Abstract
Evidence has shown that multidisciplinary tumor board conferences (MTBCs) improve patient management for various cancer types. However, few retrospective studies have investigated MTBC efficacy for patients with gynecologic cancers. Here, we prospectively aimed to evaluate how MTBCs influence patient management in gynecologic oncology. This prospective study included 85 consecutive cases that were presented at gynecologic oncology MTBCs in our tertiary university hospital between January 2015 and April 2016. The primary endpoint was treatment plan change rate, which included both major and minor changes. Major changes were defined as exchange, addition, or subtraction of treatment modality. Minor changes included all other, such as intramodality changes or treatment time changes. The secondary endpoints were the change rates of diagnosis, diagnostic work-up, and radiological and pathological findings.
The treatment plan change rate, irrespective of changes in diagnostic work-up, was 27.1%, which included 10.6% major and 16.5% minor changes. Among the treatment plan changes, changes in the treatment plan change rate alone were noted in 16.5% of cases, and changes in diagnosis and radiological findings occurred in 7.1% and 3.5% of cases, respectively. Diagnosis and radiological findings, irrespective of changes in diagnostic work-up, were also changed in 9.4% and 10.6% of cases, respectively. However, there were no changes in pathological findings. Moreover, there was a change of diagnostic method for further work-up in 23.5% of cases. The implementation rate of MTBC-determined treatment changes was 91.8%. Gynecologic oncology MTBCs resulted in considerable changes in treatment plans. Diagnosis, diagnostic work-up, and radiological findings were influenced by MTBCs. The data emphasize the importance of adopting a multidisciplinary team approach for gynecologic cancer management.
Keywords: conference, diagnosis, diagnostic techniques and procedures, oncology, therapeutics
1. Introduction
Theoretically, a multidisciplinary team approach is essential for quality control of cancer management. Evidence has suggested that a multidisciplinary team approach improves patient management for various cancers.[1,2] For breast cancer especially, a multidisciplinary team approach is considered the standard of care, globally.[3] Some prospective studies have demonstrated that multidisciplinary tumor board conferences (MTBCs) significantly influence the treatment plans or diagnoses of patients with various cancers.[4–8] In these studies, the treatment plan change rate after MTBC ranged from 18.5% to 36%,[4–8] and the diagnostic change rate after MTBC ranged from 11.0% to 14.5%.[4,5]
In gynecologic oncology, implementation of a multidisciplinary team approach is becoming more prevalent. However, only a few retrospective studies have investigated the influence of MTBCs on patients with gynecologic cancers.[9–11] In these studies, treatment plan changes occurred in 4.8% to 19.8% of patients with diagnostic changes, and the addition of chemotherapy or surgery was the most frequent treatment change.
We hypothesized that MTBCs might induce significant treatment plan changes in patients with gynecologic cancers. The purpose of this study was to prospectively evaluate the influence of MTBCs on the management of patients with gynecologic cancers using treatment plan change as the primary outcome measure and changes in diagnosis, diagnostic work-up, and radiological and pathological findings as secondary outcome measures.
2. Methods
This prospective study included 85 consecutive patients whose information was presented at gynecological oncology MTBCs of Seoul National University Bundang Hospital between January 2015 and April 2016. This study was approved by the Institutional Review Board of our tertiary university hospital (No. B-1411-274-006) on December 10, 2014. The inclusion criterion was that patient information was presented to the MTBC via their gynecological oncologist because of a gynecologic cancer or tumor. Cases with preconference treatment plans and diagnoses were presented to the MTBC. Patients who did not agree with the use of their information were excluded, although their cases were discussed in the MTBC. Written informed consent was obtained for all included cases.
Multidisciplinary tumor board conferences usually occurred weekly or biweekly for a duration of 30 minutes. Four gynecologic oncologists, 2 radiologists, a nuclear medicine physician, 2 pathologists, and fellows training in their departments attended as baseline members. Moreover, special experts in fields related to gynecologic oncology (eg, radiation oncologists, urologists, general surgeons, and orthopedists, among others) were invited to the MTBCs whenever their opinions were required. Gynecologic oncologists who were responsible for establishing the initial diagnosis and treatment plan referred cases to the MTBCs to evaluate the treatment plan, diagnosis, and/or diagnostic method. Newly diagnosed cases were presented to the MTBCs after standardized baseline work-ups. In our gynecologic department, slides for all cases transferred from other hospitals are reassessed by the pathologists of our hospital who are specialized in gynecologic pathology. Therefore, cases with pathologic findings that were referred to the MTBCs had been evaluated by the pathologists who were members of the MTBCs. Information about each case for the MTBCs was delivered to radiologists, pathologists, and special experts 3 days before each conference.
For data collection, a gynecologic oncology fellow and the faculty member who referred each case completed a case report form. After the MTBC, case report forms were compared and recorded in their final form. Each patient had a preconference treatment plan, diagnosis, and diagnostic work-up. Irrespective of indications presented to the MTBC, treatment plan, diagnosis, and diagnostic work-up were discussed for all cases. Radiographic imaging and pathological findings were reviewed by specialists and discussed by the attending physicians. After the MTBC, any changes in treatment plan, diagnosis, diagnostic work-up, radiographic imaging, and pathologic findings were recorded.
An efficacy assessment was conducted for all cases. The primary outcome was the treatment plan change rate. The secondary outcomes included the change rates of diagnosis, diagnostic work-up, and radiological and pathological findings.
Treatment plan changes were defined as changes from the initial treatment modalities that were planned by the treating gynecologic oncologist to any altered treatment modalities recommended at the MTBC. Treatment plan changes were classified as major or minor. Major changes were defined as an exchange in treatment modality (eg, radiotherapy to surgery) or the addition or subtraction of a treatment modality (eg, surgery alone to surgery plus chemotherapy). Minor changes included any changes that were not major changes, such as intramodality changes (eg, change in chemotherapy regimen or period, or change in method or extent of surgery or radiotherapy) and other specific changes (eg, change in treatment time, such as delays in treatment initiation, or transfer to other departments).
Diagnostic changes were defined as any changes from the initial diagnosis in tumor type, site, grade, or stage. More severe diagnostic changes included increases in the diagnosed disease severity (eg, upstaging or recurrence). Less severe diagnostic changes included decreases in the diagnosed disease severity (eg, recurrence to nonrecurrence; malignant to benign). Diagnostic work-up changes were defined as changes in any additional diagnostic methods recommended at the MTBC. In such cases, a treatment plan was recommended during the same conference depending on the expected diagnosis from the additional diagnostic work-up. For these cases, we identified which treatment plan was applied according to the final diagnosis after the additional diagnostic work-up. Therefore, calculation of treatment plan change rate only included cases in which the treatment plan changed definitively after additional diagnostic work-up (as opposed to the cases in which treatment plan changes were proposed based on projected future test results, but that were not altered after additional work-up). Changes in radiological and pathological findings included any changes in findings.
2.1. Sample size
In patients with gynecologic cancers, data concerning treatment plan changes concomitant with diagnostic changes were available from 3 retrospective studies.[9–11] Of these studies, only that by Cohen et al[10] reported treatment plan changes (5.9%) in cases with diagnostic changes based on a review of the radiological and pathological findings. Other studies reported treatment plan changes (4.8%–19.8%) in cases with diagnostic changes based on a review of the pathological findings alone.[9,10] Therefore, the result (P) from the study by Cohen et al was used to estimate the expected magnitude of change.[10] A precision (D) of 5% was used. Our necessary sample size was calculated as follows: N = 1.962 × P × (1 − P)/D2 = 1.962 × (0.059) × (1 − 0.059)/(0.05)2.[12] As a result, 85 cases were included in the trial.
3. Results
A mean of 3.3 ± 1.3 cases were discussed at each of the 36 MTBCs. Of the 117 cases referred to the MTBC, 85 (72.6%) were enrolled in this study after obtaining informed consent. Study cases were presented at the MTBCs to determine treatment plan (54 [63.5%]), diagnosis (18 [21.2%]), diagnostic method (2 [2.4%]), diagnosis and treatment plan (10 [11.8%]), or diagnostic method and treatment plan (1 [1.2%]). Radiological findings for all cases and pathological findings for the 63 (74.1%) cases with pathology findings were reviewed.
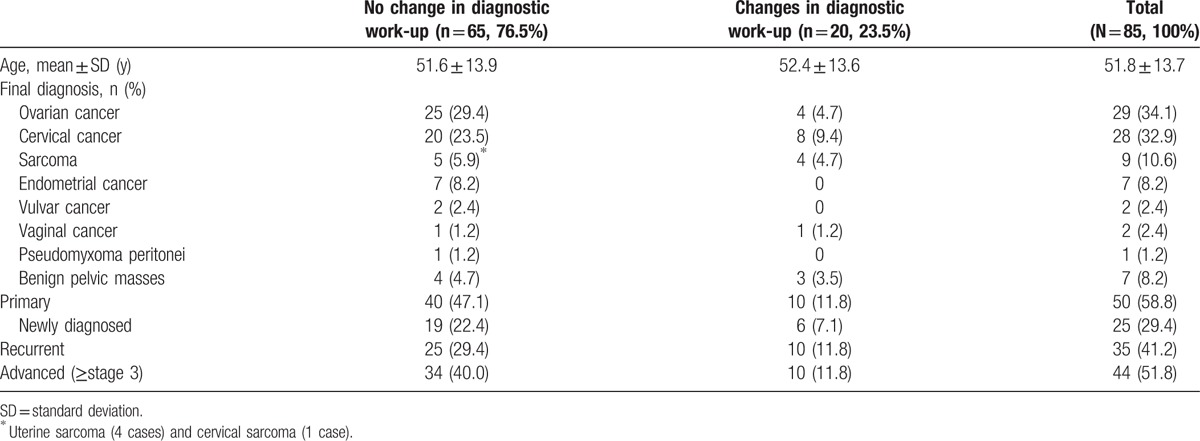
The patients’ characteristics are shown in Table 1. Ovarian cancer was the most common cancer type, followed by cervical cancer, sarcoma, endometrial cancer, vulvar cancer, vaginal cancer, and pseudomyxoma peritonei. Seven cases of benign pelvic masses were also included. Study cases included 50 (58.8%) primary cancers cases, including 25 (29.4%) newly diagnosed cases and 35 (41.2%) recurrent cancer cases.
Table 1.
Characteristics of study patients.
Changes after MTBC are shown in Fig. 1 and Table 2. Of the 23 (27.1%) cases with changes in treatment plan, irrespective of changes in diagnostic work-up, 14 (16.5%) involved changes in treatment plan alone. Changes in both diagnosis and treatment plan occurred in 6 (7.1%) cases, and changes in both radiological findings and treatment plan occurred in 3 (3.5%) cases. Changes in treatment plan alone without diagnostic work-up changes occurred in 11 (12.9%) cases. Diagnoses and radiological findings, irrespective of changes in diagnostic work-up, were changed in 8 (9.4%) and 9 (10.6%) cases, respectively. Changes in both diagnosis and radiological findings, without changes in treatment plan, occurred in 1 (1.2%) case. There were no changes in pathologic findings. Moreover, changes in diagnostic work-up occurred in 20 (23.5%) cases. In these cases, changes in treatment plan alone and changes in both diagnosis and treatment plan occurred in 3 (3.5%) cases each. Diagnoses and radiological findings were also changed in 3 (3.5%) cases each (Fig. 1 and Table 2).
Figure 1.
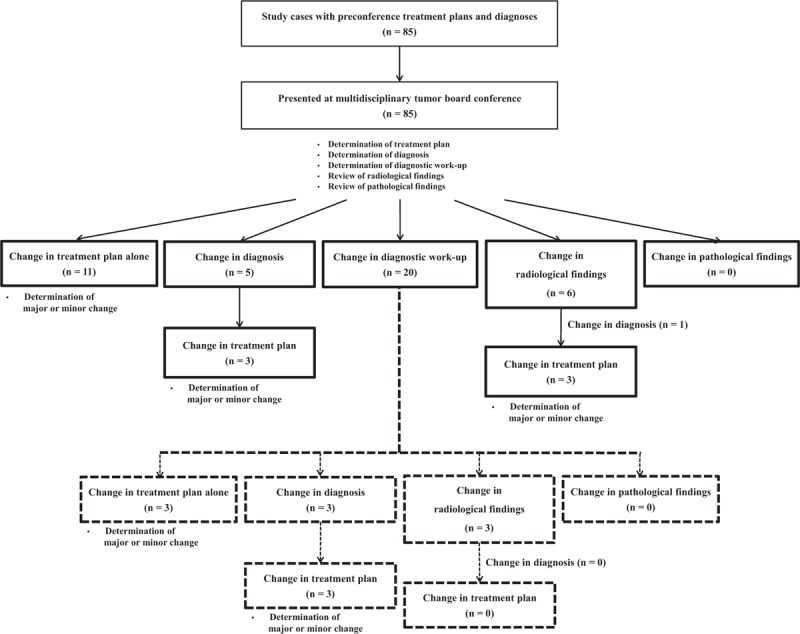
Outcomes of multidisciplinary tumor board conferences.
Table 2.
Description of significant determinations during multidisciplinary tumor board conferences.
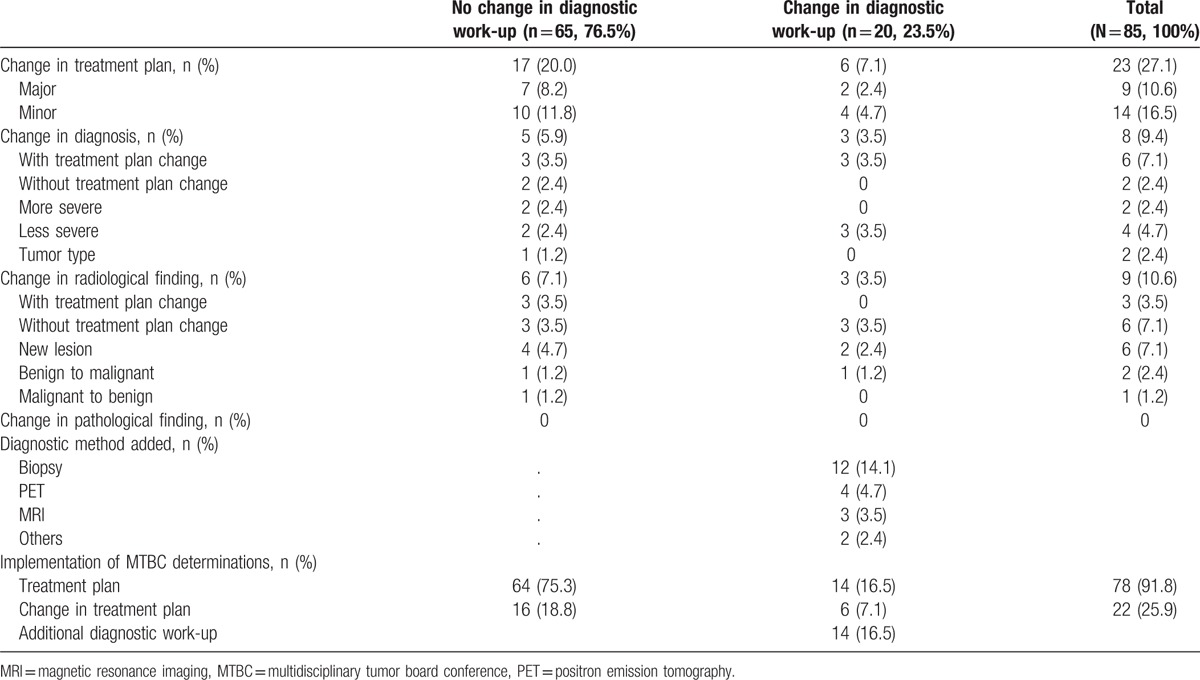
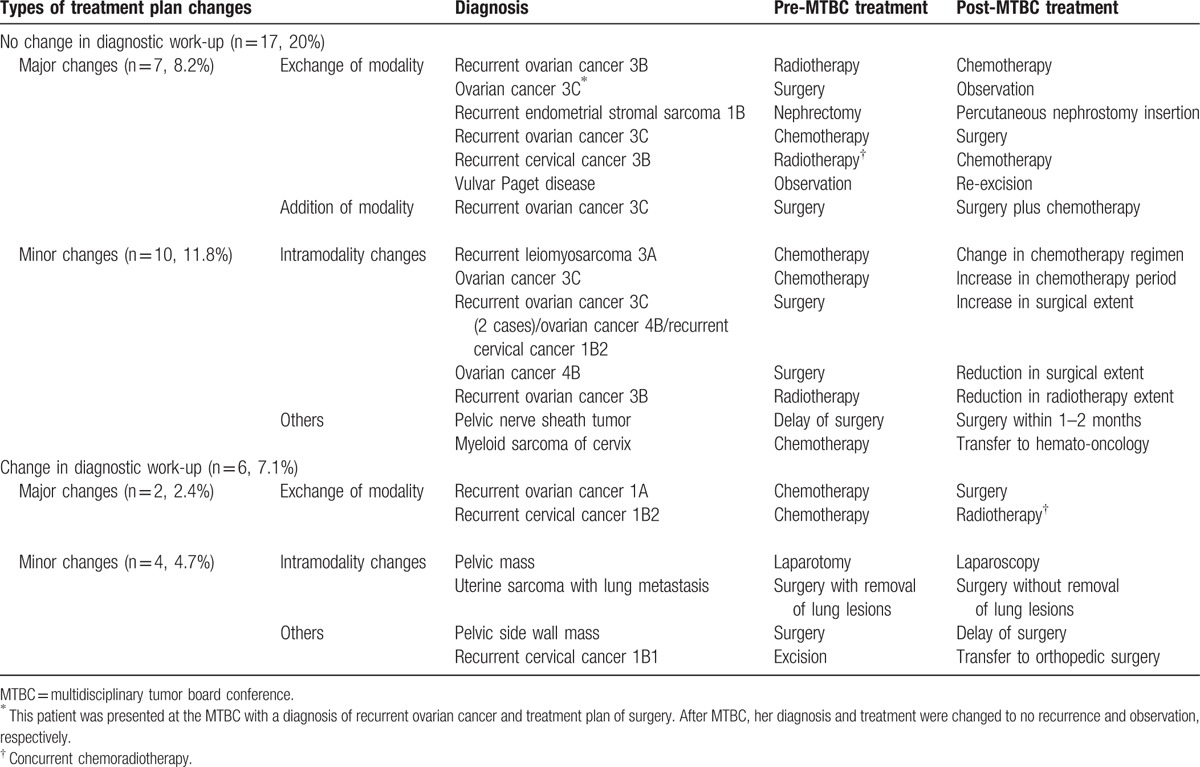
Minor treatment plan changes occurred more frequently than major changes. For major treatment plan changes, modality exchange was the most common change, followed by addition of a modality (Tables 2 and 3). Diagnostic changes without diagnostic work-up changes included changes to a more severe disease diagnoses (1 case each of stage 1 to stage 3, and nonrecurrence to recurrence), changes to a less severe disease diagnoses (1 case each of recurrence to nonrecurrence, and intestinal perforation to non-perforation), and 1 case of a change in diagnosis from cervical cancer to endometrial cancer. Diagnostic changes after diagnostic work-up changes included changes to a less severe disease alone (1 case of metastasis to primary cancer and 2 cases of cancer to benign disease). Upon radiographic imaging review, new lesion detection was the most common change, followed by changes in diagnosis from benign to malignant disease or from malignant to benign disease. Biopsy was the most commonly recommended additional diagnostic method, followed by positron emission tomography, magnetic resonance imaging (MRI), and others. In cases without diagnostic work-up changes, 1 (1.2%) case of treatment plan change was not implemented because of patient death. In 2 (2.4%) cases, after making the decision for diagnostic work-up changes, additional diagnostic work-up was not implemented resulting in failures to perform the treatment plan because it was impossible to conduct a lesion biopsy. Moreover, in 4 (4.7%) cases, for which additional diagnostic work-ups were recommended, they were not implemented, resulting in the adoption of pre-MTBC treatment plans because the patient did not accept the MTBC determinations (Table 2).
Table 3.
Summary of treatment plan changes.
4. Discussion
In the present prospective trial, we evaluated 85 consecutive cases who were presented at the gynecologic oncology MTBCs of a tertiary university hospital. After MTBCs, a large number of treatment plan changes were made, irrespective of changes in diagnosis, diagnostic work-up, radiological findings, or pathological findings. Moreover, a portion of diagnoses and an especially large portion of diagnostic methods were changed. Interestingly, some radiological findings were changed, whereas there were no changes in pathologic findings.
In the present study, treatment plan, diagnosis, or diagnostic work-up changes occurred in 42.4% of cases presented at MTBCs. This proportion of changes was greater compared with that in a previous prospective study in patients with head and neck tumors, which reported a change of 26.7% in treatment, diagnosis, or diagnostic work-up after MTBCs.[4] This discrepancy might be due to the use of different definitions for treatment plan changes between the studies. The present study included any treatment plan changes undertaken after MTBCs, whereas the previous study included only major changes such as exchange, addition, or subtraction of treatment modality.[4]
In the present study, the initial treatment plan was changed in 27.1% of cases, which was similar to the results of previous prospective studies for various cancers (18.5%–36%).[4–8] Of those treatment plan changes, minor changes, such as intramodality changes and other specific changes in initial treatment, occurred more commonly than major changes (60.9% vs 39.1%). By contrast, a previous prospective study in patients with upper gastrointestinal tract malignancies reported major and minor change proportions of 87.4% and 12.6%, respectively.[6] These discrepancies might be attributed to differences in the proportions of patients with recurrent disease between the studies (present vs previous study, 41.2% vs 13.1%).[6] Moreover, in the present study, treatment modality exchange was the most common major change, which differs from a previous prospective study in patients with head and neck tumors, which reported that treatment modality addition was the most common major change.[4] These discrepancies likely reflect differences in the proportion of patients with newly diagnosed disease between the studies (present vs previous study, 29.4% vs 100%).[4]
In the present study, treatment plan changes alone and changes in both treatment plan and diagnosis, irrespective of changes in diagnostic work-up, corresponded with the changes reported in prospective studies in patients with nongynecologic malignancies (16%–23.4% and 3%–8.9%, respectively), such as head and neck cancer or urologic malignancies.[4,5] Moreover, our finding had similar results to previous retrospective studies in patients with gynecologic cancers who reported 4.8% to 19.8% changes in both treatment plan and diagnosis.[9–11]
Previous prospective studies involving patients with nongynecologic malignancies and retrospective studies of patients with gynecologic cancers reported post-MTBC diagnostic changes of 11.0% to 14.5% and 9% to 21.5%, respectively,[4,5,9–11] which were similar to the findings of the present study.
In the present study, further diagnostic work-ups were recommended after MTBCs in 23.5% of cases, which was similar to the 7% to 32.5% change reported in previous prospective studies in patients with nongynecologic malignancies.[4–6] Additionally, the 7.1% change in both diagnostic work-up and treatment plan in the present study was comparable to that reported in patients with upper gastrointestinal malignancies (11.5%).[6]
A previous prospective study involving patients with primary rectal cancer reported an 11.9% treatment plan change that was attributed to changes in MRI findings.[7] Retrospective studies performed in patients with gynecologic cancers revealed a 3.2% to 10% change in radiological findings and a 5.9% to 27% change in pathological findings. Moreover, these radiological and pathological changes resulted in treatment plan changes in 1.4% and 4.5% to 19.8% of cases, respectively.[10,11] In the present study, the change in radiological findings, irrespective of treatment plan changes, was similar to those from previous studies. However, in contrast to previous studies, there were no changes in pathological findings in this study. This lack of changes might be because the pathologists in the present study have specialized expertise in gynecologic malignancies, and all cases were reviewed by same pathologists initially and at the MTBCs.
In the present study, 91.8% of MTBC determinations for treatment were implemented, which was similar to the 91.5% change reported on a large-scale retrospective study of patients with breast cancer.[3] A small-scale prospective study of patients with primary rectal cancer reported 100% compliance with the MTBC treatment recommendations.[7] Moreover, in the present study, the proportion of patients in which the MTBC determinations for additional diagnostic work-up and treatment plan were not implemented, due to patient choice, was comparable to that reported for patients with breast cancer (∼2%).[3]
In the present study, each case was evaluated for a mean of 9 minutes, although discussion time varied according to the case complexity. This duration was similar to a prospective study conducted in patients with gastrointestinal malignancies, which had a median discussion time of 3 minutes (range 0–8 minutes) after a presentation time of 3 minutes (range 1–9 minutes).[8]
Although some evidence suggests that a multidisciplinary team approach is beneficial for the management of various cancers, there are concerns about treatment delays associated with MTBCs.[6,13,14] Therefore, in the present study, gynecologic oncologists referred cases to the MTBC to reduce any treatment delays, irrespective of when informed consent was obtained from patients. When patients provided consent, they were enrolled in the present trial, which was distinct from the discussion in the MTBC.
The present study had several limitations. First, the sample size was calculated based on cases with treatment plan changes concomitant with diagnostic changes because there were no previous studies reporting a total treatment plan change in patients with gynecologic cancers. Second, because of the nature of the study, there was no control group of patients treated without discussion in the MTBC. Third, selection bias might have occurred because gynecologic oncologists selected which cases were referred to the MTBC. Fourth, decisions by MTBC might depend on personalities and preferences of attending physicians, making different recommendations for same cases according to different MTBCs. To minimize this, many physicians with various specialities attended to our MTBC and every decision was principally evidence based. For example, gynecologic oncologists basically followed the National Comprehensive Cancer Network guidelines.
Finally, prognostic outcomes according to the changes implemented after the MTBCs were not evaluated. We have planned an additional study to evaluate the prognostic influence of the MTBC over a long assessment period.
5. Conclusions
In conclusion, we prospectively demonstrated that the MTBCs led to significant alterations in treatment plans in many patients with gynecologic cancers. The influence of the gynecologic oncology MTBC was reflected in changes in diagnosis, diagnostic work-up, and radiological findings. These findings were comparable with those reported in previous prospective studies for other cancer types. Further large-scale prospective trials are warranted to evaluate the prognostic significance of gynecologic oncology MTBCs.
Acknowledgments
We are grateful to the clinical research coordinators (Seungah Woo, Soyun Joo, and Eunha Kim) at Seoul National University Bundang Hospital for their contributions, including obtaining informed consent.
Footnotes
Abbreviation: MTBCs = multidisciplinary tumor board conferences.
BL and KK contributed equally to this work and are co-first authors.
The authors report no conflicts of interest.
References
- [1].Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ 2012;344:e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev 2016;42:56–72. [DOI] [PubMed] [Google Scholar]
- [3].Rajan S, Foreman J, Wallis MG, et al. Multidisciplinary decisions in breast cancer: does the patient receive what the team has recommended? Br J Cancer 2013;108:2442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wheless SA, McKinney KA, Zanation AM. A prospective study of the clinical impact of a multidisciplinary head and neck tumor board. Otolaryngol Head Neck Surg 2010;143:650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kurpad R, Kim W, Rathmell WK, et al. A multidisciplinary approach to the management of urologic malignancies: does it influence diagnostic and treatment decisions? Urol Oncol 2011;29:378–82. [DOI] [PubMed] [Google Scholar]
- [6].van Hagen P, Spaander MC, van der Gaast A, et al. Impact of a multidisciplinary tumour board meeting for upper-GI malignancies on clinical decision making: a prospective cohort study. Int J Clin Oncol 2013;18:214–9. [DOI] [PubMed] [Google Scholar]
- [7].Snelgrove RC, Subendran J, Jhaveri K, et al. Effect of multidisciplinary cancer conference on treatment plan for patients with primary rectal cancer. Dis Colon Rectum 2015;58:653–8. [DOI] [PubMed] [Google Scholar]
- [8].Oxenberg J, Papenfuss W, Esemuede I, et al. Multidisciplinary cancer conferences for gastrointestinal malignancies result in measureable treatment changes: a prospective study of 149 consecutive patients. Ann Surg Oncol 2015;22:1533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Santoso JT, Schwertner B, Coleman RL, et al. Tumor board in gynecologic oncology. Int J Gynecol Cancer 2004;14:206–9. [DOI] [PubMed] [Google Scholar]
- [10].Cohen P, Tan AL, Penman A. The multidisciplinary tumor conference in gynecologic oncology: does it alter management? Int J Gynecol Cancer 2009;19:1470–2. [DOI] [PubMed] [Google Scholar]
- [11].Greer HO, Frederick PJ, Falls NM, et al. Impact of a weekly multidisciplinary tumor board conference on the management of women with gynecologic malignancies. Int J Gynecol Cancer 2010;20:1321–5. [DOI] [PubMed] [Google Scholar]
- [12].Thrusfield MV. Veterinary Epidemiology. 3rd ed.Oxford: Blackwell Science; 2007. [Google Scholar]
- [13].Leo F, Venissac N, Poudenx M, et al. Multidisciplinary management of lung cancer: how to test its efficacy? J Thorac Oncol 2007;2:69–72. [DOI] [PubMed] [Google Scholar]
- [14].Grotenhuis BA, van Hagen P, Wijnhoven BP, et al. Delay in diagnostic workup and treatment of esophageal cancer. J Gastrointest Surg 2010;14:476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


