Abstract
Background
Adverse aortic remodeling, such as dilation, is associated with multiple cardiovascular disease (CVD) risk factors. We sought to determine whether measures of enlarged aortic diameters improve prediction of incident adverse CVD events above standard CVD risk factors in a community-dwelling cohort.
Methods and Results
Participants from the Framingham Offspring and Third Generation Cohorts (N=3318, aged 48.9±10.3 years) who underwent non-contrast thoracic and abdominal multidetector computed tomography (MDCT) during 2002-2005, had complete risk factor profiles, and were free of clinical CVD, were included in this study. Diameters were measured at four anatomically-defined locations: the ascending (AA) and descending (DTA) thoracic and the infrarenal (IRA) and lower abdominal (LAA) aorta. Adverse events comprised CVD death, myocardial infarction, coronary insufficiency, index admission for heart failure, and stroke. Each aortic segment was dichotomized as enlarged (diameter ≥ upper 90th percentile for age, sex, and BSA) or not enlarged; the hazard of an adverse event for an enlarged segment was determined using multivariable-adjusted Cox proportional hazards models. Over a mean 8.8±2.0 years of follow-up, there were 177 incident adverse CVD events. In models adjusted for traditional CVD risk factors, enlarged IRA (HR=1.57, 95%CI=1.06-2.32) and LAA (HR=1.53, 95%CI=1.00-2.34) were associated with an increased hazard of CVD events. Enlarged AA and DTA were not significantly associated with CVD events.
Conclusions
Among community-dwelling adults initially free of clinical CVD, enlarged IRA and LAA, on non-contrast MDCT scans, are independent predictors of incident adverse CVD events above traditional risk factors alone.
Keywords: aortic diameters, computed tomography, cardiovascular disease, epidemiology
Aortic diameters at various anatomic locations are easily identified and measured on thoracic and abdominal multidetector computed tomography (MDCT) scans. Enlarged aortic diameters have been identified as a risk factor for aortic aneurysm and aortic dissection based on previously described referent values for age, sex, and body surface area (BSA).1,2 Prior studies have demonstrated that aneurysmal aortic dilation may also be associated with cardiovascular disease (CVD) risk factors and may portend an increased risk of adverse cardiovascular outcomes.3–5 Increased, but not necessarily aneurysmal, aortic dilation may share common risk factors with cardiovascular disease.6 Because of this commonality, we hypothesized that enlarged aortic diameters are independent predictors of incident adverse CVD events, and have clinical value for prognostication of adverse CVD events beyond vascular events such as aortic dissection.
While cardiovascular disease risk factor correlates of enlarged aortic diameters have been previously described in the Framingham Heart Study,6 it is unclear whether aortic diameters are associated with incident adverse CVD events in a community-dwelling cohort. We measured aortic diameters at multiple levels (ascending (AA) thoracic aorta, descending (DTA) thoracic aorta, infrarenal abdominal (IRA) aorta, and lower (LAA) abdominal aorta and sought to determine the association of these measures with incident adverse CVD events.
METHODS
The data, analytic methods, and study materials may be available to other researchers, for purposes of reproducing the results or replicating the procedure, contingent upon obtaining appropriate approval per the Framingham Heart Study’s Research Application Review Process & Procedures.
Study Population
Participants from the Framingham Offspring7 and Third Generation8 cohorts who underwent cardiac MDCT scanning from 2002 – 2005 and had complete risk factor profiles were included in this study. Men had to be ≥35 years of age, women were ≥40 years and non-pregnant to qualify for MDCT scanning. Non-pregnant status was verified by questionnaire and urine pregnancy test ≤24 hours prior to the MDCT study. All participants weighed < 160kg due to scanner limitations. Participants provided written informed consent, and the study was approved by the institutional review boards of the Boston University Medical Center and The Massachusetts General Hospital.6,9
Risk Factor and Endpoint Ascertainment
The Offspring and Third Generation cohorts have been previously described.7,8 For the present study, covariates were collected during the Offspring cycle 7 (1998 - 2001) or Third Generation cycle 1 (2002 - 2005) examinations that were most adjacent in time to the MDCT sub-study. Height and weight were measured with participants in light clothing. Body mass index (BMI) was calculated as weight divided by the height squared (kg/m2). Body surface area (BSA) was calculated using the Mosteller formula.10 A venous blood draw was obtained after a 12-hour overnight fast. An interim history, which included medications taken and smoking status, was obtained via interview and questionnaires. A physical examination was performed by a physician, which included two separate brachial blood pressure measurements using a mercury sphygmomanometer; the average of the two blood pressure measurements was used. Current cigarette smoking was defined by smoking ≥1 cigarette daily over the past year. Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the use of antihypertensive medication. Diabetes was defined as a fasting plasma glucose ≥126 mg/dL or treatment with insulin or anti-hyperglycemic medication. Hyperlipidemia was defined as a serum cholesterol ≥240 mg/dL or use of lipid lowering therapy.
The primary endpoint was a composite of incident events which included myocardial infarction (MI), coronary insufficiency (prolonged ischemic chest pain of > 15 minutes accompanied by documented transient ischemic S-T segment and T-wave abnormality without Q-wave abnormality or serum enzyme changes), stroke, congestive heart failure, and CVD death. If a participant experienced more than one primary event, the first event was considered in our analyses. Outcomes were adjudicated using standardized criteria by a panel of three physicians blinded to risk factor status and to CT results, after review of all available records.9 A separate review committee that included a neurologist adjudicated cerebrovascular events.
Image Acquisition and Analysis
Thoracic scanning was performed on an electrocardiographically-gated, non-contrast, 8-slice multidetector system (Lightspeed Ultra, General Electric, Milwaukee, WI) during a single, mid-inspiratory breath hold. Thoracic images were prospectively triggered in early diastole at 50% of the cardiac cycle. Contiguous, 2.5 mm thick slices (120 kVp, 320 mA if body weight < 220 lbs/400 mA if body weight > 220 lbs, gantry rotation time 500 ms, table feed 3:1) were acquired from the level of the carina to the level of the diaphragm. Each participant underwent two consecutive scans conducted during end-inspiratory breath holds for which the duration was generally 18 seconds. In the abdomen, there were twenty-five contiguous, 5-mm thick slices (120 kVp; 400 mA; gantry rotation time, 500 ms; table feed, 3:1) acquired, which covered 125 mm above the level of the first sacral vertebral body.6
Image analysis was performed on a dedicated workstation (Aquarius 3D, TeraRecon Inc., San Mateo, California). We conducted aortic diameter measures referenced to easily detected landmarks, as previously described by Rogers et al. from our group.6 The AA and DTA diameters were measured from an axial slice at the level of right pulmonary artery. The IRA was measured at one slice level 5-cm above the aorto-iliac bifurcation and the LAA was measured at the slice immediately above the aortoiliac bifurcation. At each location, aortic diameters were measured from outer wall to outer wall in both in the anteroposterior and left-right directions; the mean of the anterior-posterior and transverse measurements of each segment was used in our analyses to account for obliquity of the aorta, which is described in detail by Rogers et al. We previously reported a detailed description of absolute aortic diameter measures at the different levels and their associations with risk factors.6 These measurements were highly reproducible, as previously reported.
Statistical Analysis
Baseline characteristics of participants are presented as mean and standard deviation, median and interquartile range, or as a number and percentage as appropriate. Continuous variables between men and women were compared using the two-sample t-test. Categorical variables were compared between men and women using the Chi-squared test.
Aortic diameters vary with age, sex, and BSA. We previously defined normal reference values (for age-, sex-, and BSA-specific upper 90th percentile (P90) cutpoints to define high aortic diameters) in this population based on a healthy referent group free of prevalent CVD, hypertension, and/or previous valvular or aortic surgery.6 For the current analyses, participants with a history of adverse CVD events and valvular or aortic surgery prior to MDCT scanning were excluded.
Primary Analyses
Multivariable-adjusted Cox proportional hazards models were constructed to determine whether enlarged segmental aortic diameters were associated with an increased hazard of incident adverse CVD events. Model 1 adjusted for age and sex. Model 2 adjusted for CVD risk factors defined by the American Heart Association/American College of Cardiology,11 which included age, sex, systolic blood pressure, total and HDL cholesterol, diabetes and current smoking. In an additional exploratory model (Model 3), we adjusted for the above CVD risk factors plus the burden of coronary artery calcium (CAC). Each model also included an interaction-by-sex term as hypothesis-generating analyses. Our primary exposure consisted of dichotomized aortic diameters (≥90th percentile cutpoint). The secondary exposure was continuous aortic diameters (with hazard ratios expressed in increments of one standard deviation). We also constructed Kaplan-Meier survival curves for each segment, which compared event-free survival between individuals with and without enlarged (≥P90) aortic diameters.
We then sought to determine the incremental prognostic value of individual aortic diameter segments that were found to be independent predictors of CVD events in our Cox models. We employed the categorical net reclassification improvement (NRI)12 metric to assess the effect of individually adding enlarged AA, DTA, IRA, and LAA to Model 2, on model performance.
Secondary Analyses
While our primary analyses sought to determine whether individual, dilated segments of the aorta were predictors of incident adverse CVD events, in secondary analyses, we assessed whether the number of enlarged segments seen on MDCT scanning in an individual was also associated with an increased risk of CVD events. We constructed Cox proportional hazards models using the same covariates (Model 1, Model 2, and Model 3) from our primary analyses. Our exposure was categorized as having one enlarged segment, two enlarged segments, or three to four enlarged segments. The referent group consisted of no enlarged segments. We constructed Kaplan-Meier survival curves for each number of dilated segments. We compared event free survival for individuals without any aortic enlargement and those with a total of one, two, or three to four enlarged aortic segments.
RESULTS
Baseline characteristics of the 3318 participants are described in Table 1. Men comprised 1686 (51%) of the total participants. Women were slightly older than men. The prevalence of diabetes, hypertension, and use of lipid lowering therapy was higher in men than women. Coronary artery calcium prevalence was greater among men (54.4%) than women (33.1%). Aortic diameter measures at each segment were larger in men than in women. Over a mean of 8.8± 2.0 years, there were 177 incident adverse CVD events. Myocardial infarction (N=66) and stroke (N=60) accounted for 71% (126/177) of CVD events. Overall, women had fewer events than men (men=99, women=78 events, respectively).
Table 1.
Baseline characteristics.
| Men | Women | P-value | |
|---|---|---|---|
| N | 1686 | 1632 | |
| Offspring | 44.6% | 55.4% | |
| Third Generation | 54.2% | 45.8% | |
| Age, years | 48.9±10.3 | 51.7±9.7 | <0.001 |
| Height, m | 1.77±0.07 | 1.63±0.06 | <0.001 |
| Weight, kg | 89.0±15.3 | 71.8±16.1 | <0.001 |
| BSA, m2 | 2.08±0.19 | 1.79±0.21 | <0.001 |
| Body mass index, kg/m2 | 28.4±4.5 | 27.0±5.8 | <0.001 |
| Systolic blood pressure, mmHg | 123±14 | 120±17 | <0.001 |
| Diastolic blood pressure, mmHg | 79±9 | 74±9 | <0.001 |
| Total Cholesterol, mg/dL | 196±34 | 198±36 | 0.14 |
| HDL_C, mg/dL | 46±12 | 62±17 | <0.0001 |
| Total:HDL_C ratio | 4.5± 1.4 | 3.4 ± 1.1 | <0.001 |
| Trigylcerides, mg/dL | 113 [76, 171] | 93 [66, 135] | <0.001 |
| Fasting plasma glucose, mg/dL | 102 ± 23 | 95 ± 17 | <0.001 |
| Hypertension | 29.6% | 26.6% | 0.06 |
| Hypertension treatment | 16.8% | 18.7% | 0.17 |
| Cholesterol treatment | 15.0% | 10.5% | 0.001 |
| Diabetes | 5.3% | 3.8% | 0.03 |
| Diabetes treatment | 2.9% | 2.5% | 0.59 |
| Current Smoking | 12.1% | 11.4% | 0.56 |
| Prevalent CAC | 54.4% | 33.1% | <0.001 |
HDL=High Density Lipoprotein
Primary Results
Table 2 describes the association of enlarged (≥P90) aortic diameters with incident adverse CVD events. In Model 2, enlarged (dichotomized) IRA (Hazard Ratio [HR] =1.57, 95% Confidence Interval [CI] =1.06-2.32) and LAA (HR=1.53, 95%CI=1.00-2.34) were both associated with an increased hazard of CVD events above traditional CVD risk factors. In Model 3, IRA and LAA remained positively associated with CVD events even after addition of CAC to standard CVD risk factors (Table 3). Testing for interaction-by-sex for each aortic segment resulted in a significant association for LAA in Model 1 but not in multivariable-adjusted models (Models 2 & 3).
Table 2.
Cox proportional hazards regression models for association of high (>90th percentile cutpoint) aortic diameter with incident cardiovascular disease events.
| Unadjusted Model | Model 1* | Model 2† | Model 3‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P-value | Interaction by sex P-value |
HR (95% CI) |
P-value | Interaction by sex P-value |
HR (95% CI) |
P-value | Interaction by sex P-value |
HR (95% CI) |
P-value | Interaction by sex P-value |
|
| AA | 1.51 (1.03–2.23) |
0.04 | 0.08 | 1.36 (0.92–2.00) |
0.12 | 0.96 | 1.33 (0.90–1.98) |
0.15 | 0.72 | 1.34 (0.90–1.99) |
0.14 | 0.71 |
| DTA | 2.05 (1.44–2.93) |
<.001 | 0.18 | 1.45 (1.01 – 2.07) |
0.04 | 0.53 | 1.36 (0.94–1.97) |
0.10 | 0.88 | 1.39 (0.96–2.01) |
0.08 | 0.42 |
| IRA | 2.22 (1.53–3.21) |
<.001 | 0.13 | 1.74 (1.20–2.52) |
0.004 | 0.79 | 1.57 (1.06–2.32) |
0.02 | 0.76 | 1.59 (1.08–2.35) |
0.02 | 0.66 |
| LAA | 1.73 (1.15–2.60) |
0.009 | 0.57 | 1.43 (0.95–2.16) |
0.08 | 0.005 | 1.53 (1.00–2.34) |
0.05 | 0.29 | 1.56 (1.02–2.38) |
0.04 | 0.07 |
Model 1= Adjusted for age and sex
Model 2= Adjusted for Model 1 + systolic BP, total cholesterol, HDL cholesterol, hypertension treatment, current smoking, and diabetes
Model 3= Adjusted for Model 2 + Coronary artery calcium
AA=ascending thoracic aorta; DTA=descending thoracic aorta; IRA=infrarenal abdominal aorta; LAA=lower abdominal aorta
Table 3.
Cox proportional hazards regression models for association of continuous aortic dilation (per one standard deviation) with incident adverse cardiovascular disease events.
| Unadjusted Model | Model 1* | Model 2† | Model 3‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P-value | Interaction by sex P-value |
HR (95% CI) |
P-value | Interaction by sex P-value |
HR (95% CI) |
P-value | Interaction by sex P-value |
HR (95% CI) |
P-value | Interaction by sex P-value |
||
| AA, per SD | 1.74 (1.53–1.99) |
<.001 | 0.78 | 1.10 (0.93–1.30) |
0.25 | 0.54 | 1.08 (0.92–1.27) |
0.35 | 0.93 | 1.02 (0.87–1.21) |
0.77 | 0.58 | |
| DTA, per SD | 1.90 (1.74–2.07) |
<.001 | 0.02 | 1.35 (1.17–1.57) |
<0.001 | 0.26 | 1.23 (1.06–1.43) |
0.006 | 0.32 | 1.20 (1.03–1.41) |
0.02 | 0.67 | |
| IRA, per SD | 1.62 (1.48–1.77) |
<.001 | 0.82 | 1.17 (1.03–1.33) |
0.01 | 0.32 | 1.10 (0.98–1.23) |
0.11 | 0.14 | 1.07 (0.94–1.20) |
0.30 | 0.16 | |
| LAA, per SD | 1.49 (1.37–1.60) |
<.001 | 0.31 | 1.18 (1.06–1.32) |
0.003 | 0.09 | 1.14 (1.03–1.26) |
0.01 | 0.048 | 1.12 (1.01–1.25) |
0.03 | 0.049 | |
Model 1= Adjusted for age and sex
Model 2= Adjusted for Model 1 + systolic BP, total cholesterol, HDL cholesterol, hypertension treatment, current smoking, and diabetes
Model 3= Adjusted for Model 2 + Coronary artery calcium
AA=ascending thoracic aorta; DTA=descending thoracic aorta; IRA=infrarenal abdominal aorta; LAA=lower abdominal aorta; SD=standard deviation
We conducted parallel analyses to evaluate the association of our secondary exposure of continuous (per one standard deviation) aortic diameters with incident adverse CVD events (Table 3). In multivariable models 2 and 3, there was a significant association between continuous LAA and incident adverse CVD events (Table 3). There was also an interaction-by-sex for LAA in Models 2 and 3 (Table 3). Continuous IRA was not associated with adverse CVD events (Table 3). This finding differs from the overall strong relationship seen between dichotomized IRA and CVD events. While dichotomized DTA was not associated with CVD events, greater continuous DTA was in Model 2 and Model 3.
Upon addition of dichotomized IRA (NRI =1.93%; 95% CI=−0.6%-4.7%) and LAA (NRI=0.59%; 95% CI=−1.4%-3.2%) to the traditional CVD risk factors in Model 2, we found that the incremental prognostic value of these segments was not statistically significant. However, the addition of continuous LAA to Model 2 (NRI =18.62%; 95% CI=12.2%-27.2%) resulted in appropriate movement of participants to a higher risk category compared to the model that did not include continuous LAA. The addition of continuous DTA appeared to not have any incremental prognostic value (NRI=0.00%; 95%CI=−3.2%-3.2%) above traditional CVD risk factors. In Figure 1, we illustrate via Kaplan-Meier survival curves that event-free survival is decreased for individuals with enlarged aortic diameters (≥P90).
Figure 1.
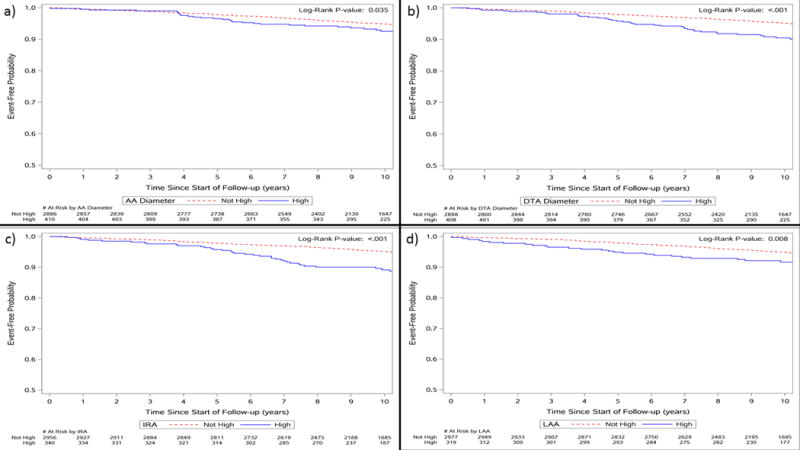
Kaplan Meier curves showing increased event-free survival for participants with and without enlarged (a) ascending (AA), (b) descending thoracic (DTA), (c) infrarenal (IRA), and (d) lower abdominal (LAA) aortic segments.
Secondary Results
Table 4 describes the results of Cox proportional hazards models constructed to identify whether participants with multiple enlarged aortic segments are at increased risk of CVD events. Among individuals with two segments that were enlarged (≥P90), the model adjusted for traditional CVD risk factors (Model 2) demonstrated that the risk of incident adverse CVD event was 2.3-fold higher when compared to individuals without any enlarged aortic diameter segments. Among participants with three or four enlarged segments, hazard ratios were indicative of a positive relationship with CVD events; however, the associations did not achieve statistical significance. In Figure 2, Kaplan-Meier survival curves illustrated that event-free survival was the lowest among individuals with 3-4 enlarged aortic diameters.
Table 4.
Cox proportional hazards models for association number of enlarged (>90th percentile cutpoint for age, sex, and body surface area) segments of the aorta with incident adverse cardiovascular disease events.
| Number of enlarged aortic diameter segments | |||||||
|---|---|---|---|---|---|---|---|
| 0 (N=2345) | 1 (N=596) | P-value | 2 (N=217) | P-value | 3–4 (N=138) | P-value | |
| No Events | 105 | 31 | 25 | 16 | |||
| Event Rate | 4.5% | 5.2% | 11.5% | 11.6% | |||
| Unadjusted Model | – | 1.16 (0.77–1.73) | 0.48 | 2.67 (1.73–4.13) | <0.001 | 2.73 (1.61–4.61) | <0.001 |
| Model 1* | – | 1.03 (0.69–1.54) | 0.88 | 2.28 (1.47–3.53) | <0.001 | 1.63 (0.96–2.78) | 0.07 |
| Model 2† | – | 0.93 (0.62–1.41) | 0.74 | 2.22 (1.42–3.47) | <0.001 | 1.62 (0.93–2.83) | 0.09 |
| Model 3‡ | – | 0.97 (0.64–1.46) | 0.88 | 2.32 (1.48–3.63) | <0.001 | 1.65 (0.94–2.88) | 0.08 |
Model 1= Adjusted for age and sex
Model 2= Adjusted for Model 1 + systolic BP, total cholesterol, HDL cholesterol, hypertension treatment, current smoking, and diabetes
Model 3= Adjusted for Model 2 + Coronary artery calcium
Figure 2.
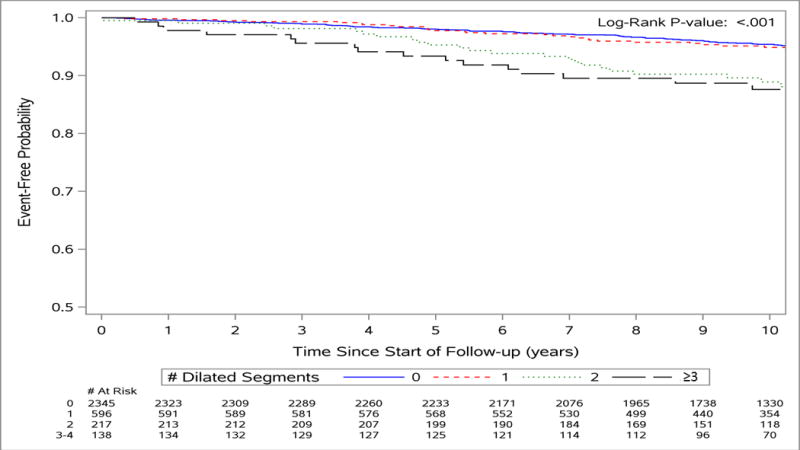
Kaplan Meier curves showing increased event-free survival for participants with and without multiple (i.e. 0, 1, 2, 3-4) enlarged aortic segments.
DISCUSSION
In this study of 3318 community-dwelling adults, high (≥ P90 aortic diameters stratified by age, sex, and BSA) abdominal aortic diameters, specifically IRA and LAA, were associated with an increased hazard of incident adverse CVD events in multivariable Cox proportional hazards models adjusted for traditional CVD risk factors. In multivariable models that further adjusted for CAC in addition to traditional CVD risk factors, CAC did not attenuate hazard ratios for the association of IRA and LAA with CVD events. In parallel analyses for continuous aortic diameters, the relationship for LAA with CVD events remained significant. In our primary analysis, dichotomized, enlarged thoracic aortic diameters (AA and DTA) were not associated with an increased hazard of CVD events in multivariable-adjusted models, although the hazard ratios indicated a positive relationship. Continuous DTA was associated with an increased risk of incident adverse CVD events. Based on NRI analyses, we found that continuous LAA significantly augmented prediction CVD events above traditional CVD risk factors. While a similar relationship was seen using the NRI metric for continuous DTA, dichotomized IRA, and dichotomized LAA, it did not achieve statistical significance for these segments. Additionally, the number of enlarged segments in an individual (i.e. ≥two segments) may confer an increased risk of adverse CVD events compared to individuals without any enlargement of the aorta.
In the context of the current literature
The distribution of aortic diameters and normal reference values measured on CT scanning have been described previously.13–16 Prior literature has also demonstrated that there is an association between CVD risk factors and aortic dilation.6,17 Abdominal aortic aneurysm as a risk factor for non-aneurysmal CVD has also been previously described.3–5 The data are sparse on the clinical and prognostic value of enlarged aortic diameter measurements on CT scanning in a community-dwelling population.
Thoracic aortic diameters, such as those from the AA and DTA, have been weakly associated with CVD events in previous literature. Gondrie et al. found that the ascending aorta conferred a borderline risk of CVD with every millimeter of diameter squared increase (HR 1.002; 95% CI 1.00–1.004) and the descending aorta was associated with a risk of CVD for every millimeter increase in diameter (HR 1.04; 95%CI 1.03–1.07). However, their study could not adjust for additional potential confounders as covariate data were limited to information available on radiology referral forms.18 Our study extends the findings of Gondrie et al. by adjusting for confounders including systolic blood pressure, total and HDL cholesterol, diabetes and current smoking. Within our study cohort, thoracic aortic dilation has been previously associated with CVD risk factors including age, male gender, and hypertension.6 After adjusting for traditional CVD risk factors, high thoracic aortic diameters were not associated with CVD events, but hazard ratios did demonstrate a positive relationship, and continuous DTA was associated with CVD events.
The abdominal aorta (IRA and LAA) was strongly associated with CVD events. This is consistent with findings from the Tromsø Study, which included 6640 participants, aged 25 to 84, who had maximal infrarenal aortic diameters measured on abdominal ultrasound. Over a 10-year follow-up, diameters ranging from 27-29mm increased the risk of CVD mortality (mortality rate ratio= 1.92; 95%CI 1.16-3.19) in age- and sex-adjusted models when compared to referent diameters from 21-23mm. Diameters ≥30 mm were associated with a significantly higher risk of CVD mortality (mortality rate ratio= 9.24; 95%CI 4.07–20.97). Interaction-by-sex testing yielded an association with total mortality that was stronger in men versus women, but this was not apparent for the outcome of CVD mortality.19 Our data demonstrates an association of abdominal aortic dilation at two different levels with CVD events that are inclusive of CVD death in addition to non-fatal MI, stroke, heart failure and coronary insufficiency. Our findings are generally concordant with the notion that adverse remodeling, resulting in aortic dilation, leads to adverse events, and there may be a sex related differences in risk of adverse events.
While the Tromsø study provides important insights into the prognostic value of aortic dilation, there was a paucity of mortality events, and this may have influenced its results.19 Norman et al included a larger cohort of men (N= 12203) in their study who were aged 65-83 and underwent abdominal aortic aneurysm screening via infrarenal abdominal ultrasound. Diameters of 23-26mm (HR=1.26; 95% CI 1.09-1.44) and 27-30mm (HR=1.35; 95% CI 1.09-1.67) were associated with higher all-cause mortality in multivariable models.20 While the sample size in the Tromsø study was robust, it excluded women. The present study investigated the prognostic value of aortic diameters at different levels of the abdominal aorta in a population consisting of community-dwelling men and women.
In the Cardiovascular Health Study, among 4734 participants aged ≥65 years, ultrasound-measured abdominal aortic diameters ranging from 20-30mm were associated with a 17% increased hazard of total mortality (HR 1.17; 95 %CI 1.02–1.35) when compared to a referent group of participants with aortic diameters < 20mm.21 The mean age of the population analyzed within the Cardiovascular Health Study was substantially greater (Men=75.2y, Women=74.6y) than in our Framingham MDCT cohort (Men=48.9y, Women=51.7y). The present study extends the age range examined in prior studies while still demonstrating an association between enlarged aortic diameters and CVD events.
Recently, Duncan et al. demonstrated that among men screened for abdominal aortic aneurysm (N=8146) who were aged 65-74 and were followed for a median of 7.4 years, after adjusting for potential confounders, a significant relationship between non-aneurysmal dilation and CVD death was not seen. Diameters of ≥25 mm conferred an increased risk of first hospital admission due to hypertensive disease (HR 1.29; 95%CI 1.08 to 1.54), ischemic heart disease (HR 1.33; 95%CI 1.08 to 1.64), and heart failure (HR 1.55; 95%CI 1.12 to 2.14) when compared to referent diameters of ≤24mm. Our findings in the Framingham cohort are compatible with those of Duncan et al because we previously found enlarged aortic diameters were associated with hypertension, and in the present study we identified an association with CVD events inclusive of heart failure, MI, and coronary insufficiency. The study by Duncan et al was limited by exclusion of women (on the assumption that aortic dilation is more common among men).22
Our study extends the literature by investigating the association of increased aortic diameters on non-contrast MDCT scanning with CVD events. While ultrasound is readily available, CT scanning is routinely obtained for countless indications in the clinical setting from which the aorta is easily visualized and aortic diameters can be easily measured at multiple segments. Unlike ultrasound, CT imaging is less affected by obesity and body habitus, overlaying rib and lung tissue in the thorax, and bowel gas in the abdomen.2 Our data do not allow us to directly extrapolate our findings to aortic diameters measured on ultrasound. Furthermore, while prior studies have demonstrated an association of aortic diameters with adverse events, literature on the incremental prognostic value of individual, enlarged aortic segments is limited. Similarly, our secondary analyses add another dimension to the current literature by demonstrating that multiple, enlarged segments identified on CT scanning of an individual may independently predict the risk of CVD events.
Potential Mechanisms
As demonstrated previously,6 aortic dilation is associated with traditional CVD risk factors. These include hypertension, age, male sex, and smoking. The propensity for aortic dilation to occur in the abdominal aorta, specifically at the level of the IRA, upon exposure to CVD risk factors has been previously described.23 This relationship has been attributed to smaller diameters in addition to differing connective tissue content (higher elastin and lower collagen) in the abdominal aorta compared to the thoracic aorta.24 Because of these unique histological characteristics in concert with a greater tendency for atherosclerosis25 and a strong association with risk factors that differ from those associated with the thoracic aorta, i.e. smoking,6 the IRA may be more sensitive to multiple stressors resulting in connective tissue breakdown and remodeling that leads to aortic dilation.23 While the IRA is more often described in the literature, a similar mechanism may be attributed to the LAA. The association of enlarged aortic diameters, IRA and LAA specifically, may be a marker of pre-existing sub-clinical CVD that may provide additional information for predicting the risk of clinically evident CVD.
Clinical Implications
Aortic diameters can be measured accurately and reproducibly on MDCT ordered for any clinical indication, and may provide additional information to appropriately identify individuals at increased risk for CVD. Rogers et al reported cutpoints and a nomogram that may be used clinically among community-dwelling adults.6 That said, we do not propose CT scanning be performed solely to measure aortic diameters for CVD risk stratification, but we note that aortic diameters obtained incidentally on scans performed for other indications may have additional value for assessment of CVD risk.
Strengths and Limitations
The Framingham Heart Study cohorts comprise community-dwelling adults followed closely with meticulous collection of clinical characteristics through history and risk factor ascertainment by trained investigators rather than through self-report. Each adverse CVD event was adjudicated after extensive review of all available records by a panel of physician-investigators using standardized criteria, as opposed to based solely on participant self-report or billing codes. Previous analyses of this study population have demonstrated that aortic diameter measurements from non-contrast CT images are highly reproducible at the levels of the AA, DTA, IRA, and LAA.6 However, our study is not without limitations. Data on the occurrence of aorta and valve surgery are not reported in our study as these participants were excluded. Obliquity and tortuosity of the aorta may influence diameter measurements on axial CT imaging; however, in our image analyses, we attempted to mitigate these effects as described by Rogers et al. Finally, the Framingham Offspring and Third Generation cohorts are predominantly middle-aged and overwhelmingly of European ethnicity; this may limit generalization to other age-groups or races.
CONCLUSION
Aortic diameters increase with greater age and burden of common CVD risk factors. We found that enlarged abdominal aortic diameters are associated with excess CVD events over and above both traditional CVD risk factors, and may have value in augmenting CVD risk stratification of individuals who undergo routine CT evaluation for various other clinical indications without requiring additional testing.
Clinical Perspective.
Adverse aortic remodeling (dilation) is been associated with traditional cardiovascular disease (CVD) risk factors. The present study demonstrates that enlarged segments (i.e. ≥90th percentile diameter for age, sex, and body surface area) of the infrarenal and the lower abdominal aorta are independent predictors of incident adverse CVD events in community-dwelling adults. Presently, the infrarenal aortic diameter is screened for abdominal aortic aneurysm in men in routine clinical practice. However, we demonstrate that enlargement of other regions in the aorta, namely the descending thoracic, infrarenal, and lower abdominal segments, may prognosticate incident adverse CVD events. These segmental aortic diameters can be easily and accurately ascertained from tomographic imaging of the chest and abdomen obtained for any clinical indication. Because aortic diameters are commonly measured on axial imaging and do not require additional analysis, these measurements may provide valuable clinical information that can be used to identify and appropriately categorize individuals at risk of developing CVD events. However, we do not propose obtaining CT imaging of the chest or the abdomen specifically for the measurement of aortic diameters; however, when imaging is available in the appropriate clinical setting, aortic diameter measurements may provide an additional important information on a patient.
Acknowledgments
SOURCES OF FUNDING
This project was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (contract numbers N01-HC-25195 and N01-HC-38038).
Footnotes
DISCLOSURES
None.
References
- 1.Hirsch AT, Haskal Z, Hertzer N, Bakal C, Creanger M, Halperin J, Hiratzka L, Murphy W, Olin J, Puschett J, Rosenfield K, Sacks D. ACC/AHA 2005 practice Guidelines for the Management of Patients With Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic) Circulation. 2005;113:1474–1547. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 2.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM. Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;121:e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 3.Sohrabi S, Wheatcroft S, Barth JH, Bailey MA, Johnson A, Bridge K, Griffin K, Baxter PD, Scott DJA. Cardiovascular risk in patients with small and medium abdominal aortic aneurysms, and no history of cardiovascular disease. Br J Surg. 2014;101:1238–43. doi: 10.1002/bjs.9567. [DOI] [PubMed] [Google Scholar]
- 4.Brady AR, Fowkes FGR, Thompson SG, Powell JT. Aortic Aneurysm Diameter and Risk of Cardiovascular Mortality. Arterioscler Thromb Vasc Biol. 2001;21:1203–1207. doi: 10.1161/hq0701.091999. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Arnold AM, Burke GL, O’Leary DH, Manolio TA. Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: The cardiovascular health study. Ann Intern Med. 2001;134:182–190+I28. doi: 10.7326/0003-4819-134-3-200102060-00008. [DOI] [PubMed] [Google Scholar]
- 6.Rogers IS, Massaro JM, Truong QA, Mahabadi AA, Kriegel MF, Fox CS, Thanassoulis G, Isselbacher EM, Hoffmann U, O’Donnell CJ. Distribution, determinants, and normal reference values of thoracic and abdominal aortic diameters by computed tomography (from the framingham heart study) Am J Cardiol. 2013;111:1510–1516. doi: 10.1016/j.amjcard.2013.01.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 8.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB, Fox CS, Larson MG, Murabito JM, O’Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: Design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102:1136–41. 1141.e1. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosteller RD. Simplified Calculation of Body Suface Area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 11.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 12.Pencina MJ, D’Agostino RB, Pencina KM, Janssens ACJW, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–81. doi: 10.1093/aje/kws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Jacobs A, Weinsaft JW, Shaw LJ, Berman DS, Gilmore A, Callister TQ, Min JK. Assessment of the thoracic aorta by multidetector computed tomography: Age- and sex-specific reference values in adults without evident cardiovascular disease. J Cardiovasc Comput Tomogr. 2008;2:298–308. doi: 10.1016/j.jcct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Kälsch H, Lehmann N, Möhlenkamp S, Becker A, Moebus S, Schmermund A, Stang A, Mahabadi AA, Mann K, Jöckel KH, Erbel R, Eggebrecht H. Body-surface adjusted aortic reference diameters for improved identification of patients with thoracic aortic aneurysms: Results from the population-based Heinz Nixdorf Recall study. Int J Cardiol. 2013;163:72–78. doi: 10.1016/j.ijcard.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 15.Wolak A, Gransar H, Thomson LEJ, Friedman JD, Hachamovitch R, Gutstein A, Shaw LJ, Polk D, Wong ND, Saouaf R, Hayes SW, Rozanski A, Slomka PJ, Germano G, Berman DS. Aortic Size Assessment by Noncontrast Cardiac Computed Tomography: Normal Limits by Age, Gender, and Body Surface Area. JACC Cardiovasc Imaging. 2008;1:200–209. doi: 10.1016/j.jcmg.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Mao SS, Ahmadi N, Shah B, Beckmann D, Ngo L, Flores FR, Gao Y, Budoff MJ. Normal Thoracic Aorta Diameter on Cardiac Computed Tomography in Healthy Asymptomatic Adult. Impact of Age and Gender. 2009;15:827–834. doi: 10.1016/j.acra.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laughlin GA, Allison MA, Jensky NE, Aboyans V, Wong ND, Detrano R, Criqui MH. Abdominal aortic diameter and vascular atherosclerosis: The multi-ethnic study of atherosclerosis. Eur J Vasc Endovasc Surg. 2011;41:481–487. doi: 10.1016/j.ejvs.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gondrie MJ, a van der Graaf Y, Jacobs PC, Buckens SCFM, Mali WPTM. The prognostic value of vascular diameter measurements on routine chest computed tomography in patients not referred for cardiovascular indications. J Comput Assist Tomogr. 2011;35:734–41. doi: 10.1097/RCT.0b013e318231824a. [DOI] [PubMed] [Google Scholar]
- 19.Forsdahl SH, Solberg S, Singh K, Jacobsen BK. Abdominal aortic aneurysms, or a relatively large diameter of non-aneurysmal aortas, increase total and cardiovascular mortality: The Tromsø study signe. Int J Epidemiol. 2010;39:225–232. doi: 10.1093/ije/dyp320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norman P, Le M, Pearce C, Jamrozik K. Infrarenal aortic diameter predicts all-cause mortality. Arterioscler Thromb Vasc Biol. 2004;24:1278–1282. doi: 10.1161/01.ATV.0000131261.12051.7f. [DOI] [PubMed] [Google Scholar]
- 21.Freiberg MS, Arnold AM, Newman AB, Edwards MS, Kraemer KL, Kuller LH. Abdominal aortic aneurysms, increasing infrarenal aortic diameter, and risk of total mortality and incident cardiovascular disease events: 10-year follow-up data from the Cardiovascular Health Study. Circulation. 2008;117:1010–7. doi: 10.1161/CIRCULATIONAHA.107.720219. [DOI] [PubMed] [Google Scholar]
- 22.Duncan JL, Harrild KA, Iversen L, Lee AJ, Godden DJ. Long term outcomes in men screened for abdominal aortic aneurysm : prospective cohort study. Bmj. 2012;2958:1–9. doi: 10.1136/bmj.e2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–828. doi: 10.1161/01.CIR.0000154569.08857.7A. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, Bolen MA, Connolly HM, Cuellar-Calabria H, Czerny M, Devereux RB, Erbel RA, Fattori R, Isselbacher EM, Lindsay JM, McCulloch M, Michelena HI, Nienaber CA, Oh JK, Pepi M, Allen JTSM. Multimodality Imaging of Diseases of the Thoracic Aorta in Adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:119–182. doi: 10.1016/j.echo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Jaffer FA, O’Donnell CJ, Larson MG, Chan SK, Kissinger KV, Kupka MJ, Salton C, Botnar RM, Levy D, Manning WJ. Age and sex distribution of subclinical aortic atherosclerosis: A magnetic resonance imaging examination of the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2002;22:849–854. doi: 10.1161/01.atv.0000012662.29622.00. [DOI] [PubMed] [Google Scholar]


