Abstract
Current literature reveals three types of paroxysmal atrioventricular block (AVB) that can cause syncope:
Intrinsic paroxysmal atrioventricular block is due to an intrinsic disease of the AV conduction system; this type of “cardiac syncope” is also called Stokes-Adams attack;
Extrinsic vagal paroxysmal atrioventricular block is linked to the effect of the parasympathetic nervous system on cardiac conduction and is one of the mechanisms involved in “reflex syncope.”
Extrinsic idiopathic paroxysmal atrioventricular block is associated with low levels of endogenous adenosine and is supposed to be one of the mechanisms involved in “low-adenosine syncope.”
These three types of paroxysmal AVB present different clinical and electrocardiographic features. Additionally, the efficacy of cardiac pacing and theophylline therapy to prevent syncopal recurrences is also different for these three types of AVB.
Keywords: Paroxysmal atrioventricular block, Syncope, Adenosine, Theophylline, Idiopathic atrioventricular block
1. Introduction
Syncope due to paroxysmal atrioventricular block (AVB) occurs because of a sudden change from apparently physiological atrioventricular conduction to transient second- or third-degree heart block, which leads to ventricular asystole.
Syncope is the main accompanying symptom in approximately 40% patients affected by recent-onset persistent AVB [1], [2], [3]. However, the prevalence of syncope due to paroxysmal AVB is probably under-reported [4]. In recent years, newly available long-term ECG monitoring devices have increased the diagnostic yield [5], [6].
2. Types of paroxysmal AV block
Three types of paroxysmal AVB are currently described in literature:
-
•
Intrinsic paroxysmal atrioventricular block (I-AVB) is due to an intrinsic disease of the AV conduction system; this type of “cardiac syncope,” is also called Stokes-Adams attack.
-
•
Extrinsic vagal paroxysmal atrioventricular block (EV-AVB) is linked to parasympathetic influence on cardiac conduction and is one of the mechanisms involved in “reflex syncope.”
-
•
Extrinsic idiopathic paroxysmal atrioventricular block (EI-AVB) is associated with low values of endogenous adenosine and is supposed to be one of the mechanisms involved in “low adenosine syncope.”
The management (diagnosis and therapy) of patients with syncope suspected of being due to paroxysmal AVB depends on the type of block.
The distinguishing features of the three forms of paroxysmal AVB are summarized in Table 1 and Fig. 1.
Table 1.
Comparison of the 3 forms of paroxysmal AVB.
| Features | Intrinsic AV block (I-AVB) | Extrinsic vagal AV block (EV-AVB) | Extrinsic idiopathic AV block (EI-AVB) |
|---|---|---|---|
| Cardiac syncope | Reflex syncope | adenosine syncope | |
| ECG | |||
| Sinus rhythm | BBB frequent | BBB infrequent | Narrow QRS |
| Before AVB |
|
|
|
| During asystolic AVB | Sinus rate increase | Sinus rate slowing | Sinus rate unchanged |
| (P-P cycle decrease) | (P-P cycle increase) | (P-P cycle unchanged) | |
| End of AVB |
|
Sinus rate acceleration (P-P cycle decrease) | Sinus rate unchanged (P-P cycle unchanged) |
| Follow-up | Progression to persistent AVB | No progression to persistent AVB | No progression to persistent AVB |
| Syncope | |||
| History of syncope | Short (mostly<1 year) | Long (since youth) | Short (average 2 years) |
| Prodromes | No or very short (≤5 s) prodromes | Always present >10 s | No or very short (≤5 s) prodromes |
| Structural heart disease | Mostly present | Mostly absent | Absent |
| Age on presentation | Elderly | Any age | Any age, mostly over 40 years |
| Efficacy of pacemaker therapy | Effective | Partially effective | Effective |
| Efficacy of theophylline therapy | Ineffective | Partially effective | Effective |
| Investigations | |||
| Plasma adenosine value | Normal | High | Low or very low |
| Adenosine (ATP) test | Usually negative | May be positive | Frequently positive (asystolic 3rd degree block) |
| Tilt table test | Usually negative | Mostly positive | Mostly negative |
| Electrophysiological study | Frequently positive | Negative | Negative |
| Carotid sinus massage | Usually negative | Frequently positive | Negative |
Abbreviations: AVB=atrioventricular block; ECG=electrocardiography; BBB=bundle branch block; APB=atrial premature beat; VPB=ventricular premature beat
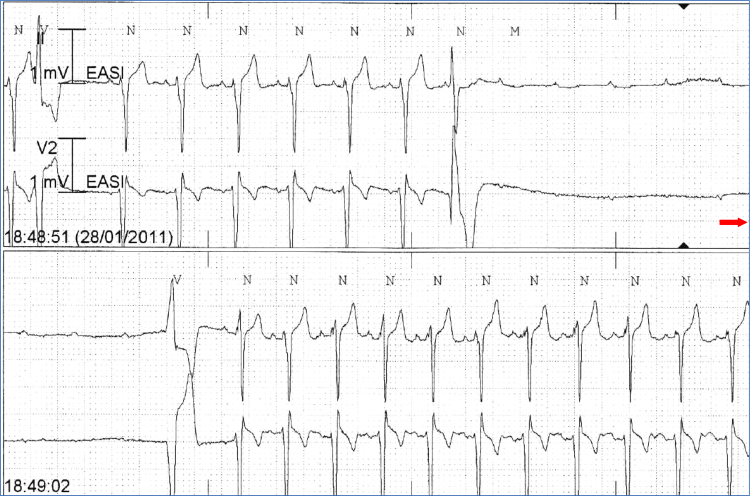
Fig. 1.
Continuous tracing shows an episode of intrinsic AVB (I-AVB) due to His-Purkinje conduction disease. AVB starts suddenly and ends with a ventricular premature beat; P-P cycle length remains constant throughout the recording. AVB=atrioventricular block.
I-AVB is a manifestation of an intrinsic disease of the atrioventricular conduction system. It usually occurs in patients older than 60 years and is associated with bundle branch block (BBB) and/or an underlying heart disease [4], [7]. A short history of syncope is usually reported, the onset of syncopal episodes typically occurring within 1 year before the ECG diagnosis. I-AVB is characterized by progression toward permanent AVB [2], [4], [7]. Electrocardiographically, this block starts mostly with an extrasystole, and the frequency of sinus node stimulation may be increased (tachy-dependent AVB) or decreased (brady-dependent AVB) (Fig. 1). The sudden onset of AVB explains why prodromes are absent or shorter than 5 s [8].
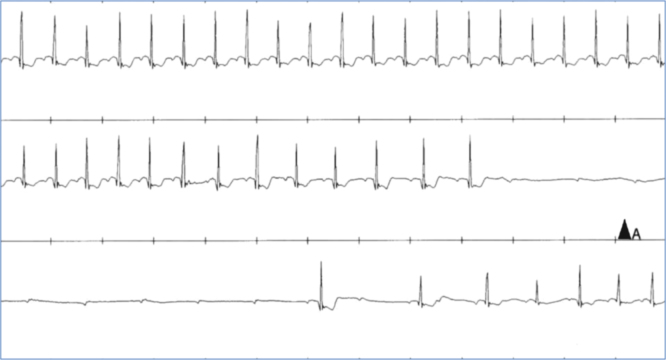
EV-AVB is typically associated with well-identifiable triggers (central, i.e. emotional distress, or peripheral, i.e. prolonged standing) and characteristic symptoms of autonomic activation (i.e. feeling of warmth, an odd sensation in the abdomen, and lightheadedness or dizziness, nausea, and sweating) [9]. Electrocardiographically, it is associated with two vagal effects on the conduction system: a gradual slowing of the sinus rate (P-P interval) and a delayed atrioventricular conduction (PR prolonging, or Wenckeback) followed by sinus arrest or complete AVB [4], [10], [11], [12] (Fig. 2). Consequently, prodromes are much longer than those in I-AVB [13]. A long history of syncope, with onset in young middle age, is usually reported. EV-AVB does not progress to stable AVB because it is not an expression of anatomical involvement of the atrioventricular conduction system [14].
Fig. 2.
Continuous tracing shows an episode of extrinsic vagal paroxysmal AV block (EV-AVB). A gradual slowing of the sinus rate (P-P interval) before and during the asystolic pause and a delay of AV conduction (prolonging PR) followed by complete AV block is typical of vagal origin. AV=atrioventricular.
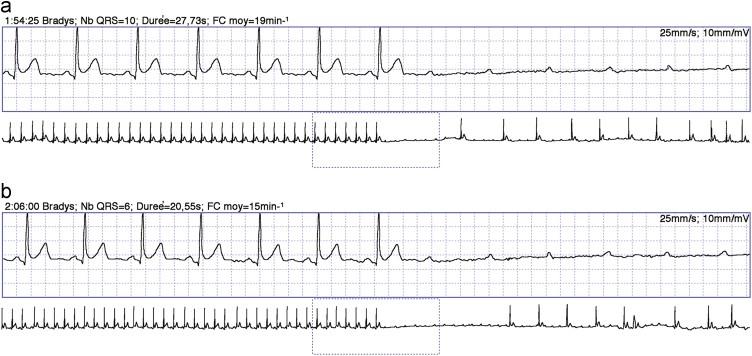
The clinical observation of clinical and electrophysiological features in some patients with AVB that are different from those with the two above-mentioned forms of block has led to the identification of a third distinct clinical entity. EI-AVB is characterized by a long-standing history of recurrent syncope without prodromes and is associated with normal heart and normal electrocardiogram [15]; additionally, EI-AVB does not progress to persistent forms [16]. In such clinical conditions, an abrupt-onset AVB without significant rhythm disturbances before or during the attack (type IC block according to ISSUE classification) is pathognomonic (Fig. 3). The sudden onset of the AVB explains why prodromes are absent or shorter than 5 s. Typically, EI-AVB is associated with low baseline adenosine plasma levels and shows a high susceptibility to exogenous adenosine. EI-AVB has been found in 8% patients with syncope and normal ECG but without structural heart disease (corresponding to 15% of those with ECG documentation of syncope) [17].
Fig. 3.
Continuous Holter tracing shows two episodes of extrinsic idiopathic paroxysmal AV block (EI-AVB) that occurred a few minutes apart. The two episodes were very similar and were characterized by sudden-onset complete AV block without changes in the P-P cycle length, which constantly remained 720 ms (top, trace), and long ventricular asystoles of 7 and 11 s, respectively (bottom, compressed trace). From Ref16, with permission. AV=atrioventricular.
No single symptom or sign is diagnostic for a specific type of AVB. An intrinsic AVB is more likely in patients with baseline BBB, although the presence of BBB does not rule out an EV-AVB; indeed, > 40% patients with BBB have reflex syncope, and in another 15%, the cause of syncope remains unknown [18]. Differential diagnosis may be difficult in cases with slight increases in PP intervals, which is consistent with sinus arrhythmia. According to some authors [19], [20], an increase in this interval of only 40 ms suggests that the block is of vagal origin. This value of 40 ms is arbitrary, and a much more evident slowing of the sinus rate should probably be documented [14]. The coexistence of two types of AVB mechanisms, i.e. vagal and adenosine, may be observed (Fig. 4). Absent or short prodromes are common to both I-AVB [8] and EI-AVB [16], whereas in patients without structural heart disease, prodromes lasting more than 10 s are associated with reflex syncope [13]. Moreover, it is well known that the perception of symptoms varies from patient to patient and that a phenomenon of retrograde amnesia frequently occurs in patients with syncope [9]. "High-risk" features that suggest a diagnosis of I-AVB are the presence of a definite structural heart disease, and syncope during exertion, or while lying in supine position, or preceded by sudden-onset palpitations. "Low-risk" features, which suggest EV-AVB, are the absence of heart disease, a long-standing history of recurrent syncope, the occurrence of syncope after exertion or after prolonged standing or in crowded places, during a meal, on head rotation or pressure on the carotid sinus, or after a sudden unexpected pain, unpleasant sight, sound, or smell [9].
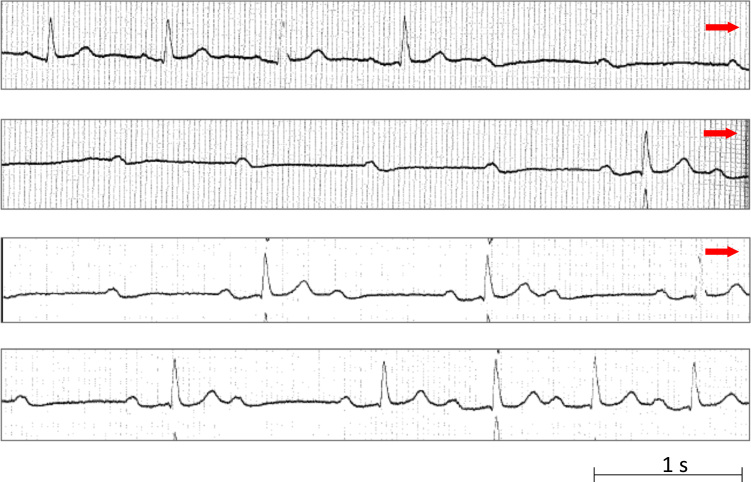
Fig. 4.
Continuous tracing shows an episode of paroxysmal AV block of uncertain mechanism in a patient with normal heart and normal ECG. In this patient, AV block occurred without P-P cycle changes, but PR interval was prolonged before block from 0.20 s to 0.28 s in the beat immediately preceding the asystolic pause; furthermore, the asystolic pause was followed by 2:1 AV block with prolonged PR interval up to 0.36 s in the conducted beats. PR prolongation is suggestive of vagal AV block (EI-AVB). Nevertheless, in this patient, AV block occurred without P-P cycle changes, which is suggestive of idiopathic AVB; the low adenosine value of 0.10 μmol/L (normal value: 0.40–0.80 μmol/L) observed in this patient supports this mechanism. AV=atrioventricular; ECG=electrocardiography.
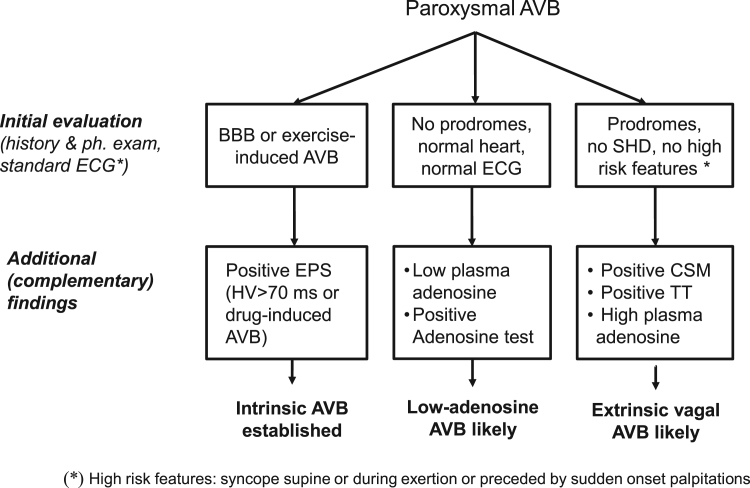
In brief, the diagnosis is made using ECG findings and clinical and laboratory features reported in Table 1 and Fig. 5.
Fig. 5.
Distinctive features of the three types of paroxysmal AVB AVB=atrioventricular block; BBB= bundle branch block; CSM= carotid sinus massage; TT= tilt testing; SHD= structural heart disease; EPS = electrophysiological study.
3. Role of electrophysiological study (EPS) and prolonged ECG monitoring in patients with unexplained syncope and BBB
I-AVB should be considered as a possibility in patients with unexplained syncope and BBB [18]. At follow-up, the rate of development of stable AVB has been seen to increase from 2% in patients with BBB without syncope to 17% in patients with BBB and syncope [21] and up to 37% in those with a positive EPS or those who have undergone prolonged ECG monitoring [22], [23]. Consequently, approximately 50% patients with unexplained syncope and BBB were reported to require cardiac pacing at the end of a standardized evaluation [18], [22], [24].
The diagnostic work-up of patients with unexplained syncope and BBB should start with a few days of ECG monitoring [9]. Early recording improves the diagnostic yield [6] and is justified by the need to avoid immediate risk to the patient [25].
After initial negative (in-hospital) ECG monitoring, EPS is recommended in patients with preserved ejection fraction. EPS is no longer indicated in patients with severely depressed ejection fraction, as in these cases, implantation of an implantable cardioverter defibrillator (ICD) should be indicated irrespective of the mechanism of syncope [9]. The following EPS findings are considered diagnostic: baseline HV interval ≥70 ms and second- or third-degree His-Purkinje block during incremental atrial pacing or after intravenous class IC antiarrhythmic drug challenge [9]. These criteria have an acceptable positive predictive value [23], [26], [27], but low sensitivity: false negative responses have been found in 33–45% patients with syncope, BBB and negative EPS [22], [24]. In other words, a negative EPS cannot rule out the presence of paroxysmal AVB in patients with BBB; further investigations, i.e. implantable loop recorder (ILR), are therefore warranted in case of negative EPS to establish a symptom-rhythm correlation [5], [22], [23], [28]. ILR is able to establish a symptom-rhythm correlation in one out of three patients (the most frequent finding being I-AVB: 63–71% of recorded events) [22], [24].
The decision of whether to perform EPS before ILR implantation depends on the global risk-benefit assessment of an invasive procedure. Although the risk of syncopal recurrence after the index episode is strictly related to the number of syncopes [22], [29], ILR may be useful even after the first episode, as the risk of third-degree AVB block is 10.7% /year in patients with BBB [30].
4. Treatment
4.1. Cardiac pacing
4.1.1. I-AVB
There is general agreement that, in patients with documented I-AVB, cardiac pacing relieves syncopal recurrences and improves survival [9], [31]. In paced patients with documented I-AVB, syncopal recurrences are virtually eliminated, being 1% over 5 years of follow-up [1]. By contrast, in patients paced for unexplained syncope and BBB (AVB still undocumented), the recurrence rate of syncopal events is 7% over 2 years of follow-up [24] when EPS is positive, 13.5–14% over 5 years of follow-up when EPS is not performed [1], [32] and 25–27% over 31 months of follow-up when pacing is administered empirically without EPS or ILR documentation [23], [32].
4.1.2. EV-AVB
Cardiac pacing has much lower efficacy in preventing syncopal recurrences in patients affected by EV-AVB even if a spontaneous asystolic reflex has been documented. No studies have specifically investigated the effect of cardiac pacing in patients with EV-AVB. Indeed, all studies on cardiac pacing have involved patients affected by vasovagal syncope, with EV-AVB patients constituting a minority of the population. Therefore, we can only infer that the result of pacing in the EV-AVB subgroup is not different from that of the overall population. In the ISSUE 2, SUP 2, and ISSUE 3 trials [29], [33], [34], dual-chamber cardiac pacing in patients with syncope and documentation of asystolic pause (either sinus arrest or AVB) by means of ECG monitoring was associated with a not infrequent recurrence rate of syncopal events (12–25% at 2-year follow-up). Although cardiac pacing is the most effective therapy when bradycardia is responsible for syncope, syncope may recur because of the coexistence of a vasodepressor reflex, which is present to some degree in virtually all patients. The absence of the above-mentioned "hypotensive susceptibility," as evidenced by a negative tilt test result, has been associated with higher efficacy of pacing in preventing syncopal recurrences, similar to that observed in documented I-AVB [35].
4.1.3. EI–AVB
No trial has been performed. Permanent cardiac pacing was successful in preventing syncopal recurrences during long-term follow-up in two small observational cohorts of EI-AVB patients [16], [36].
Table 2 summarizes the expected benefit of pacing in patients with different types of AVB.
Table 2.
Expected benefit of pacing in patients with different types of AVB.
| Clinical forms | Expected syncope recurrence rate at 2 years follow-up on cardiac pacing |
|---|---|
|
High efficacy (≤5% recurrence rate) |
|
Moderate efficacy (5–20% recurrence rate) |
|
Low efficacy (>20% recurrence rate) |
Abbreviations: AVB=atrioventricular block; ECG=electrocardiography; I-AVB=intrinsic atrioventricular block; EI-AVB=extrinsic idiopathic atrioventricular block; EV-AVB=extrinsic vagal atrioventricular block; BBB=bundle branch block
4.2. Theophylline
Since patients with low plasma adenosine levels are highly susceptible to exogenous and endogenous adenosine, the effect of treatment with theophylline, a non-selective adenosine receptor antagonist, in the prevention of syncopal recurrences has been investigated. In two recent small observational studies, oral theophylline appeared to be effective over a mean follow-up of 16 and 17 months in patients with an established diagnosis of EI-AVB and may be considered an alternative to permanent pacing in such patients [36], [37].
No studies on the role of theophylline in I-AVB are available.
A few small observational studies on patients with EV-AVB treated with theophylline have recorded a recurrence rate ranging between 12% and 22% [38], [39], [40]. In a randomized controlled trial [41], theophylline proved ineffective in preventing reflex syncope in patients affected by sick sinus syndrome compared with the no-treatment arm.
Conflict of interest
All authors declare no conflict of interest related to this study.
References
- 1.Aste M., Oddone D., Donateo P. Syncope in patients paced for atrioventricular block. Europace. 2016;18:1735–1739. doi: 10.1093/europace/euv425. [DOI] [PubMed] [Google Scholar]
- 2.Langenfeld H., Grimm W., Maisch B. Course of symptoms and spontaneous ECG in pacemaker patients: a 5-year follow-up study. Pacing Clin Electrophysiol. 1988;11:2198–2206. doi: 10.1111/j.1540-8159.1988.tb05986.x. [DOI] [PubMed] [Google Scholar]
- 3.Proclemer A., Ghidina M., Gregori D. Trend of the main clinical characteristics and pacing modality in patients treated by pacemaker: data from the Italian Pacemaker Registry for the quinquennium 2003–07. Europace. 2010;12:202–209. doi: 10.1093/europace/eup346. [DOI] [PubMed] [Google Scholar]
- 4.Lee S., Wellens J.J., Josephson M. Paroxysmal atrioventricular block. Heart Rhythm. 2009;6:1229–1234. doi: 10.1016/j.hrthm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Brignole M., Vardas P., Hoffmann E. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace. 2009;11:671–687. doi: 10.1093/europace/eup097. [DOI] [PubMed] [Google Scholar]
- 6.Locati E.T., Moya A., Oliveira M. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace. 2016;18:1265–1272. doi: 10.1093/europace/euv311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Sherif N., Jalife J. Paroxysmal atrioventricular block: are phase 3 and phase 4 block mechanisms or misnomers? Heart Rhythm. 2009;6:1514–1521. doi: 10.1016/j.hrthm.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calkins H., Shyr Y., Frumin H. The value of history in the differentiation of syncope due to ventricular tachycardia, atrioventricular block and neurocardiogenic syncope. Am J Med. 1995;98:365–373. doi: 10.1016/S0002-9343(99)80315-5. [DOI] [PubMed] [Google Scholar]
- 9.Moya A., Sutton R., Ammirati F. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC) Eur Heart J. 2009;30:2631–2671. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sud S., Klein G., Skanes A. Implications of mechanism of bradycardia on response to pacing in patients with unexplained syncope. Europace. 2007;9:312–318. doi: 10.1093/europace/eum020. [DOI] [PubMed] [Google Scholar]
- 11.Brignole M., Moya A., Menozzi C. Proposed electrocardiographic classification of spontaneous syncope documented by an implantable loop recorder. Europace. 2005;7:14–18. doi: 10.1016/j.eupc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Zysko D., Gajek J., Kozluk E. Electrocardiographic characteristics of atrioventricular block induced by tilt testing. Europace. 2009;11:225–230. doi: 10.1093/europace/eun299. [DOI] [PubMed] [Google Scholar]
- 13.Alboni P., Brignole M., Menozzi C. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol. 2001;37:1921–1928. doi: 10.1016/s0735-1097(01)01241-4. [DOI] [PubMed] [Google Scholar]
- 14.Alboni P., Holz A., Brignole M. Vagally mediated atrioventricular block: pathophysiology and diagnosis. Heart. 2013;99:904–908. doi: 10.1136/heartjnl-2012-303220. [DOI] [PubMed] [Google Scholar]
- 15.Brignole M., Deharo J.C., Guieu R. Syncope and Idiopathic (Paroxysmal) AV Block. Cardiol Clin. 2015;33:441–447. doi: 10.1016/j.ccl.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Brignole M., Deharo J.C., De Roy L. Syncope due to idiopathic paroxysmal atrioventricular block: long-term follow-up of a distinct form of atrioventricular block. J Am Coll Cardiol. 2011;58:167–173. doi: 10.1016/j.jacc.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Brignole M., Sutton R., Menozzi C. Lack of correlation between the responses to tilt testing and adenosine triphosphate test and the mechanism of spontaneous neurally mediated syncope. Eur Heart J. 2006;27:2232–2239. doi: 10.1093/eurheartj/ehl164. [DOI] [PubMed] [Google Scholar]
- 18.Donateo P., Brignole M., Alboni P. A standardized conventional evaluation of the mechanism of syncope in patients with bundle branch block. Europace. 2002;4:357–360. doi: 10.1053/eupc.2002.0265. [DOI] [PubMed] [Google Scholar]
- 19.Barold S.S., Hayes D.L. Second degree atrioventricular block: a reappraisal. Mayo Clin Proc. 2001;76:44–57. doi: 10.4065/76.1.44. [DOI] [PubMed] [Google Scholar]
- 20.Lange H.W., Ameisen O., Mack R. Prevalence and clinical correlates of non-Wenckebach, narrow-complex second-degree atrioventricular block detected by ambulatory ECG. Am Heart J. 1988;115:114–120. doi: 10.1016/0002-8703(88)90526-1. [DOI] [PubMed] [Google Scholar]
- 21.McAnulty J.H., Rahimtoola S.H., Murphy E. Natural history of high-risk bundle-branch block: final report of a prospective study. N Engl J Med. 1982;307:137–143. doi: 10.1056/NEJM198207153070301. [DOI] [PubMed] [Google Scholar]
- 22.Brignole M., Menozzi C., Moya A. Mechanism of syncope in patients with bundle branch block and negative electrophysiological test. Circulation. 2001;104:2045–2050. doi: 10.1161/hc4201.097837. [DOI] [PubMed] [Google Scholar]
- 23.Kalscheur M.M., Donateo P., Wenzke K.E. Long-term outcome of patients with bifascicular block and unexplained syncope following cardiac pacing. Pacing Clin Electrophysiol. 2016;39:1126–1131. doi: 10.1111/pace.12946. [DOI] [PubMed] [Google Scholar]
- 24.Moya A., García-Civera R., Croci F. Diagnosis, management, and outcomes of patients with syncope and bundle branch block. Eur Heart J. 2011;32:1535–1541. doi: 10.1093/eurheartj/ehr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Croci F., Brignole M., Alboni P. The application of a standardized strategy of evaluation in patients with syncope referred to three Syncope Units. Europace. 2002;4:351–355. doi: 10.1053/eupc.2002.0267. [DOI] [PubMed] [Google Scholar]
- 26.Gronda M., Magnani A., Occhetta E. Electrophysiologic study of atrio-ventricular block and ventricular conduction defects. G Ital Cardiol. 1984;14:768–773. [PubMed] [Google Scholar]
- 27.Bergfeldt L., Edvardsson N., Rosenqvist M. Atrioventricular block progression in patients with bifascicular block assessed by repeated electrocardiography and a bradycardia-detecting pacemaker. Am J Cardiol. 1994;74:1129–1132. doi: 10.1016/0002-9149(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 28.Krahn A.D., Klein G.J., Yee R. Randomized assessment of syncope trial: conventional diagnostic testing versus a prolonged monitoring strategy. Circulation. 2001;104:46–51. doi: 10.1161/01.cir.104.1.46. [DOI] [PubMed] [Google Scholar]
- 29.Brignole M., Sutton R., Menozzi C. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J. 2006;27:1085–1092. doi: 10.1093/eurheartj/ehi842. [DOI] [PubMed] [Google Scholar]
- 30.Da costa A., Defaye P., Romeyer-Bouchard C. Clinical impact of the implantable loop recorder in patients with isolated syncope, bundle branch block and negative workup: a randomized multicenter prospective study. Arch Cardiovasc Dis. 2013;106:146–154. doi: 10.1016/j.acvd.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Brignole M., Auricchio A., Baron-Esquivias G. ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on Cardiac Pacing and Resynchronization Therapy of the EuropeanSociety of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Europace. 2013;2013(15):1070–1118. doi: 10.1093/europace/eut206. [DOI] [PubMed] [Google Scholar]
- 32.Santini M., Castro A., Giada F. Prevention of syncope through permanent cardiac pacing in patients with bifascicular block and syncope of unexplained origin: the PRESS study. Circ Arrhythm Electrophysiol. 2013;6:101–107. doi: 10.1161/CIRCEP.112.975102. [DOI] [PubMed] [Google Scholar]
- 33.Brignole M., Ammirati F., Arabia F. Assessment of a standardized algorithm for cardiac pacing in older patients affected by severe unpredictable reflex syncopes. Eur Heart J. 2015;36:1529–1535. doi: 10.1093/eurheartj/ehv069. [DOI] [PubMed] [Google Scholar]
- 34.Brignole M., Donateo P., Tomaino M. Benefit of pacemaker therapy in patients with presumed neurally mediated syncope and documented asystole is greater when tilt test is negative: an analysis from the third International Study on Syncope of Uncertain Etiology (ISSUE-3) Circ Arrhythm Electrophysiol. 2014;7:10–16. doi: 10.1161/CIRCEP.113.001103. [DOI] [PubMed] [Google Scholar]
- 35.Brignole M., Arabia F., Ammirati F. Standardized algoritm for cardiac pacing in older patients affected by severe unpredictable reflex syncope: 3–year insights from the Syncope Unit Project 2 (SUP 2) study. Europace. 2016;18:1427–1433. doi: 10.1093/europace/euv343. [DOI] [PubMed] [Google Scholar]
- 36.Brignole M., Guieu R., Tomaino M. Mechanism of syncope without prodromes with normal heart and normal electrocardiogram. Heart Rhythm. 2017;14:234–239. doi: 10.1016/j.hrthm.2016.08.046. [DOI] [PubMed] [Google Scholar]
- 37.Brignole M., Solari D., Iori M. Efficacy of theophylline in patients affected by low adenosine syncope. Heart Rhythm. 2016;13:1151–1154. doi: 10.1016/j.hrthm.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Šinkovec M., Grad A., Rakovec P. Role of endogenous adenosine in vaso-vagal syncope. Clin Auton Res. 2001;11:155–161. doi: 10.1007/BF02329923. [DOI] [PubMed] [Google Scholar]
- 39.Brignole M., Gaggioli G., Menozzi C. Adenosine-induced atrioventricular block in patients with unexplained syncope:the diagnostic value of ATP testing. Circulation. 1997;96:3921–3927. doi: 10.1161/01.cir.96.11.3921. [DOI] [PubMed] [Google Scholar]
- 40.Benditt D.G., Benson W., Kreitt J. Electrophysiologic effects of theophylline in young patients with recurrent symptomatic bradyarrhythmias. Am J Cardiol. 1983;52:1223–1229. doi: 10.1016/0002-9149(83)90578-7. [DOI] [PubMed] [Google Scholar]
- 41.Sra J., Jazayeri M., Avitali B. Comparison of cardiac pacing with drug therapy in the treatment of neuro-cardiogenic (vasovagal) syncope with bradycardia or asystole. N Engl J Med. 1993;328:1085–1090. doi: 10.1056/NEJM199304153281504. [DOI] [PubMed] [Google Scholar]