Introduction
Penile intraepithelial neoplasia (PIN) is a rare disease that can be associated with great morbidity and mortality. Historically, the term PIN has been used to refer to 3 premalignant penile lesions: Bowen disease (BD), erythroplasia of Queyrat (EQ), and Bowenoid papulosis (BP). Although these clinical variants share similar histopathologic features of squamous cell carcinoma in situ—namely, transepidermal keratinocyte atypia, numerous mitotic figures, and nuclear crowding—they vary in their clinical presentation. Although BD typically presents as a well-demarcated, scaly plaque on keratinized penile skin, EQ is characterized by moist, red plaques located on the mucosal surfaces of the glans and prepuce. By contrast, BP typically presents with multiple, flesh-colored papillomatous papules on the penile shaft, glans, or foreskin.1
Because the risk of progression from PIN to invasive carcinoma is estimated to be between 10% and 30%, PIN has classically required surgical treatment.2 However, the resulting morbidity from surgical interventions has led to the judicious use of non-invasive alternatives to achieve efficacy while preserving appearance and function. The chemotherapeutic agent 5-fluorouracil (5-FU) has since become a popular, first-line topical treatment option. However, despite early cohort studies reporting response rates approaching 100% at 5 years, a more recent study presenting 42 cases of PIN treated with 5-FU found that only 50% and 31% of patients achieved a complete and partial response, respectively.3 Thus, the demand for better cure rates for PIN persists.
Although both cryotherapy and imiquimod have been used to treat PIN, their efficacy as monotherapies is rather tepid. A study of 299 patients with extragenital BD found a greater risk of recurrence after cryotherapy (13.4%) compared with 5-FU (9%) and surgical excision (5.5%).4 Similarly, a recent literature review highlighting 48 cases of PIN treated with imiquimod monotherapy found the complete response rate to be 63% and the no-response rate to be 29% after an average of 8.4 months (range, 1-48 months).5 However, because cryotherapy and imiquimod have separate mechanisms of action (the former exerts its therapeutic effect by inducing cellular apoptosis, whereas imiquimod enhances cell-mediated immune responses), we hypothesized that combining the 2 therapies might have a complementary effect. Thus, we report our experience with 8 cases of PIN treated with combined cryotherapy and topical imiquimod.
Materials and methods
A retrospective case study was performed on patients with PIN diagnosed from January 2010 to March 2017 by the Dermatology service at Memorial Sloan Kettering Cancer Center. Only cases that were subsequently treated with cryotherapy were included. The following clinical characteristics were assessed: age, anatomic location, lesion type, human papilloma virus status, HIV status, circumcision status, time to clinical regression, other concurrent treatment modalities, and follow-up time.
Results
Eight cases were identified that met the aforementioned inclusion criteria. Table I summarizes their relevant clinical history. The mean age was 45.1 years (range, 26-65). The clinical presentations included BD (5 cases), EQ (2 cases) and BP (1 case), all of which were histopathologically confirmed. Of the 5 cases tested for HPV, all were found to be positive for the high-risk subtypes HPV 16/18. Two patients had concurrent squamous cell carcinoma involving the rectal and anal skin (cases 2 and 6), and in 3 cases, the patients had HIV and were treated with highly active antiretroviral therapy concomitantly (cases 1, 2, and 6).
Table I.
Patient clinical information
| Patient no. | Age | Anatomic location | Lesion type | HPV status | HIV status | Foreskin | No. of cryotherapy sessions | Imiquimod | 5-FU | Other treatments | Time to clearance (mo) | Follow-up (mo) | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | Shaft | EQ | Not tested | + | C | 11 | 2-5 times per wk for 12 mo | bid for 2 wks | None | 39 | 12 | No |
| 2 | 50 | Foreskin, shaft | BD | HPV 16/18 | + | UC | 2 | 3-5 times per wk for 2 mo | bid for 2 wks | Circumcision | 17 | 40 | No |
| 3 | 28 | Base and shaft | BP | HPV 16/18 | − | C | 5 | 2-5 times per wk for 7 mo | None | Mohs | 14 | 28 | No |
| 4 | 54 | Shaft and glans | EQ | Not tested | − | UC | 6 | 1-5 times per wk for 10 mo | None | None | 12 | 26 | No |
| 5 | 38 | Shaft | BD | Not tested | − | C | 3 | 3-5 times per wk for 4 mo | None | None | 5 | 5 | No |
| 6 | 60 | Shaft | BD | HPV 16/18 | + | C | 6 | 1-5 times per wk for 13 mo | bid for 2 wks | None | 20 | 59 | No |
| 7 | 40 | Shaft and glans | BD | HPV 16/18 | − | UC | 4 | 2-5 times per wk for 6 mo | bid for 2 wks | Laser, circumcision | 17 | 7 | No |
| 8 | 26 | Shaft | BD | HPV 16/18 | − | C | 4 | 3 times weekly for 8 mo | None | None | 4 | 24 | No |
5-FU, 5-fluorouracil; BP, Bowenoid papulosis; BD, Bowen disease; EQ, erythroplasia of Queyrat; C, circumcised; UC, uncircumcised.
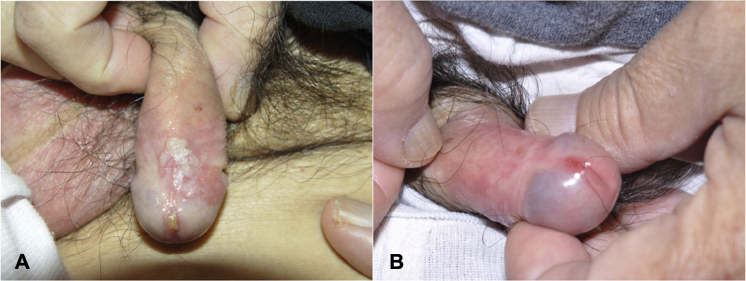
All 8 patients had cryotherapy as frontline therapy. In 1 case (case 3), the patient first had Mohs micrographic surgery at an outside health care facility, but because the lesion persisted, cryotherapy was initiated at our institution. In all cases, the recommended treatment was in-office cryotherapy (2 freeze/thaw cycles consisting of 10 seconds of funneled spray application of liquid nitrogen per lesion per cycle) followed by at-home treatment with topical imiquimod 3 to 5 times a week for at least 8 weeks; the frequency of imiquimod administration was reduced to 1 to 3 times per week in the maintenance phase of the treatment course to prevent recurrence. The average number of cryotherapy sessions administered was 5.1 (range, 2-11). The average number of months using topical imiquimod was 7.8 months (range, 2-13). All patients exhibited a complete response to therapy; additionally, during the follow-up period of 59 months (range, 5-59 months), none of the patients exhibited signs of recurrence. Of note, at the conclusion of the case study, cases 1 and 4 were still undergoing maintenance therapy. The successful resolution of PIN in patient 1 is depicted in Fig 1.
Fig 1.
Patient 1. A, Before treatment. EQ lesion on the shaft and glans of the penis of a 65-year-old man. B, After the combination therapy of cryotherapy (11 sessions) and imiquimod (12 months after initiation of treatment).
Other concurrent treatment modalities used included 5-FU twice daily for 2 weeks (cases 1, 2, 6, and 7), elective circumcision (cases 2 and 7), and laser therapy (case 7). The median follow-up was 24 months (range, 5-59 months). The median time to clearance was 15 months (range, 4-39 months). In addition, patients were advised to use concurrent hydrocortisone 2.5% cream, petroleum jelly, ketoconazole cream, and mupirocin ointment during the treatment period to decrease skin irritation. Although patients reported mild redness and irritation at the site of imiquimod application, no severe skin reactions were reported.
Discussion
PIN has a 10% to 30% rate of advancement to SCC; moreover, the metastatic potential after advancement to invasive malignancy is 3% to 5%.2, 6 In an effort to eradicate PIN while limiting anatomic disfigurement and loss of function, clinicians have sought alternatives to surgical excision, including laser ablation, Mohs micrographic surgery, electrodessication and curettage, cryotherapy, and chemotherapeutic/immune modulating topical creams (5-FU and imiquimod). Although the 2014 British Association of Dermatologists' recommendations for the management of PIN is helpful in guiding the choice of such noninvasive therapy, there is no gold standard treatment for PIN, nor are there randomized, controlled trials available upon which to base treatment decisions.7, 8 Moreover, given the limited efficacy of the most common noninvasive treatment modality, 5-FU, a clear need for a viable, robust and noninvasive treatment option for PIN remains.3
Both cryotherapy and topical imiquimod have been evaluated as monotherapies for PIN in recent years. Cryotherapy exerts its therapeutic effect by inducing ice crystal formation, vascular stasis, and, ultimately, cellular apoptosis.9 By contrast, imiquimod acts as an immune response modulator by binding the toll-like receptor 7 and elaborating interferon-α, interleukin-1α and interleukin-12 production.10 In a recent review of cryotherapy used for BD (both genital and extragenital), Neubert and Lehmann11 identified that lower recurrence rates could be achieved by longer and repeated freezing cycles but with the increased risk of prolonged wound healing and poor cosmetic outcome such as scaring and hypopigmentation. Similarly, the utility of imiquimod monotherapy can be limited by severe skin reactions including severe erythema, superficial erosions, and tingling/itching/pain at the site of application.5 Moreover, as was mentioned above, the reported efficacies of both cryotherapy and imiquimod monotherapy are limited.4, 5
However, given that cryotherapy and immunotherapy have differing mechanisms of action, we hypothesized that combining the 2 therapies might have a complementary effect. From the molecular perspective, there seems to be an advantage to combining these 2 methods; while cryotherapy exerts its therapeutic effect by inducing a cellular stress response and apoptosis, imiquimod simultaneously enhances cell-mediated immune responses to maximize clearance of dysplastic cells. Additional advantages to combining said therapies include that both are easily available, inexpensive, noninvasive, and lack severe systemic toxicity.
Importantly, the clearance rate in our case series exceeds that of the individual monotherapies. Although it is possible that our robust clearance rate may be attributed to the concomitant use of 5-FU or laser therapy in 4 of 8 patients, the limited use of these added therapies (5-FU was applied for a maximum of 2 weeks in 4 patients, 1 session of laser therapy was used in 1 patient) suggests that the observed improvement in clearance rate is at least in part owing to the synergistic effects of cryotherapy and imiquimod. Additionally, none of our patients reported any severe skin irritation secondary to imiquimod application. We speculate that the reason for this favorable response is 2-fold: not only were our patients instructed to wait at least 3 to 4 days after cryotherapy before applying the imiquimod, but we advised them to use hydrocortisone and skin moisturizer during the treatment period to ameliorate any skin irritation. Given the favorable response and purported benefits of combination therapy, we propose the use of cryotherapy with topical imiquimod in the treatment of PIN. Given that this is a small case series, we plan to validate this finding in a larger cohort.
Footnotes
Funding sources: Memorial Sloan Kettering Cancer Center Dermatology Research Fund.
Conflicts of interest: None declared.
References
- 1.Porter W.M., Francis N., Hawkins D., Dinneen M., Bunker C.B. Penile intraepithelial neoplasia: clinical spectrum and treatment of 35 cases. Br J Derm. 2002;147:1159–1165. doi: 10.1046/j.1365-2133.2002.05019.x. [DOI] [PubMed] [Google Scholar]
- 2.Arya M., Kalsi J., Kelly J., Muneer A. Malignant and premalignant lesions of the penis. BMJ. 2013;346:f1149. doi: 10.1136/bmj.f1149. [DOI] [PubMed] [Google Scholar]
- 3.Alnajjar H.M., Lam W., Bolgeri M., Rees R.W., Perry M.J., Watkin N.A. Treatment of carcinoma in situ of the glans penis with topical chemotherapy agents. Eur Urol. 2012;62(5):923–928. doi: 10.1016/j.eururo.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 4.Hansen J.P., Drake A.L., Walling H.W. Bowen's disease: a four-year retrospective review of epidemiology and treatment at a university center. Dermatol Surg. 2008;34(7):878–883. doi: 10.1111/j.1524-4725.2008.34172.x. [DOI] [PubMed] [Google Scholar]
- 5.Deen K., Burdon-Jones D. Imiquimod in the treatment of penile intraepithelial neoplasia: An update. Australas J Dermatol. 2017;58(2):86–92. doi: 10.1111/ajd.12466. [DOI] [PubMed] [Google Scholar]
- 6.Kao G.F. Carcinoma arising in Bowen's disease. Arch Dermatol. 1986;122:1124–1126. [PubMed] [Google Scholar]
- 7.Morton C.A., Whitehurst C., Moore J. Photodynamic therapy vs cryotherapy in the treatment of Bowen's disease. Clin Exp Dermatol. 1996;21:79. [PubMed] [Google Scholar]
- 8.Cox N.H., Eedy D.J., Morton C.A. Guidelines for management of Bowen's disease: 2006 update. Br J Dermatol. 2007;156:11–21. doi: 10.1111/j.1365-2133.2006.07610.x. [DOI] [PubMed] [Google Scholar]
- 9.Baust J.G., Gage A.A. The molecular basis of cryosurgery. BJU Int. 2005;95(9):1187–1191. doi: 10.1111/j.1464-410X.2005.05502.x. [DOI] [PubMed] [Google Scholar]
- 10.Hengge U.R., Benninghoff B., Ruzicka T. Topical immunomodulators – progess treating inflammation, infection and cancer. Lancet Inf Dis. 2001;1:189–198. doi: 10.1016/s1473-3099(01)00095-0. [DOI] [PubMed] [Google Scholar]
- 11.Neubert T., Lehmann P. Bowen's disease – a review of newer treatment options. Ther Clin Risk Manag. 2008;4(5):1085–1095. [PMC free article] [PubMed] [Google Scholar]