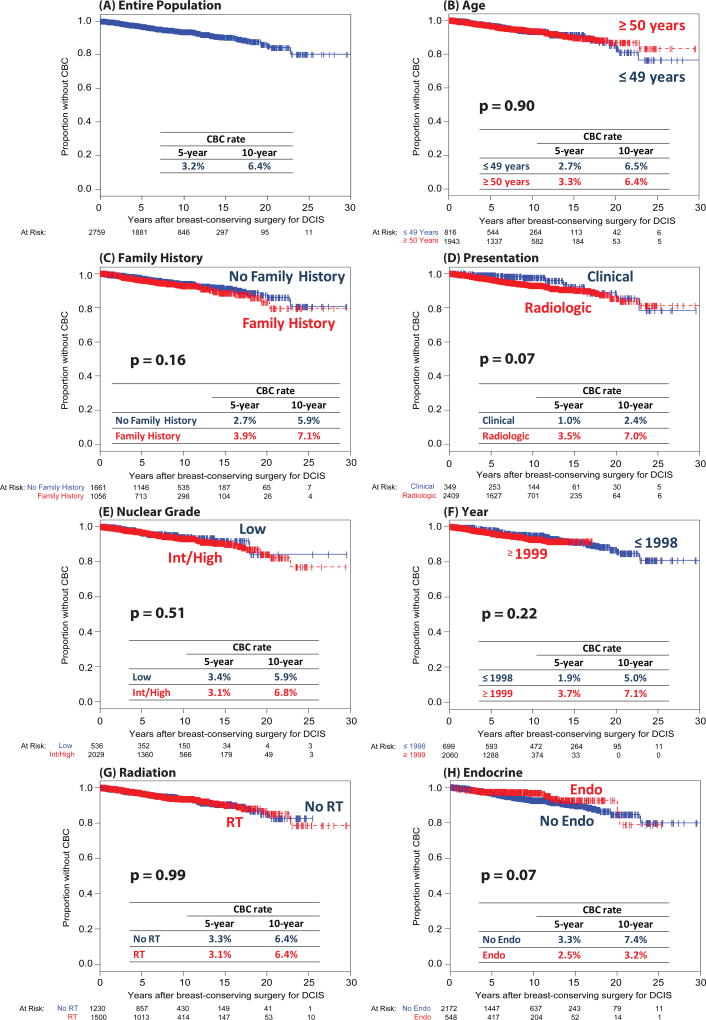
Of 2759 patients identified, 151 developed CBC and 344 IBTR. 5- and 10-year Kaplan-Meier CBC rates were 3.2% and 6.4%. Overall, 10-year IBTR rates were 2.5-fold higher than CBC rates, and without radiation, 4-fold higher. On CRR, 5- and 10-year rates were 2.9% and 5.8% for CBC, and 7.8% and 14.5% for IBTR. CBC risk (Kaplan-Meier and CRR multivariable analysis) and invasive CBC risk (CRR multivariable analysis) were not significantly associated with age, family history, presentation, nuclear grade, year of surgery, or radiation. By Kaplan-Meier, endocrine therapy was associated with lower CBC risk (HR 0.57, p=0.03). 10-year risk of subsequent CBC in the subset of patients who developed IBTR was similar to the cohort as a whole (8.1% vs. 6.4%).
Conclusions
Rates of CBC were low across all groups, including those who experienced IBTR. CBC was not associated with factors that increase IBTR risk. While factors associated with IBTR risk are important in decision-making regarding management of the index DCIS, they are not an indication for contralateral prophylactic mastectomy.
Keywords: ductal carcinoma in situ, contralateral breast cancer risk, ipsilateral breast tumor recurrence, bilateral mastectomy, characteristics of initial DCIS, breast-conserving surgery
BACKGROUND
The use of bilateral mastectomy has increased over the past two decades for women with unilateral breast cancer.1,2 This trend has been seen in patients with early stage breast cancer and particularly in those with ductal carcinoma in situ (DCIS), in whom the rate of bilateral mastectomy nearly tripled from 2005 to 2013.1,3,4
Disease-specific survival after treatment of DCIS is over 98% at 10 years.5,6 Despite the overall excellent prognosis, many patients with DCIS overestimate their risk of local recurrence, metastatic disease, and death from breast cancer.7 Similarly, patients frequently misperceive their risk of contralateral breast cancer (CBC)8,9, potentially leading to decisions in favor of bilateral mastectomy.
The risk of developing CBC for average-risk women with breast cancer is low, estimated to range from 0.1–0.6% per year, and has decreased in recent years.10–12 However, these estimates are generally based on the risk of CBC after invasive breast cancer rather than DCIS. Furthermore, few studies have examined the risk of CBC in women undergoing breast-conserving surgery (BCS) for DCIS, a group inherently different than those treated with unilateral mastectomy. Similarly, there has been little published on factors predictive of CBC following DCIS.
We sought to quantify the risk of CBC in women with DCIS treated with BCS and to compare this with the risk of ipsilateral breast tumor recurrence (IBTR) in the same population. Our secondary aim was to assess if risk factors for IBTR were also associated with increased risk of CBC. Our goal was to provide data to aid in shared decision-making regarding the surgical approach to unilateral DCIS for women in whom BCS is feasible.
METHODS
Following Institutional Review Board approval, all patients with a contralateral breast at risk for the subsequent development of breast cancer were identified from a prospectively maintained database of DCIS patients treated with BCS at Memorial Sloan Kettering Cancer Center from 1978–2011. Those with a diagnosis of CBC prior to or synchronous with the diagnosis of DCIS and those who underwent contralateral mastectomy were excluded.
Clinicopathologic factors were collected based on the index DCIS, including age at diagnosis (≤49 or ≥50 years), presentation (radiologic or clinical), family history of breast cancer (one or more first- or second-degree relative), nuclear grade (low or intermediate/high), treatment time period (≤1998 or ≥1999), and use of adjuvant radiation and endocrine therapy for the index DCIS. Cases of markedly atypical ductal hyperplasia bordering on or focally reaching DCIS were included as low grade DCIS.
The primary endpoint was time from definitive surgery for the initial DCIS to diagnosis of CBC, including either DCIS or invasive breast cancer. The Kaplan-Meier method was used to calculate 5- and 10- year CBC estimates for the entire population and by each clinicopathologic factor for the index DCIS. Differences in CBC rates were assessed using the logrank test. Multivariable Cox models were fit to evaluate the relationship between clinicopathologic factors and CBC risk. In a subset analysis, patients who experienced an IBTR following BCS for DCIS were analyzed to describe their risk of CBC after IBTR, with time to event defined as the time interval from IBTR to subsequent CBC.
Competing risk analysis was used to evaluate differences in the risk of CBC with that of IBTR.13 IBTR included ipsilateral recurrence in the breast, in the axilla, or, in a single case, as metastatic disease in the absence of locoregional recurrence or CBC. In this competing risk analysis, the endpoint was defined as the time interval from definitive surgery for the index DCIS to the first event, either CBC or IBTR. A single patient with subsequent synchronous IBTR and CBC diagnosed on the same day was excluded from the competing risk analysis. Gray’s test was used to evaluate the association of each factor with the cumulative incidence of CBC or IBTR. Competing risk multivariable regression was then used to evaluate factors associated with CBC or with IBTR in a single model.14 A separate competing risk analysis examined invasive CBC or IBTR outcomes. Competing risk multivariable regression evaluated the association of index DCIS characteristics with subsequent invasive CBC or IBTR.
All analyses were performed in SAS version 9.4 and R version 3.1.1
RESULTS
From 1978 to 2011, there were 2759 DCIS patients who underwent BCS with a contralateral breast at risk for the development of breast cancer. Median follow-up was 6.8 years (range 0.01–29.6 years); 846 patients had ≥10 years of follow-up. 151 patients developed CBC and 344 patients developed IBTR. Of these, 34 patients developed both CBC and IBTR. Of the 151 patients who developed CBC, 107 were invasive and 42 were DCIS; the type of CBC was unknown in 2 cases. Of the 344 IBTRs, 144 were invasive and 192 were DCIS; the type of IBTR was unknown in 8 cases.
Characteristics of the index DCIS for entire cohort and those with CBC, IBTR, and both CBC and IBTR are summarized in Table 1.
Table 1.
Demographic characteristics of the entire population, and by contralateral breast cancer and ipsilateral recurrence as first subsequent breast event
| Total Population N= 2759 |
CBC N = 151* |
IBTR N = 344** |
Both CBC and IBTR N = 34§ |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | % | N | % | N | % | N | % | |
| Age, yrs | ||||||||
| ≤49 | 816 | 29.6% | 47 | 31.1% | 128 | 37.2% | 12 | 35.3% |
| ≥50 | 1943 | 70.4% | 104 | 68.9% | 216 | 62.8% | 22 | 64.7% |
|
| ||||||||
| Family history | ||||||||
| No | 1661 | 60.2% | 85 | 56.3% | 206 | 59.9% | 20 | 58.8% |
| Yes | 1056 | 38.3% | 64 | 42.4% | 134 | 39.0% | 14 | 41.2% |
| Unknown | 42 | 1.5% | 2 | 1.3% | 4 | 1.1% | 0 | 0% |
|
| ||||||||
| Presentation | ||||||||
| Clinical | 349 | 12.7% | 15 | 9.9% | 68 | 19.8% | 8 | 23.5% |
| Radiologic | 2409 | 87.3% | 136 | 90.1% | 276 | 80.2% | 26 | 76.5% |
| Unknown | 1 | 0.04% | 0 | 0% | 0 | 0% | 0 | 0% |
|
| ||||||||
| Nuclear grade | ||||||||
| Low | 536 | 19.4% | 24 | 15.9% | 50 | 14.5% | 5 | 14.7% |
| Intermediate/high | 2029 | 73.6% | 111 | 73.5% | 250 | 72.7% | 25 | 73.5% |
| Unknown | 194 | 7.0% | 16 | 10.6% | 44 | 12.8% | 4 | 11.8% |
|
| ||||||||
| Year of surgery | ||||||||
| ≤1998 | 699 | 25.3% | 57 | 37.8% | 158 | 45.9% | 19 | 55.9% |
| ≥1999 | 2060 | 74.7% | 94 | 62.2% | 186 | 54.1% | 15 | 44.1% |
|
| ||||||||
| Radiation | ||||||||
| No | 1230 | 44.6% | 70 | 46.4% | 201 | 58.4% | 20 | 58.8% |
| Yes | 1500 | 54.4% | 81 | 53.6% | 143 | 41.6% | 14 | 41.2% |
| Unknown | 29 | 1.0% | 0 | 0% | 0 | 0% | 0 | 0% |
|
| ||||||||
| Endocrine therapy | ||||||||
| No | 2172 | 78.7% | 127 | 84.1% | 299 | 86.9% | 29 | 85.3% |
| Yes | 548 | 19.9% | 23 | 15.2% | 44 | 12.8% | 5 | 14.7% |
| Unknown | 39 | 1.4% | 1 | 0.7% | 1 | 0.3% | 0 | 0% |
CBC N = 151 includes all patients who had a CBC, including N=34 that had IBTR
IBTR N = 344 includes all patients who had an IBTR, including N=34 that had CBC
N = 34 includes patients who had both an IBTR and CBC; these patients are also included in the CBC and IBTR columns
CBC = contralateral breast cancer
IBTR = ipsilateral breast tumor recurrence
Contralateral breast cancer risk
The 5- and 10-year cumulative incidence rates of CBC were 3.2% and 6.4%, respectively (Figure 1A). On univariate Kaplan-Meier analysis, CBC risk was not significantly associated with the following index DCIS characteristics: age, family history, presentation, nuclear grade, year of surgery, or radiation (Figure 1B–1G). Those who received endocrine therapy trended toward a lower risk of CBC (10-year rate 3.2% vs. 7.4%, p=0.07) (Figure 1H). On multivariable analysis, the use of endocrine therapy was significantly associated with lower CBC risk, with a rate decreased by almost half (HR 0.57, 95% CI 0.35–0.93, p=0.03) (Table 2).
Fig. 1.
Univariate analysis of characteristics at initial diagnosis of DCIS as risk factors for subsequent contralateral breast cancer
CBC = contralateral breast cancer
Table 2.
Multivariable analysis of characteristics at initial diagnosis of DCIS as risk factors for subsequent contralateral breast cancer
| Multivariable Cox Regression | ||||
|---|---|---|---|---|
|
|
||||
| HR | 95% CI | p-value | ||
| Age (years) | ≤49 | 1.00 | ||
| ≥50 | 0.97 | 0.67–1.40 | 0.85 | |
|
| ||||
| Family history | No | 1.00 | ||
| Yes | 1.11 | 0.78–1.57 | 0.56 | |
|
| ||||
| Presentation | Radiologic | 1.00 | ||
| Clinical | 0.58 | 0.30–1.11 | 0.10 | |
|
| ||||
| Nuclear grade | Low | 1.00 | ||
| Intermediate/high | 1.26 | 0.79–2.01 | 0.34 | |
|
| ||||
| Year of surgery | ≤1998 | 1.00 | ||
| ≥1999 | 1.39 | 0.90–2.15 | 0.14 | |
|
| ||||
| Radiation | No | 1.00 | ||
| Yes | 0.81 | 0.56–1.17 | 0.26 | |
|
| ||||
| Endocrine therapy | No | 1.00 | ||
| Yes | 0.57 | 0.35–0.93 | 0.03 | |
HR = hazard ratio
CI = confidence interval
We performed a subset analysis in the 331 patients who developed an IBTR as a first event after their initial DCIS to determine if their risk of subsequent CBC was greater than that of the cohort as a whole. Their CBC risk at 5- and 10-years after IBTR was 3.7% and 8.1%, respectively.
Competing risk analysis of CBC vs. IBTR
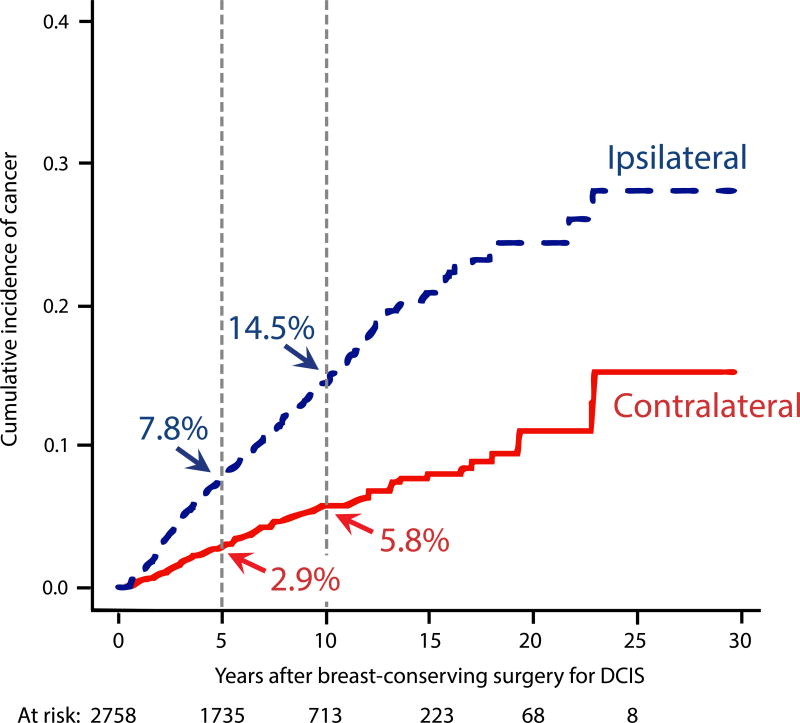
Competing risk analysis demonstrated 5- and 10-year CBC rates of 2.9% and 5.8%, compared to 7.8% and 14.5% for IBTR, respectively (Figure 2A). On univariate competing risk analysis, the only factor significantly associated with the risk of CBC was radiologic presentation of the initial DCIS, with a 10-year CBC risk of 6.3%, compared with a 2.3% risk in those whose initial DCIS was diagnosed clinically (p=0.01) (Table 3). Several factors were associated with the risk of IBTR. Of note, the 10-year IBTR risk was 2.5-fold higher than that of CBC for the entire population (Figure 2A), and for the subset not receiving radiation, it was nearly 4-fold higher (Table 3).
Fig. 2.
(A) Competing risk cumulative incidence of contralateral breast cancer and ipsilateral breast tumor recurrence.
Table 3.
Competing risk analysis univariate 10-year cumulative incidence rates and multivariable hazard ratios for contralateral breast cancer and ipsilateral recurrence by characteristics at initial diagnosis of DCIS
| Competing risk cumulative incidence rates (univariate analysis) |
Competing risk regression hazard ratios (multivariable analysis) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 10-yr CBC risk |
p- value* |
10-yr IBTR risk |
p- value** |
CBCHR | p- value |
IBTR HR |
p- value |
|
| Age, yrs | ||||||||
| ≤49 | 6.1% | 0.9 | 17.6% | 0.002 | ref | 0.72 | ref | 0.002 |
| ≥50 | 5.6% | 13.2% | 0.93 | 0.68 | ||||
|
| ||||||||
| Family history | ||||||||
| No | 5.4% | 0.3 | 14.0% | 0.47 | ref | 0.7 | ref | 0.54 |
| Yes | 6.2% | 15.1% | 1.08 | 1.08 | ||||
|
| ||||||||
| Presentation | ||||||||
| Radiologic | 6.3% | 0.01 | 13.6% | 0.001 | ref | 0.06 | ref | 0.013 |
| Clinical | 2.3% | 20.1% | 0.47 | 1.49 | ||||
|
| ||||||||
| Nuclear grade | ||||||||
| Low | 5.2% | 0.4 | 12.7% | 0.1 | ref | 0.49 | ref | 0.002 |
| Intermediate/high | 6.2% | 14.5% | 1.21 | 1.65 | ||||
|
| ||||||||
| Year of surgery | ||||||||
| ≤1998 | 4.1% | 0.09 | 19.3% | < 0.0001 | ref | 0.1 | ref | 0.037 |
| ≥1999 | 6.6% | 13.2% | 1.45 | 0.75 | ||||
|
| ||||||||
| Radiation | ||||||||
| No | 5.2% | 0.2 | 19.5% | < 0.0001 | ref | 0.82 | ref | <0.0001 |
| Yes | 6.3% | 10.4% | 1.05 | 0.46 | ||||
|
| ||||||||
| Endocrine therapy | ||||||||
| No | 6.5% | 0.2 | 16.5% | < 0.0001 | ref | 0.07 | ref | 0.004 |
| Yes | 3.2% | 7.8% | 0.61 | 0.59 | ||||
p-value for each co-variate for risk of CBC; calculated using Gray’s test
p-value for each co-variate for risk of IBTR; calculated using Gray’s test
HR = hazard ratio
IBTR = ipsilateral breast tumor recurrence
CBC = contralateral breast cancer
Multivariable analysis utilizing competing risk regression identified no characteristics of the initial DCIS to be statistically significantly associated with the risk of subsequent CBC, although several factors were associated with risk of IBTR (Table 3).
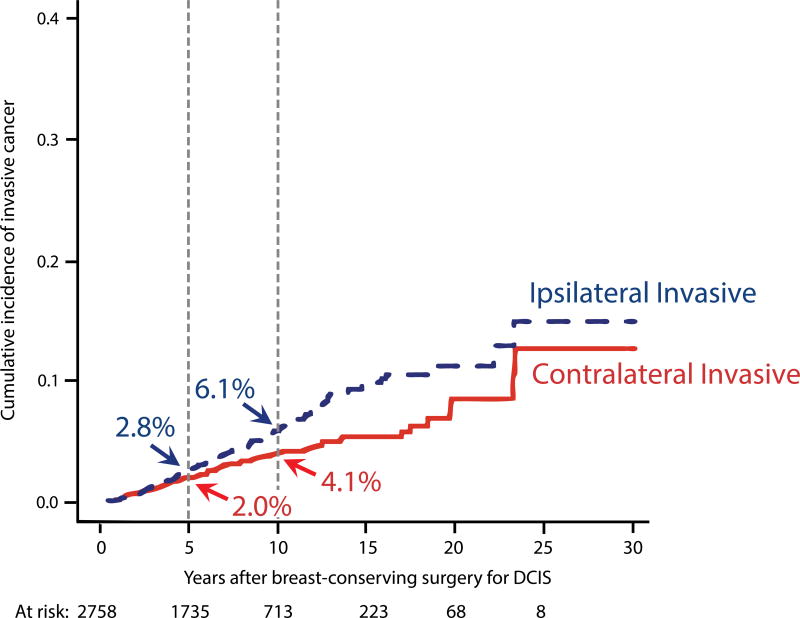
Competing risk analysis of invasive CBC vs. invasive IBTR
On competing risk analysis, the 10-year invasive CBC rate was 4.1%; the invasive IBTR rate was 6.1% (Figure 2B). Using multivariable competing risk regression to examine association of initial DCIS characteristics with the development of subsequent invasive CBC or IBTR, only the receipt of endocrine therapy trended towards association with a lower risk of invasive CBC (HR 0.52, p=0.058). However, a lower risk of invasive IBTR was found among those with age ≥50 (HR 0.61, p=0.009) and radiation (HR 0.44, p<0.0001). Women who received endocrine therapy had a non-significantly lower rate of invasive IBTR (HR 0.66, p=0.11).
DISCUSSION
Although disease-specific survival for DCIS is excellent and concerns exist regarding its overtreatment, an increasing number of patients are opting for more extensive surgery to treat DCIS. While the use of unilateral mastectomy for DCIS has decreased slightly over the last two decades, the rate of bilateral mastectomy for DCIS has more than doubled.1,4 A variety of factors likely influence patients’ decisions to choose bilateral mastectomy, including anxiety, confusion regarding the lack of impact of contralateral mastectomy on IBTR risk, overestimation of CBC risk, desire for reconstructive symmetry, and the elimination of the need for future surveillance and possibly future adjuvant therapy.15–18
Our aim was to better understand the risk of CBC in women with DCIS treated with BCS. In our cohort of 2759 patients with DCIS, we found the rate of CBC was 3.2% and 6.4% at 5- and 10-years, respectively. On competing risk analysis, the 10-year risk of IBTR was 2.5-fold greater than the risk of CBC for the entire cohort, and among the subset of women not receiving radiation for the initial DCIS, the risk of IBTR was nearly 4-fold higher than CBC (19.5% vs 5.2%).
The low rates of CBC observed in the current data are similar to those in prior studies. The rate of CBC after early stage breast cancer was 0.6% per year for patients in the SEER database between 1973 and 1996.11 In the limited literature reporting specifically on the risk of CBC among women with DCIS, all treated in the 1990s and earlier, 5- and 10-year rates of CBC were 3.1–4.3% and 6.0–6.8%.11,19,20 Results from our cohort, which includes patients with DCIS treated from 1978 to 2011, confirms the low rate of CBC after DCIS for those treated over a span of greater than 30 years and in a more contemporary setting than previous reports. Further, our analysis examined the influence of treatment time period and did not demonstrate a significant change in rate of CBC in more recent years, although rates of IBTR have fallen significantly.21
Based on studies of patients with invasive breast cancer, we hypothesized that the risk of CBC would be higher in those with a family history of breast cancer and those at younger ages when diagnosed with DCIS.22–24 However, we did not find either factor to be significantly associated with the development of subsequent CBC. Innos et al. found a significantly elevated risk of contralateral invasive cancer (incidence rate ratio 1.35, 95% CI 1.11–1.66) but not contralateral DCIS (incidence rate ratio 0.87, 95% CI 0.64–1.18) among those with their initial DCIS diagnosed at age ≥65, as compared to age 50–64.20 Similarly, Li et al. found diagnosis of DCIS at age ≥60 (HR 1.3–1.5, p<0.05) to be significantly associated with increased risk of subsequent CBC when compared to younger ages.25 Unlike our analysis, neither of these studies adjusted for the use of endocrine therapy, which is less commonly utilized in older women.26–28
In our cohort, after adjustment for multiple factors on multivariable analysis, use of endocrine therapy for the index DCIS was associated with a 43% lower risk of CBC (HR 0.57, p=0.03). This is consistent with the two randomized studies that examined the use of tamoxifen in women undergoing BCS for DCIS, which found a 32% (NSABP B-24) and 56% (UK-ANZ trial) reduction in CBC.29,30
On competing risk analysis, the 10-year risk of IBTR was 2.5 times greater than the risk of CBC, 14.5% vs. 5.8%. On multivariable competing risk analysis, none of the characteristics of the index DCIS were significantly associated with subsequent CBC risk. In contrast, the risk of IBTR was significantly higher when the index DCIS was diagnosed at a younger age, presented clinically, was of intermediate/high grade, or treated prior to 1999, all of which are consistent with prior literature.21,26,31–36 Similar to results from the large, prospective, randomized trials demonstrating that radiation and endocrine therapy decrease IBTR by approximately 50% and 30%, respectively29,30,37–39, we found that radiation halved the risk of IBTR (HR 0.46, p<0.0001) and endocrine therapy reduced it by approximately 40% (HR 0.59, p=0.004).
Since the primary goal of treating DCIS is to prevent an invasive recurrence, we examined the risk of subsequent invasive CBC and IBTR after DCIS. The 10-year rate was 4.1% for invasive CBC and 6.1% for invasive IBTR. Women who received endocrine therapy had a borderline significant lower risk of invasive CBC (HR 0.52, p=0.058), of similar magnitude to that seen in the UK-ANZ trial (HR 0.47, p=0.03).29 Consistent with prior findings from our institution and from the NSABP, younger age and not receiving radiation were associated with a higher risk of invasive IBTR.26,30 Receiving endocrine therapy was associated with a non-significant reduction in risk of invasive IBTR of magnitude similar to that observed in NSABP B-24 (current series: HR 0.66, p=0.11; B-24: HR 0.68, p=0.03).30
Many patients who experience an IBTR will be treated with an ipsilateral mastectomy. Since undergoing unilateral mastectomy increases the chance that a woman will choose a contralateral prophylactic mastectomy (CPM),16 we examined the risk of CBC after IBTR. In this subset, the 10-year rate of CBC was low (8.1%) and similar to that of the overall cohort (6.4%), suggesting that women who experience IBTR are not at a significantly higher risk of future CBC that would mandate CPM.
Our study was retrospective in nature and subject to all associated limitations. The use of radiation and endocrine therapy are correlated with tumor and patient risk factors because they were at the discretion of the treating physician and patient, and reflect perceived risk of recurrence. However, our robust prospectively maintained database, including detailed clinicopathologic data, allowed us to perform multivariable analysis to adjust for these and other factors that could be confounded and affect risk. Further, our patient population represents a large group of women with DCIS eligible for BCS and includes a more contemporary cohort than previous studies examining the risk of CBC.
It is essential that a woman considering CPM be aware of her risk of CBC, the excellent overall survival for treated DCIS, the lack of survival benefit with CPM, the increased complications associated with bilateral as compared to unilateral mastectomy, and the expected aesthetic results.40–43 CPM increases the risk of major complications that require a return to the operating room or hospitalization, implant loss, and skin necrosis, as well as minor complications such as seromas, hematomas, cellulitis, and delayed wound healing.42 Up to 20–30% patients who have undergone reconstruction report that outcomes such as cosmetic appearance, numbness or tingling, and sexuality were worse than expected.44–46 For these reasons, the use of bilateral mastectomy should be carefully considered, with a discussion of appropriate CBC risk estimates, in particular for patients who are eligible for BCS and have a low risk of CBC.
In summary, rates of CBC were low across all patient groups and irrespective of age, family history, and characteristics of initial DCIS. Factors associated with an increased risk of IBTR were not predictive of greater CBC risk. For a woman undergoing BCS for DCIS, the 10-year IBTR rate is 2.5-fold higher than the CBC rate, and for a woman not receiving radiation, it is nearly 4-fold higher than the CBC rate. In the subset of women who experienced an IBTR, the subsequent CBC risk was still low and comparable to that in the larger cohort. Factors associated with higher IBTR risk may be important in decision-making regarding management of the index DCIS, but are not an indication for CPM. A discussion of the risks and benefits of various treatment options, including risk estimates of CBC and IBTR for each, should inform women with DCIS who are candidates for breast conservation.
Acknowledgments
The preparation of this study was supported by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Footnotes
Disclosures: The authors have no conflicts of interest to declare.
This paper will be presented at the American Society of Breast Surgeons 18th Annual Meeting on April 28, 2017 as an oral podium presentation.
References
- 1.Steiner CA, Weiss AJ, Barrett ML, Fingar KR, Davis PH. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet] Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. Feb. Trends in Bilateral and Unilateral Mastectomies in Hospital Inpatient and Ambulatory Settings, 2005–2013: Statistical Brief #201. (Updated Mar 1) [PubMed] [Google Scholar]
- 2.Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998–2007. Ann Surg Oncol. 2010 Oct;17(10):2554–62. doi: 10.1245/s10434-010-1091-3. [DOI] [PubMed] [Google Scholar]
- 3.Pesce CE, Liederbach E, Czechura T, Winchester DJ, Yao K. Changing surgical trends in young patients with early stage breast cancer, 2003 to 2010: a report from the National Cancer Data Base. J Am Coll Surg. 2014 Jul;219(1):19–28. doi: 10.1016/j.jamcollsurg.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 4.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009 Mar 20;27(9):1362–7. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 5.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol. 2015 Oct;1(7):888–96. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 6.Worni M, Akushevich I, Greenup R, et al. Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ. J Natl Cancer Inst. 2015 Dec;107(12):djv263. doi: 10.1093/jnci/djv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakovitch E, Franssen E, Kim J, et al. A comparison of risk perception and psychological morbidity in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res Treat. 2003 Feb;77(3):285–93. doi: 10.1023/a:1021853302033. [DOI] [PubMed] [Google Scholar]
- 8.Abbott A, Rueth N, Pappas-Varco S, Kuntz K, Kerr E, Tuttle T. Perceptions of contralateral breast cancer: an overestimation of risk. Ann Surg Oncol. 2011 Oct;18(11):3129–36. doi: 10.1245/s10434-011-1914-x. [DOI] [PubMed] [Google Scholar]
- 9.Partridge A, Adloff K, Blood E, et al. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst. 2008 Feb 20;100(4):243–51. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 May 14–20;365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 11.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003 Jul 15;56(4):1038–45. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 12.Nichols HB, Berrington de Gonzalez A, Lacey JV, Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011 Apr 20;29(12):1564–9. doi: 10.1200/JCO.2010.32.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16(3):1141–54. [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 15.Ager B, Butow P, Jansen J, Phillips KA, Porter D. Contralateral prophylactic mastectomy (CPM): A systematic review of patient reported factors and psychological predictors influencing choice and satisfaction. Breast. 2016 Aug;28:107–20. doi: 10.1016/j.breast.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Covelli AM, Baxter NN, Fitch MI, McCready DR, Wright FC. 'Taking control of cancer': understanding women's choice for mastectomy. Ann Surg Oncol. 2015 Feb;22(2):383–91. doi: 10.1245/s10434-014-4033-7. [DOI] [PubMed] [Google Scholar]
- 17.Tracy MS, Rosenberg SM, Dominici L, Partridge AH. Contralateral prophylactic mastectomy in women with breast cancer: trends, predictors, and areas for future research. Breast Cancer Res Treat. 2013 Aug;140(3):447–52. doi: 10.1007/s10549-013-2643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi M, Hunt KK, Arun BK, et al. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prev Res (Phila) 2010 Aug;3(8):1026–34. doi: 10.1158/1940-6207.CAPR-09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claus EB, Stowe M, Carter D, Holford T. The risk of a contralateral breast cancer among women diagnosed with ductal and lobular breast carcinoma in situ: data from the Connecticut Tumor Registry. Breast. 2003 Dec;12(6):451–6. doi: 10.1016/s0960-9776(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 20.Innos K, Horn-Ross PL. Risk of second primary breast cancers among women with ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2008 Oct;111(3):531–40. doi: 10.1007/s10549-007-9807-1. [DOI] [PubMed] [Google Scholar]
- 21.Subhedar P, Olcese C, Patil S, Morrow M, Van Zee KJ. Decreasing Recurrence Rates for Ductal Carcinoma In Situ: Analysis of 2996 Women Treated with Breast-Conserving Surgery Over 30 Years. Ann Surg Oncol. 2015 Oct;22(10):3273–81. doi: 10.1245/s10434-015-4740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein JL, Lapinski RH, Thakore SS, Doucette JT, Thompson WD. The descriptive epidemiology of second primary breast cancer. Epidemiology. 2003 Sep;14(5):552–8. doi: 10.1097/01.ede.0000072105.39021.6d. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein JL, Thompson WD, Risch N, Holford TR. The genetic epidemiology of second primary breast cancer. Am J Epidemiol. 1992 Oct 15;136(8):937–48. doi: 10.1093/oxfordjournals.aje.a116566. [DOI] [PubMed] [Google Scholar]
- 24.Reiner AS, John EM, Brooks JD, et al. Risk of asynchronous contralateral breast cancer in noncarriers of BRCA1 and BRCA2 mutations with a family history of breast cancer: a report from the Women's Environmental Cancer and Radiation Epidemiology Study. J Clin Oncol. 2013 Feb 01;31(4):433–9. doi: 10.1200/JCO.2012.43.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li CI, Malone KE, Saltzman BS, Daling JR. Risk of invasive breast carcinoma among women diagnosed with ductal carcinoma in situ and lobular carcinoma in situ, 1988–2001. Cancer. 2006 May 15;106(10):2104–12. doi: 10.1002/cncr.21864. [DOI] [PubMed] [Google Scholar]
- 26.Cronin PA, Olcese C, Patil S, Morrow M, Van Zee KJ. Impact of Age on Risk of Recurrence of Ductal Carcinoma In Situ: Outcomes of 2996 Women Treated with Breast-Conserving Surgery Over 30 Years. 2016 Sep;23(9):2816–24. doi: 10.1245/s10434-016-5249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flanagan MR, Rendi MH, Gadi VK, Calhoun KE, Gow KW, Javid SH. Adjuvant Endocrine Therapy in Patients with Ductal Carcinoma In Situ: A Population-Based Retrospective Analysis from 2005 to 2012 in the National Cancer Data Base. Ann Surg Oncol. 2015 Oct;22(10):3264–72. doi: 10.1245/s10434-015-4668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols HB, Bowles EJ, Islam J, et al. Tamoxifen Initiation After Ductal Carcinoma In Situ. Oncologist. 2016 Feb;21(2):134–40. doi: 10.1634/theoncologist.2015-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011 Jan;12(1):21–9. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011 Mar 16;103(6):478–88. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006 Jul 20;24(21):3381–7. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 32.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999 Jun 12;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 33.Ringberg A, Nordgren H, Thorstensson S, et al. Histopathological risk factors for ipsilateral breast events after breast conserving treatment for ductal carcinoma in situ of the breast--results from the Swedish randomised trial. Eur J Cancer. 2007 Jan;43(2):291–8. doi: 10.1016/j.ejca.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Rudloff U, Brogi E, Brockway JP, et al. Concurrent lobular neoplasia increases the risk of ipsilateral breast cancer recurrence in patients with ductal carcinoma in situ treated with breast-conserving therapy. Cancer. 2009 Mar 15;115(6):1203–14. doi: 10.1002/cncr.24166. [DOI] [PubMed] [Google Scholar]
- 35.Van Zee KJ, Liberman L, Samli B, et al. Long term follow-up of women with ductal carcinoma in situ treated with breast-conserving surgery: the effect of age. Cancer. 1999 Nov 01;86(9):1757–67. doi: 10.1002/(sici)1097-0142(19991101)86:9<1757::aid-cncr18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 36.Vicini FA, Shaitelman S, Wilkinson JB, et al. Long-term impact of young age at diagnosis on treatment outcome and patterns of failure in patients with ductal carcinoma in situ treated with breast-conserving therapy. Breast J. 2013 Jul-Aug;19(4):365–73. doi: 10.1111/tbj.12127. [DOI] [PubMed] [Google Scholar]
- 37.Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012 Apr 20;30(12):1268–73. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donker M, Litiere S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013 Nov 10;31(32):4054–9. doi: 10.1200/JCO.2013.49.5077. [DOI] [PubMed] [Google Scholar]
- 39.Warnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J Clin Oncol. 2014 Nov 10;32(32):3613–8. doi: 10.1200/JCO.2014.56.2595. [DOI] [PubMed] [Google Scholar]
- 40.Crosby MA, Garvey PB, Selber JC, et al. Reconstructive outcomes in patients undergoing contralateral prophylactic mastectomy. Plast Reconstr Surg. 2011 Nov;128(5):1025–33. doi: 10.1097/PRS.0b013e31822b6682. [DOI] [PubMed] [Google Scholar]
- 41.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010 Nov;10(11):Cd002748. doi: 10.1002/14651858.CD002748.pub3. [DOI] [PubMed] [Google Scholar]
- 42.Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. 2013 Dec;20(13):4113–20. doi: 10.1245/s10434-013-3108-1. [DOI] [PubMed] [Google Scholar]
- 43.Silva AK, Lapin B, Yao KA, Song DH, Sisco M. The Effect of Contralateral Prophylactic Mastectomy on Perioperative Complications in Women Undergoing Immediate Breast Reconstruction: A NSQIP Analysis. Ann Surg Oncol. 2015 Oct;22(11):3474–80. doi: 10.1245/s10434-015-4628-7. [DOI] [PubMed] [Google Scholar]
- 44.Altschuler A, Nekhlyudov L, Rolnick SJ, et al. Positive, negative, and disparate--women's differing long-term psychosocial experiences of bilateral or contralateral prophylactic mastectomy. Breast J. 2008 Jan-Feb;14(1):25–32. doi: 10.1111/j.1524-4741.2007.00521.x. [DOI] [PubMed] [Google Scholar]
- 45.Frost MH, Hoskin TL, Hartmann LC, Degnim AC, Johnson JL, Boughey JC. Contralateral prophylactic mastectomy: long-term consistency of satisfaction and adverse effects and the significance of informed decision-making, quality of life, and personality traits. Ann Surg Oncol. 2011 Oct;18(11):3110–6. doi: 10.1245/s10434-011-1917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med. 2013 Sep 17;159(6):373–81. doi: 10.7326/0003-4819-159-6-201309170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]