Abstract
Background
Gene environmental interaction analysis can identify novel genetic factors for blood pressure. We performed genome-wide analyses to identify genomic loci that interact with potassium to influence blood pressure (BP) using single marker (one and two degrees of freedom [DF] joint tests) and gene-based tests among Chinese participants of the Genetic Epidemiology Network of Salt-Sensitivity (GenSalt) study.
Methods and Results
Among 1,876 GenSalt participants, the average of three urine samples was used to estimate potassium excretion. Nine BP measurements were taken using a random-zero-sphygmomanometer. A total of 2.2 million SNPs were imputed using Affymetrix 6.0 genotype data and the Chinese Han of Beijing and Japanese of Tokyo HapMap reference panel. Promising findings (P <1.00×10−4) from GenSalt were evaluated for replication among 775 Chinese participants of the Multi-ethnic Study of Atherosclerosis (MESA). SNP and gene-based results were meta-analyzed across the GenSalt and MESA studies to determine genome-wide significance. The one DF tests identified interactions for ARL15 rs16882447 on systolic BP (P=2.83×10−9) and RANBP3L rs958929 on pulse pressure (P=1.58×10−8). The two DF tests confirmed the ARL15 rs16882447 signal for systolic BP (P=1.15×10−9). Genome-wide gene-based analysis identified CC2D2A (P=2.59×10−7) at 4p15.32 and the BNC2 (P=4.49×10−10) at 9p22.2 for systolic BP, GGNBP1 (P=1.18×10−8) and LINC00336 (P=1.36×10−8) at 6p21 for diastolic BP, DAB1 (P=1.05×10−13) at 1p32.2 and MIR4466 (P=5.34×10−8) at 6q25.3 for pulse pressure. BNC2 (P=3.57×10−8) gene was also significant for mean arterial pressure.
Conclusions
We identified 2 novel BP loci and 6 genes through the examination of SNP- and gene-based interactions with potassium.
Keywords: potassium, genome-wide analysis, blood pressure, interaction, gene-based analysis
Journals Subject Terms: Genetic, Association Studies, High Blood Pressure, Diet and Nutrition
Introduction
Blood pressure (BP) is determined by genetic factors, environment factors, and their interactions. Genome-wide association studies (GWAS) have identified many genetic loci that are robustly associated with BP.1–5 However, these findings together only explain a small proportion of the inter-individual variation of BP.6 A large number of genetic loci are yet to be identified 6. It has been proposed that exploring the interaction between genes and environmental risk factors for BP may help to identify novel genetic loci underlying BP regulation.7–9 In addition, gene-based analysis methods testing the joint contributions of single SNPs with modest effect may have higher power to detect BP loci.10, 11
Dietary potassium intake has been demonstrated to decrease BP in clinical trials.12 Since genetic factors may modify the effects of potassium on BP, examination of gene-dietary potassium intake interactions may help to identify novel genetic variants and genes underlying BP regulation.13 However, few studies have explored gene-potassium interactions using single variant and gene-based analyses.14, 15 The objective of this analysis was to identify novel genetic variants and genes influencing BP regulation by conducting genome-wide SNP-based and gene-based gene-potassium interaction analyses of systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP), and pulse pressure (PP) phenotypes among 1,876 Chinese participants of the Genetic Epidemiologic Network of Salt Sensitivity (GenSalt) study.
Methods
The data, analytic methods, and study materials will be made available to other researchers for reproducing the results or replicating the procedure upon request, discussion, and agreement with the investigators.
Study population
GenSalt is a family-based dietary intervention study designed to identify genes that influence BP responses to environmental risk factors for hypertension in human populations. The study includes 1,906 Han Chinese participants aged 16 years and older from 633 families recruited from 6 study sites in rural, north China.16 In the current study, genotypes, BP, and covariates at baseline are available for a total of 1,876 participants (98.4%).
Institutional review boards at the Tulane University Health Sciences Center, Washington University School of Medicine, University of Texas School of Public Health, Fu Wai Hospital and Chinese National Human Genome Center at Beijing, and Chinese Academy of Medical Sciences approved the GenSalt study. Written informed consents for the baseline observation were obtained from each participant.
Genotyping and Genotype Imputation
A total of 868,158 autosomal SNPs across the entire genome were genotyped using the Affymetrix 6.0 platform for each participant. After excluding monomorphic SNPs and SNPs with Hardy-Weinberg Equilibrium P<1×10−6, missing rate>25% or MAF<1%, a total of 820,017 genotyped SNPs remained for genotype imputation. Imputation of 2,416,663 SNPs from the HapMap release 22 build 36 Chinese Han of Beijing and Japanese of Tokyo (CHB+JPT) reference panel was conducted using MACH software (version 1.0).17 After imputation, SNPs with r2<0.30, MAF<1%, or HWE P<1×10−6 were removed, and a total of 2,216,774 SNPs, with fractional values ranging from 0 to 2, remained for the analysis.
Urinary Potassium and Covariates Measurement
Dietary potassium intake was measured from 24-hour urinary potassium level. During the three-day baseline examination, each GenSalt participant collected three urine samples, one 24-hour urine sample and 2 overnight urine samples. Among a random sample of 238 participants, 24-hour urine samples were collected in two containers, one for day time and the other for overnight. Using this random sample, an equation was generated to estimate 24-hour urinary potassium level from the overnight urine sample, and this equation was applied to the remaining overnight urine samples to estimate 24-hour urinary potassium levels. The mean of the one measured 24-hour urinary potassium level and two estimated 24-hour urinary potassium levels was used in the current analysis.
Demographic variables, including age and gender, and medication use were collected using standard questionnaires by trained study staff. Body weight and height were measured twice during the baseline examination with the participants in light indoor clothing without shoes. Body mass index (BMI) was calculated as kilograms per meters squared.
BP Measurement and Imputation
SBP, DBP, MAP and PP phenotypes were assessed as outcomes in the current analyses. BP was measured three times at the same time each morning during the three-day baseline examination by trained and certified observers using a random zero sphygmomanometer according to standard protocol. For participants taking anti-hypertensive medications, BP was imputed by adding 10 mm Hg to systolic BP (SBP) and 5 mm Hg to diastolic BP (DBP).18 After imputation, the mean of the 9 SBP and DBP measures were used in the current analysis. The mean SBP and DBP values were also used to calculate PP (SBP-DBP) and MAP (SBP/3 + 2*DBP/3) phenotypes.
Replication Study and Genotype Imputation
We attempted to replicate promising GenSalt findings (P<1×10−4) among Chinese participants of the Multi-Ethnic Study of Atherosclerosis (MESA). MESA participants were recruited between July 2000 and August 2002 from 6 field centers around the US. Participants were aged 45–84 and free of cardiovascular diseases at the baseline examination. Phenotype and Affymetrix 6.0 genotype data of MESA participants were made publicly available through the NCBI’s database of Genotypes and Phenotypes (dbGaP) and were downloaded for the current analysis. Among 777 Chinese MESA participants, genotype, urinary potassium, BP, and all covariables at baseline were available for 775 (99.7%) participants. Similar to GenSalt, we imputed BP for participants taking antihypertensive medication by adding 10 and 5 mm Hg to SBP and DBP, respectively. Since potassium was measured from spot urine using baseline urine samples, 24-hour urine potassium was estimated from spot urine potassium levels using Tanaka’s equation.19
A total of 909,622 autosomal SNPs across the entire genome were genotyped using the Affymetrix 6.0 platform for each participant. We excluded SNPs with Hardy-Weinberg Equilibrium P<1×10−6, missing rate>5% and MAF<1%. Minimac software20 was used to perform targeted imputation of the 3 Mb region surrounding each identified SNP based on the ALL ancestry panel from the 1000G Phase I Integrated Release Version 3 Haplotypes, which contains haplotypes of 1,092 individuals of all ethnic background. 21 After imputation, SNPs with r2<0.30, MAF<1%, or HWE P<1×10−6 were removed.
Statistical Analysis
To accommodate familial relationships in both GenSalt (discovery) and MESA (replication) studies, linear mixed effect models were used to examine single SNP- potassium interactions on BP, after adjustment for age, gender, and BMI. Principal components analysis revealed population substructure in MESA (but not GenSalt). Therefore, ancestry was also accounted for in MESA by adjusting for the first three principal components. Both 1 degrees of freedom (df) interaction and 2 df joint tests were explored. The 2 df joint test has higher power to identify variants with both moderate main effect and moderate interaction effect. 22 As shown in supplementary figures S1 – S4, genomic inflation was minimal with lamda values ranging from 1.029 for DBP in the 1 df interaction analyses to 1.075 for pulse pressure in the 2 df joint tests. Still, genomic control was applied to single SNP-based analyses results before gene-based analyses and meta-analyses. After genomic control, lead SNPs with interaction term P<1.0 ×10−4 or joint test P<1.0 ×10−4 from independent loci in the discovery stage analyses were further evaluated for replication in Chinese MESA participants. For the 1 df interaction test, inverse-variance-weighted meta-analysis was conducted to combine results from GenSalt and MESA using METAL software.23 For the 2 df joint test, meta-analysis was performed using methods described by Manning and colleagues, which were implemented in METAL software 23 with their patch source code.24 After ensuring the effect direction of interaction terms were consistent, SNPs with replication stage P<0.05 and meta-analysis P<5.0×10−8 were considered significant for the 1 df interaction test. SNPs with consistent effect directions in both the main effect and interaction term, replication stage P<0.05, and joint meta-analysis P<5.0×10−8 were considered significant for the 2 df joint test. We also conducted a sensitivity analysis restricting the replication study to Chinese MESA participants not taking any antihypertensive medication.
For the gene-based analyses, SNPs within the 5 kilo bp flanking regions of a gene were first mapped to the gene according to physical positions. SNPs within 5 kilo bp flanking regions of two genes were assigned to both genes. P values of both 1 df interaction and 2 df joint tests in single marker analyses were used to generate gene-based P values using the extended Simes procedure (GATES) method for gene-based association testing.10 Genes with P<1.0×10−4 in the discovery stage gene-based interaction analysis were further evaluated for replication among Chinese MESA participants. Specifically, SNPs from promising genes were tested for SNP-potassium interactions using the methods described in the above single marker analysis, and P values of these SNPs were used to generate gene-based p values using GATES method.10 Fisher’s method was applied to combine gene-based p values across GenSalt and MESA.25 Genes with replication stage P<0.05 and combined P<5.0×10−6 were considered significant. Gene-based analysis combines contributions of all variants within a gene, therefore, has higher power to detect genes interacting with dietary potassium intake on BP regulation compared to single marker analyses.
Results
As shown in Table 1, participants of both GenSalt and the MESA replication sample had, on average, optimal BP and BMI levels. However, the Chinese MESA participants were older, had a higher proportion with hypertension, and had relatively higher potassium intake. Discovery stage genome-wide analysis results using both 1 df interaction and 2 df joint tests are shown in Figures S5–S8. A total of 5,331 variants from 1092 loci (r2<0.3) achieved P<1×10−4 and were tested for replication among MESA participants.
Table 1.
Characteristics of GenSalt and Chinese MESA participants
| GenSalt (n=1876) | MESA (n=775) | |
|---|---|---|
| Age, y, mean (SD) | 38.7 (9.5) | 62.4 (10.4) |
| Women, n (%) | 883 (47.2) | 394 (50.8) |
| Hypertension, n (%) | 178 (9.5) | 316 (40.8) |
| Antihypertensive medication use, n (%) | 7 (0.4) | 225 (29.0) |
| BMI, kg/m2, mean (SD) | 23.3 (3.2) | 24 (3.3) |
| 24-h urinary K, mmol, mean (SD) | 36.9 (9.6) | 45.4 (7.8) |
| Baseline SBP, mmHg, mean (SD) | 116.9 (14.2) | 127.4 (23.8) |
| Baseline DBP, mmHg, mean (SD) | 71.0 (9.7) | 74.8 (12.0) |
SD=standard deviation; SBP=systolic blood pressure; DBP=diastolic blood pressure; BMI=body mass index;
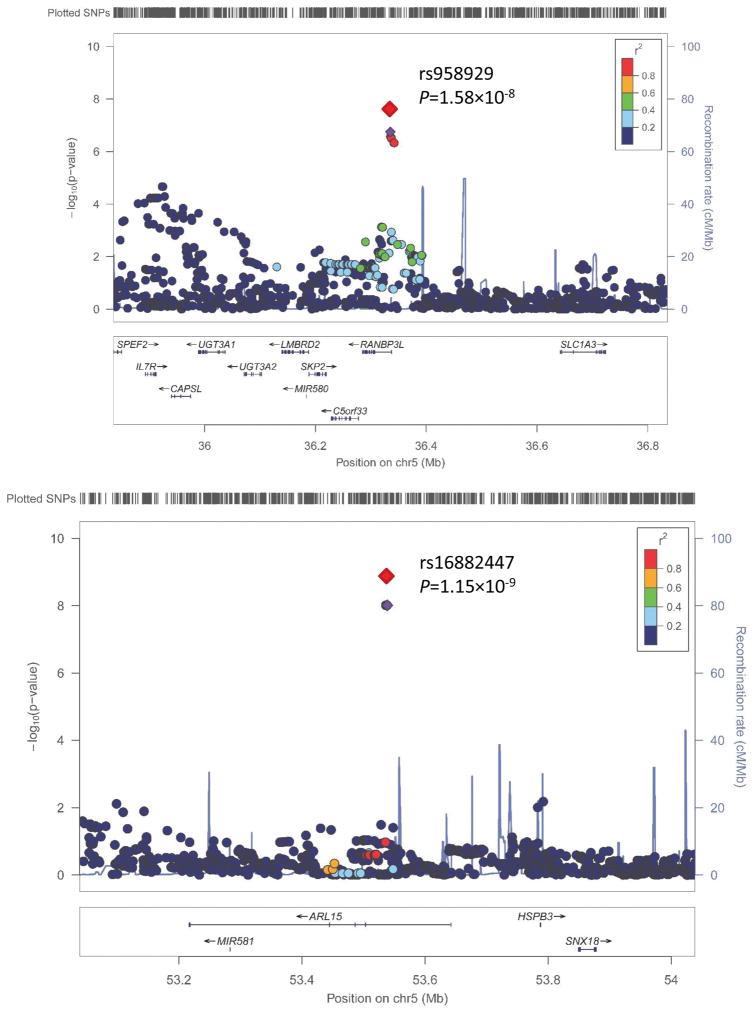
Two novel loci which achieved genome-wide significance in the combined analyses using the 1 df interaction test are presented in Table 2 and supplementary Figures S9–12. Novel ARL15 gene variant rs16882447 interacted with potassium on SBP (GenSalt P=1.99×10−7, MESA P=2.28×10−3, Meta-analysis P=2.83×10−9). In addition, significant gene-potassium interaction on pulse pressure was identified for RANBP3L gene variant rs958929 (GenSalt P=1.79×10−7, MESA P=1.66×10−2, Meta-analysis P=1.58×10−8). Variant rs16882447 was also identified in the 2 df joint test (GenSalt P=9.79×10−9, MESA P=8.19×10−3, Meta-analysis P=1.15×10−9) as shown in Table 3. Regional association plots for the 2 novel loci achieving genome-wide significance in meta-analyses are presented in Figure 1. Results of sensitivity analyses conducted among Chinese MESA participants not taking antihypertension medication were similar to those conducted among the overall replication sample (supplementary Table S1).
Table 2.
Results Achieving Genome-wide Significance in Gene-Potassium Interactions Analysis Using 1 Degrees of Freedom Test
| rsID | Chr | Position (build 36) | EA | EAF | Region | Genes | Studies | Imputation quality (r2) | Beta | SE | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Systolic Blood Pressure | |||||||||||
| rs16882447 | 5 | 53538659 | A | 0.010 | Intronic | ARL15 | GenSalt | 0.98 | −0.82 | 0.16 | 1.99E-07 |
| MESA | 0.51 | −1.59 | 0.49 | 2.28E-03 | |||||||
| Meta-analysis | −0.89 | 0.15 | 2.83E-09 | ||||||||
| Pulse Pressure | |||||||||||
| rs958929 | 5 | 36335304 | C | 0.340 | Intronic | RANBP3L | GenSalt | 0.83 | −0.18 | 0.03 | 1.79E-07 |
| MESA | 0.38 | −0.37 | 0.15 | 1.66E-02 | |||||||
| Meta-analysis | −0.19 | 0.03 | 1.58E-08 | ||||||||
Chr=chromosome; EA=effect allele; EAF=effect allele frequency; SE=standard error
Table 3.
Results Achieving Genome-wide Significance in Gene-Potassium Interaction Analysis Using 2 Degrees of Freedom Joint Test
| rsID | Chr | Position (Build 36) | EA | EAF | Function | Gene | Studies | SNP term | Interaction term | Covariance | Joint P | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Beta | SE | Beta | SE | ||||||||||
| Systolic Blood Pressure | |||||||||||||
| rs16882447 | 5 | 53538659 | A | 0.010 | Intron | ARL15 | GenSalt | 25.02 | 5.93 | −0.82 | 0.15 | −0.88 | 9.70E-09 |
| MESA | 72.36 | 22.17 | −1.59 | 0.49 | −10.59 | 8.19E-03 | |||||||
| Meta-analysis | 26.31 | 5.65 | −0.83 | 0.15 | −0.79 | 1.15E-09 | |||||||
Chr=chromosome; EA=effect allele; EAF=effect allele frequency; SE=standard error
Figure 1.
Regional Association Plots for Loci Achieving Genome-Wide Significance in the Meta-Analysis of both 1 degrees of freedom (df) Interaction Test and 2 df Joint Test Results. For the SNPs associated with multiple phenotypes, the results for the most significant phenotype are shown. The index SNPs are shown in purple diamond; the correlation (r2) of each of the surrounding SNPs to the index SNP are shown on a scale from minimal (blue) to maximal (red), The final P values for the index SNPs are shown and also indicated by the red diamonds. The genes in the 1 Mb regions around the index SNPs (500 kb on each side) are indicated at the bottom, and recombination rates are shown in light blue line. The regional plots are drawn using LocusZoom online software
Discovery stage gene-base analysis results are shown in Figures S13–S16. Genome-wide significant findings from meta-analysis of both the 1 df and 2 df tests are shown in Table 4. In the gene-based analysis of the 1 df test, significant interactions between potassium and the BNC2 and CC2D2A genes on SBP, GGNBP1 and LINC00336 on DBP, BNC2 gene on mean arterial pressure, and DAB1 and MIR4466 on pulse pressure were identified. Gene-based analysis of the 2 df joint test confirmed the gene-BP associations for BNC2, CC2D2A, GGNBP1, and DAB1 genes, and additionally identified novel gene-potassium interactions for RANBP3L on PP.
Table 4.
Genes Achieving Genome-wide Significance in Gene-based Analysis of Gene-Potassium Interactions
| Gene | Chr | Start (Build 36) | Gene Length (bp) | GenSalt P | MESA P | Meta P |
|---|---|---|---|---|---|---|
| 1 DF Test | ||||||
|
| ||||||
| Systolic Blood Pressure | ||||||
|
| ||||||
| BNC2 | 9 | 16399500 | 461285 | 4.16E-06 | 1.51E-03 | 6.28E-09 |
| CC2D2A | 4 | 15080586 | 132105 | 1.16E-05 | 4.64E-02 | 5.38E-07 |
|
| ||||||
| Diastolic Blood Pressure | ||||||
|
| ||||||
| GGNBP1 | 6 | 33659453 | 5327 | 4.01E-07 | 2.94E-02 | 1.18E-08 |
| LINC00336 | 6 | 33661860 | 7232 | 4.35E-07 | 3.14E-02 | 1.36E-08 |
|
| ||||||
| Mean Arterial Pressure | ||||||
|
| ||||||
| BNC2 | 9 | 16399500 | 461285 | 1.75E-05 | 2.04E-03 | 3.57E-08 |
|
| ||||||
| Pulse Pressure | ||||||
|
| ||||||
| DAB1 | 1 | 57236166 | 1255758 | 1.82E-05 | 7.14E-03 | 1.30E-07 |
| MIR4466 | 6 | 1.57E+08 | 53 | 1.30E-06 | 4.11E-02 | 5.34E-08 |
| 2 DF Test | ||||||
|
| ||||||
| Systolic Blood Pressure | ||||||
|
| ||||||
| BNC2 | 9 | 16399500 | 461285 | 3.97E-07 | 1.13E-03 | 4.49E-10 |
| CC2D2A | 4 | 15080586 | 132105 | 6.97E-05 | 3.71E-03 | 2.59E-07 |
|
| ||||||
| Diastolic Blood Pressure | ||||||
|
| ||||||
| GGNBP1 | 6 | 33659453 | 5327 | 6.86E-07 | 4.06E-02 | 2.79E-08 |
|
| ||||||
| Mean Arterial Pressure | ||||||
|
| ||||||
| BNC2 | 9 | 16399500 | 461285 | 5.35E-06 | 9.58E-03 | 5.13E-08 |
|
| ||||||
| Pulse Pressure | ||||||
|
| ||||||
| DAB1 | 1 | 57236166 | 1255758 | 3.29E-12 | 3.20E-02 | 1.05E-13 |
| RANBP3L | 5 | 36284860 | 54061 | 2.27E-05 | 3.36E-02 | 7.63E-07 |
DF=degrees of freedom;
Discussion
In the first ever genome-wide gene-potassium interaction study in a Chinese population, we identified 1 novel locus, ARL15, that interacted with potassium to influence the BP phenotypes using both the 1 df interaction test and 2 df joint test. The 1 df interaction test also provide direct evidence of a previously reported BP locus at RANBP3L. Furthermore, gene-based analyses using both the 1 df and 2 df joint tests revealed 6 additional genes at 5 novel loci that were associated with BP, including: CC2D2A at 4p15.32 and BNC2 at 9p22.2 for SBP, GGNBP1 and LINC00336 at 6p21 for DBP, DAB1 at 1p32.2 and MIR4466 at 6q25.3 for pulse pressure. BNC2 gene was also significant for mean arterial pressure. These findings contribute to our understanding of the biological mechanisms underlying BP regulation.
Novel ARL15 marker rs16882447 was identified by both the 1 df interaction test and the 2 df joint test. The 2 df joint test signal was mainly driven by the interaction effect. The ARL15 gene encodes ADP ribosylation factor like GTPase 15.26 This gene has been previously identified by GWAS of related cardiometabolic traits including diabetes.27 rheumatoid arthritis.28 high density lipoprotein cholesterol.29 and adiponectin.30 More importantly, a recent GWAS by Gorski and colleagues identified that the ARL15 gene was associated with kidney function. 31 Considering the importance of kidney function in BP regulation and potassium filtration, further investigation into this gene is warranted. While the functional relevance of ARL15 to BP is unclear, this signal could reflect other genes at this locus. For example, the nearby HSPB3 gene, encoding the heat shock protein family B (small) member 3 32 represents a biologically plausible candidate for hypertension. The Hspb3 gene is highly expressed in heart and skeletal muscles 33. Animal studies showed that cardiac overloading related to hypertension increased expression of the hspb3 gene in rat models.34 Furthermore, in Bnp knockout rats, Hspb3 was overexpressed in hypertrophic left ventricular mass prior to the development of adult-onset hypertension 35. Future studies with higher genotyping resolution at this locus are warranted to identify the causal variant(s) that interact with potassium intake on hypertension.
The 1 df test also provided the first robust evidence for the relevance of the RANBP3L locus in BP regulation. A previous study utilizing a two-marker association testing approach identified a SNP pair approximately 200 kb downstream from RANBP3L for hypertension.36 However, the study did not replicate their association signals in an independent sample. Our study identified a significant interaction between intronic RANBP3L variant rs958929 and dietary potassium intake on pulse pressure, which was replicated among MESA findings. Three variants in the RANBP3L gene were positively associated with urinary potassium levels (Supplementary Table S2). Therefore, the involvement of this gene in blood pressure regulation may be mediated through its role in potassium metabolism. Our finding is further supported by a recent functional study, in which extracellular osmolality within the renal medulla up-regulated the expression of RanBP3L.37 The RANBP3L signal may have also reflect other genes in this locus. For example, a nearby gene, SKP2 encoding S-phase kinase associated protein 2, interacts with p27 and promotes p27 degradation 38. Skp2 knockout mice showed p27 accumulation in cells 38, 39, and subsequently inhibited renal tubular epithelial cell proliferation and reduced renal damage. 40 Considering the importance of kidney function in blood pressure regulation and potassium filtration, this locus warrants further investigation.
Gene-based analysis of the 1 df interaction test and 2 df joint test identified 6 additional genes (DAB1, CC2D2A, GGNBP1, LINC00336, MIR4466, BNC2) at 5 novel loci that were associated with BP. The 6 genes were not reported in previous GWAS of cardiovascular related phenotypes. However, the GGNBP1 gene was previously reported to associate with SBP in a secondary analysis using the genetic pleiotropy-informed conditional false discovery rate method.41 In addition, mutations in the CC2D2A gene have been shown to cause Joubert Syndrome, a disorder characterized by renal impairment and hypertension.42 The functional relevance of the other identified genes on BP regulation is unclear. Future studies of these genes are warranted.
Our study has several strengths. First of all, stringent quality control measures were employed for genotyping, data cleaning, covariable collection, BP measurement, and urinary potassium measurement. Second, since we limited our analysis to Chinese participants, population stratification should be minimized. Third, GenSalt was a family based study, and dietary potassium intake should have strong familial resemblance, therefore, 24-hour urinary potassium levels should have smaller variability, and subsequently we should have stronger statistical power to test gene-potassium interactions on blood pressure. Finally, a total of 9 BP were measured using random zero sphygmomanometer at the same time during the 3 day baseline examination in GenSalt. Using multiple BP measures should greatly reduce measurement error and increase statistical power to detect genetic variants for BP. However, certain limitations should also be addressed. The Chinese MESA replication sample was small, and we may not have had enough power to replicate all promising findings from the GenSalt study. In addition, 24-hour potassium level was estimated from spot urine using Tanaka’s equation in MESA. Although the method has been validated previously, any measurement error could further reduce statistical power for replicating genetic variants identified in GenSalt. In addition, MESA had a high proportion of participants taking antihypertensive medication. We imputed BP by adding 10 and 5 mm Hg to SBP and DBP, respectively. Although this approach is widely applied to genetic studies of BP, 2, 5, 43 any resulting inaccuracy may dilute associations between genetic factors and BP. Furthermore, additional factors such as age and urbanization level differ between the GenSalt and MESA samples. If these factors influence gene-potassium interactions on BP, our power to detect (or replicate) SNP-BP associations could again be reduced. Imputation quality of the significant SNPs in MESA was not very high. Thus, future replication studies with larger replication sample sizes, more homogeneous populations, better measurement of urinary potassium levels, and higher imputation quality or higher genotyping resolution are warranted. Finally, GenSalt study did not collect data on serum potassium level, so we cannot assess correlations between daily potassium intake and serum potassium levels.
In conclusion, in the first ever genome-wide gene-potassium interaction analyses of BP in a Chinese population, utilizing both single marker and gene-based analyses, we identified 6 novel loci that interacted with dietary potassium intake on SBP, and provided first robust evidence of the relevance of the RANBP3L locus in BP regulation. Both 1 df interaction and 2 df joint tests of single markers identified novel locus ARL15 rs16882447. Gene-based analyses provided consistent support for the RANBP3L gene, and identified an additional 6 genes at 5 novel loci, including DAB1, CC2D2A, MIR4466, GGNBP1, LINC00336 and BNC2. Such findings highlight the importance of examining gene-potassium interactions and conducting gene-based analysis to identify novel BP loci. Further, these findings contribute to understanding the mechanisms of BP regulation. Sequencing along with functional studies are needed to help delineate causal variants underlying the strong signals identified here.
Supplementary Material
Clinical Perspective.
Dietary potassium intake has been shown to decrease blood pressure, and blood pressure responses to dietary potassium intake vary across individuals. We conducted the first genome-wide interaction analyses to identify genes and genomic loci that modified correlations between dietary potassium intake and blood pressure in a Chinese population. The current study identified one novel locus, ARL15 rs16882447 that interacted with dietary potassium intake on systolic blood pressure in single-marker analysis. We also provided first robust evidence of the relevance of the previously reported RANBP3L rs958929 locus in blood pressure regulation in the single-marker analysis. Gene-based gene-potassium interaction analyses provided consistent support for the RANBP3L gene, and identified an additional 6 genes at 5 novel loci, including DAB1, CC2D2A, MIR4466, GGNBP1, LINC00336 and BNC2 for blood pressure phenotypes. Such findings provide strong evidence for the genetic background of the effect of dietary potassium intake on blood pressure regulation, and highlight the importance of examining gene-potassium interactions and conducting gene-based analysis to identify novel blood pressure loci. Further, these findings contribute to understanding the mechanisms of blood pressure regulation. However, Future sequencing and functional studies will be needed to better understand the causal mechanism underlying the identified interactions and to identify the causal variants underlying the gene-based signals.
Acknowledgments
The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) is supported by a cooperative agreement project grant (U01HL072507, R01HL087263, and R01HL090682) from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM109036, and by the NHLBI, NIH under Award Number 5R01HL111249-02. Support for MESA is provided by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 and CTSA UL1-RR-024156. Funding for SHARe genotyping was provided by NHLBI Contract N02-HL-64278.
Sources of Funding: The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) is supported by a cooperative agreement project grant (U01HL072507, R01HL087263, and R01HL090682) from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM109036, and by the NHLBI, NIH under Award Number 5R01HL111249-02.
Footnotes
Disclosures: None
References
- 1.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–9. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wain LV, Verwoert GC, O’Reilly PF, Shi G, Johnson T, Johnson AD, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–11. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–87. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–76. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet. 2011;43:531–8. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehret GB, Caulfield MJ. Genes for blood pressure: an opportunity to understand hypertension. European heart journal. 2013;34:951–61. doi: 10.1093/eurheartj/ehs455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murcray CE, Lewinger JP, Gauderman WJ. Gene-environment interaction in genome-wide association studies. Am J Epidemiol. 2009;169:219–26. doi: 10.1093/aje/kwn353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom JS, Ehrenreich IM, Loo WT, Lite TL, Kruglyak L. Finding the sources of missing heritability in a yeast cross. Nature. 2013;494:234–7. doi: 10.1038/nature11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li MX, Gui HS, Kwan JS, Sham PC. GATES: a rapid and powerful gene-based association test using extended Simes procedure. Am J Hum Genet. 2011;88:283–93. doi: 10.1016/j.ajhg.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, Brown KM, et al. A versatile gene-based test for genome-wide association studies. Am J Hum Genet. 2010;87:139–45. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA. 1997;277:1624–32. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- 13.He J, Kelly TN, Zhao Q, Li H, Huang J, Wang L, et al. Genome-wide association study identifies 8 novel loci associated with blood pressure responses to interventions in Han Chinese. Circ Cardiovasc Genet. 2013;6:598–607. doi: 10.1161/CIRCGENETICS.113.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park YM, Kwock CK, Kim K, Kim J, Yang YJ. Interaction between Single Nucleotide Polymorphism and Urinary Sodium, Potassium, and Sodium-Potassium Ratio on the Risk of Hypertension in Korean Adults. Nutrients. 2017:9. doi: 10.3390/nu9030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt SC, Hasstedt SJ, Wu LL, Williams RR. A gene-environment interaction between inferred kallikrein genotype and potassium. Hypertension. 1993;22:161–8. doi: 10.1161/01.hyp.22.2.161. [DOI] [PubMed] [Google Scholar]
- 16.Group GCR. GenSalt: rationale, design, methods and baseline characteristics of study participants. J Hum Hypertens. 2007;21:639–46. doi: 10.1038/sj.jhh.1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–34. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui J, Hopper JL, Harrap SB. Genes and family environment explain correlations between blood pressure and body mass index. Hypertension. 2002;40:7–12. doi: 10.1161/01.hyp.0000022693.11752.e9. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103. doi: 10.1038/sj.jhh.1001307. [DOI] [PubMed] [Google Scholar]
- 20.Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–7. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraft P, Yen YC, Stram DO, Morrison J, Gauderman WJ. Exploiting gene-environment interaction to detect genetic associations. Hum Hered. 2007;63:111–9. doi: 10.1159/000099183. [DOI] [PubMed] [Google Scholar]
- 23.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning AK, LaValley M, Liu CT, Rice K, An P, Liu Y, et al. Meta-analysis of gene-environment interaction: joint estimation of SNP and SNP × environment regression coefficients. Genet Epidemiol. 2011;35:11–8. doi: 10.1002/gepi.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. 2. Wiley; 2009. [Google Scholar]
- 26.Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 2002;99:16899–903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahajan A, Go MJ, Zhang W, Below JE, Gaulton KJ, Ferreira T, et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46:234–44. doi: 10.1038/ng.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negi S, Juyal G, Senapati S, Prasad P, Gupta A, Singh S, et al. A genome-wide association study reveals ARL15, a novel non-HLA susceptibility gene for rheumatoid arthritis in North Indians. Arthritis Rheum. 2013;65:3026–35. doi: 10.1002/art.38110. [DOI] [PubMed] [Google Scholar]
- 29.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA, et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607. doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorski M, van der Most PJ, Teumer A, Chu AY, Li M, Mijatovic V, et al. 1000 Genomes-based meta-analysis identifies 10 novel loci for kidney function. Sci Rep. 2017;7:45040. doi: 10.1038/srep45040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam WY, Wing Tsui SK, Law PT, Luk SC, Fung KP, Lee CY, et al. Isolation and characterization of a human heart cDNA encoding a new member of the small heat shock protein family--HSPL27. Biochim Biophys Acta. 1996;1314:120–4. doi: 10.1016/s0167-4889(96)00121-8. [DOI] [PubMed] [Google Scholar]
- 33.Sugiyama Y, Suzuki A, Kishikawa M, Akutsu R, Hirose T, Waye MM, et al. Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J Biol Chem. 2000;275:1095–104. doi: 10.1074/jbc.275.2.1095. [DOI] [PubMed] [Google Scholar]
- 34.Cerutti C, Kurdi M, Bricca G, Hodroj W, Paultre C, Randon J, et al. Transcriptional alterations in the left ventricle of three hypertensive rat models. Physiol Genomics. 2006;27:295–308. doi: 10.1152/physiolgenomics.00318.2005. [DOI] [PubMed] [Google Scholar]
- 35.Holditch SJ, Schreiber CA, Nini R, Tonne JM, Peng KW, Geurts A, et al. B-Type Natriuretic Peptide Deletion Leads to Progressive Hypertension, Associated Organ Damage, and Reduced Survival: Novel Model for Human Hypertension. Hypertension. 2015;66:199–210. doi: 10.1161/HYPERTENSIONAHA.115.05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slavin TP, Feng T, Schnell A, Zhu X, Elston RC. Two-marker association tests yield new disease associations for coronary artery disease and hypertension. Hum Genet. 2011;130:725–33. doi: 10.1007/s00439-011-1009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulze Blasum B, Schröter R, Neugebauer U, Hofschröer V, Pavenstädt H, Ciarimboli G, Schlatter E, Edemir B. The kidney-specific expression of genes can be modulated by the extracellular osmolality. FASEB J. 2016 doi: 10.1096/fj.201600319R. [DOI] [PubMed] [Google Scholar]
- 38.Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–4. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–81. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki S, Fukasawa H, Misaki T, Togawa A, Ohashi N, Kitagawa K, et al. The amelioration of renal damage in Skp2-deficient mice canceled by p27 Kip1 deficiency in Skp2−/− p27−/− mice. PLoS One. 2012;7:e36249. doi: 10.1371/journal.pone.0036249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreassen OA, McEvoy LK, Thompson WK, Wang Y, Reppe S, Schork AJ, et al. Identifying common genetic variants in blood pressure due to polygenic pleiotropy with associated phenotypes. Hypertension. 2014;63:819–26. doi: 10.1161/HYPERTENSIONAHA.113.02077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doherty D, Parisi MA, Finn LS, Gunay-Aygun M, Al-Mateen M, Bates D, et al. Mutations in 3 genes (MKS3, CC2D2A and RPGRIP1L) cause COACH syndrome (Joubert syndrome with congenital hepatic fibrosis) J Med Genet. 2010;47:8–21. doi: 10.1136/jmg.2009.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–87. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.