Introduction
The molecular classification of melanomas, which can have diverse clinical and histopathologic features, is defined by the acquisition of somatic mutations. Mutations such as BRAF V600E result in constitutive activation of critical signaling pathways that promote formation of melanocytic nevi.1 Acquisition of subsequent mutations induces the progression to melanomagenesis, and further accumulation of tertiary mutations might promote metastasis. These molecular pathways remain largely undiscovered. Here we describe the use of the Stanford solid tumor actionable mutation panel (STAMP), a targeted next-generation sequencing (NGS) panel comprising 130 genes selected on the basis of their known impact as actionable targets of existing and emerging anti-cancer therapies, prognostic features, and mutation recurrence frequency across patients with known cancer types, including melanoma.
Case report
A 75-year-old man with stage III anorectal mucosal melanoma had a lung metastasis, and both lesions were subjected to STAMP. The primary lesion was initially diagnosed during a hemorrhoidectomy procedure and consisted of a 1.2 × 0.6–cm polypoid, ulcerating mass with histologic findings of diffusely atypical melanocytes (Fig 1). The results of NGS demonstrated that the primary and metastatic lesion were identical with the exception of 1 detectable mutation in PTEN c.892C>T, p. Gln298Ter (Table I), resulting in a truncation. PTEN has previously been identified as a gene that might have mutations present in advanced and metastatic cutaneous melanoma but has not been described in anorectal melanoma.1, 2
Fig 1.
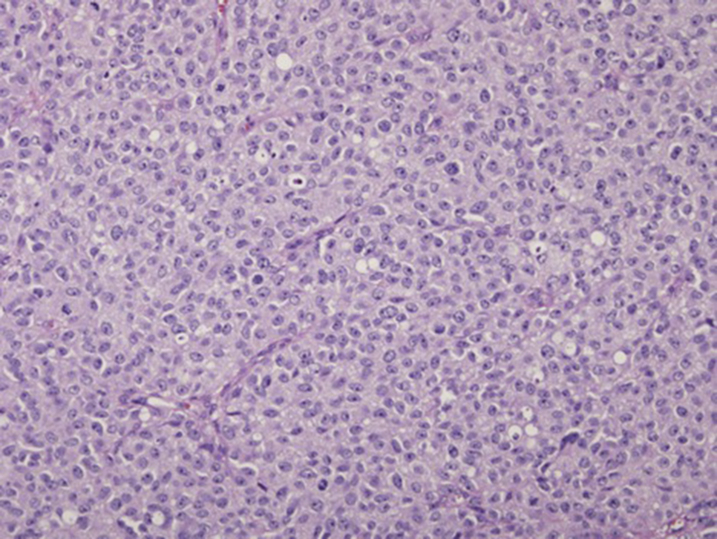
Invasive anorectal melanoma. Anorectal invasive melanoma demonstrates a diffuse proliferation of atypical melanocytes with abundant amphophilic cytoplasm and nuclear polymorphism. (Hematoxylin-eosin stain; original magnification: ×200.)
Table I.
Mutations in stage III primary mucosal anorectal melanoma and metastatic lung melanoma identified by next-generation sequencing of targeted and actionable mutations
| Gene | Variant |
|---|---|
| Known pathogenic significance | |
| PTEN∗ | p.Gln298Ter |
| NF1 | p.Met442fs |
| SF3B1 | p.Arg625His |
| Unknown significance | |
| BCR | p.Val949Ile |
| BRCA1 | p.Arg841Trp |
| MET | c.2941+49T>G |
| NFE2L2 | p.Leu266Phe |
| PTCH1 | p.Gly15_Gly17del |
Mutation present in metastatic lesion only.
Discussion
In a series of anorectal melanomas, Yang et al2 found anorectal melanomas have driver mutations in KIT, NF1, SF3B1, TP53, HRAS, BRAF, and MLH1. In a separate study of cutaneous melanoma, Shain et al1 proposed a model for the progression of cutaneous melanomas from benign nevi and found common driver mutations in BRAF, NRAS, TERT, CDKN2A, NF1, HRAS, and the ARID gene family with TP53 and PTEN mutations identified in more advanced to metastatic melanoma. Through NGS, we identified a mutation in metastatic anorectal melanoma, supporting a potential global role of PTEN in promoting melanoma metastasis.
In addition, 5 variants of unknown significance (BCR p.Val949Ile, BRCA1 p.Arg841Trp, MET c.2941+49T>G, NFE2L2 p.Leu266Phe, and PTCH1 p.Gly15_Gly17del) were identified in both primary and metastatic lesions (Table I). Overexpression of MET has previously been associated with metastatic melanoma, and this association was replicated in mouse models.3 Mutations in BRCA1 and PTCH1 have been previously described in melanoma but without detailed investigation of their role in the progression from benign nevi to melanoma and metastasis.4 Higher NFE2L2 expression has been associated with worse prognosis in melanoma,5 but analysis of novel NFE2L2 mutations in melanoma has not been conducted. There are no previous reportss on the association between mutations in BCR and melanoma. The identification of additional mutations in metastasis supports the role of NGS to guide personalized therapy for advanced lesions.
Targeted NGS presents a new opportunity to expand existing knowledge of mutations for various subtypes of melanoma to further define the mechanisms of initiation and progression. This case identifies a mutation, PTEN p. Gln298Ter (ie, unique to the metastatic site), which might represent the primary driver mutation for melanoma metastasis in this patient. Biopsies of additional metastatic sites were not available in this case; however, identification of PTEN p. Gln298Ter at additional sites would provide strong evidence for the role of PTEN p. Gln298Ter in the development of distal metastasis. This would further support the role of PTEN in driving metastasis in both primary cutaneous and anorectal melanomas.
In the clinical management of the patient in this case, the results of the STAMP panel provided guidance to continue the patient on targeted immunotherapies other than BRAF inhibitors. Current targeted treatment of advanced stage melanoma is primarily dependent on BRAF mutation status.6 In addition, PTEN inactivation has previously been noted to significantly shorten overall survival and time to metastasis to brain and liver in patients with BRAF V600 mutations, as well as increase resistance to BRAF inhibitor therapy.7 However, in patients without BRAF V600 mutations, there was not a significant association between PTEN inactivation and time to brain metastasis or length of overall survival. This BRAF mutation–negative, PTEN p. Gln298Ter patient was started on pembrolizumab, a programmed cell death 1 inhibitor and a first-line therapy for advanced stage melanoma. This patient was also found to harbor a pathogenic mutation in NF1; however, this patient was not enrolled in any experimental NF1-targeted therapies.
This case additionally presents variants in BCR, BCRA1, NFE2L2, PTCH1, and MET that require further investigation to ascertain their role in melanomagenesis and progression, as well as their clinical significance for disease prognosis and treatment. Thus, the study of these new variants holds the promise of promoting the identification of additional drug targets for the treatment of melanoma.
Acknowledgments
We would like to acknowledge Ursula Lang, MD, PhD, for her role in identifying key references for this article.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Shain A.H., Yeh I., Kovalyshyn I. The genetic evolution of melanoma from precursor lesions. New Engl J Med. 2015;373(20):1926–1936. doi: 10.1056/NEJMoa1502583. [DOI] [PubMed] [Google Scholar]
- 2.Yang H.M., Hsiao S.J., Schaeffer D.F. Identification of recurrent mutational events in anorectal melanoma. Mod Pathol. 2017;30(2):286–296. doi: 10.1038/modpathol.2016.179. [DOI] [PubMed] [Google Scholar]
- 3.Otsuka T., Takayama H., Sharp R. c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Res. 1998;58(22):5157. [PubMed] [Google Scholar]
- 4.Monnerat C., Chompret A., Kannengiesser C. BRCA1, BRCA2, TP53, and CDKN2A germline mutations in patients with breast cancer and cutaneous melanoma. Fam Cancer. 2007;6(4):453–461. doi: 10.1007/s10689-007-9143-y. [DOI] [PubMed] [Google Scholar]
- 5.Hintsala H.-R., Haapasaari K.-M., Soini Y., Karihtala P. An immunohistochemical study of NFE2L2, KEAP1 and 8-hydroxy-2'-deoxyguanosine and the EMT markers SNAI2, ZEB1 and TWIST1 in metastatic melanoma. Histol Histopathol. 2017;32(2):129–136. doi: 10.14670/HH-11-778. [DOI] [PubMed] [Google Scholar]
- 6.Luke J.J., Flaherty K.T., Ribas A., Long G.V. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14(8):463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 7.Bucheit A.D., Chen G., Siroy A. Complete loss of PTEN protein expression correlates with shorter time to brain metastasis and survival in stage IIIB/C melanoma patients with BRAF V600 mutations. Clin Cancer Res. 2014;20(21):5527. doi: 10.1158/1078-0432.CCR-14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]


