Abstract
We investigated whether serum CXC ligand 13 protein (CXCL13) levels correlate with the circulating plasmablasts and memory B-cells alteration in systemic lupus erythematosus (SLE) patients. The diagnostic use of CXCL13 concentrations in active lupus was also analyzed.
A total of 36 SLE patients and 18 healthy controls were included. Serum CXCL13 levels were examined by enzyme-linked immunosorbent assay. The frequency and absolute count of circulating plasmablasts and memory B cells were analyzed by flow cytometry. Receiver operating characteristic curves (ROC curves) were generated to analyze the utility of serum CXCL13 level and plasmablasts frequency as tools for the recognition of active SLE.
Elevation of serum CXCL13 levels, higher plasmablasts frequency, and reduction of memory B-cells count were observed in SLE patients, compared with healthy controls. Interestingly, correlational analyses showed not only significantly positive association between CXCL13 levels and SLE Disease Activity Index (SLEDAI) or plasmablasts frequency, but an inverse correlation between CXCL13 concentration and memory B-cell count. ROC curves showed that serum CXCL13 level and plasmablasts frequency were practical in identifying active disease from overall SLE patients, with considerable accuracy.
Serum CXCL13 levels correlate with the alteration of plasmablasts and memory B cells in SLE. CXCL13 may be used as a practical tool in judgment of active SLE.
Keywords: CXC ligand 13 protein, memory B cells, plasmablasts, systemic lupus erythematosus
1. Introduction
The disorder of B-cell subsets is a characteristic of systemic lupus erythematosus (SLE). Various B-cell subgroups take part in the pathological process via different mechanisms. The CXC ligand 13 protein (CXCL13), which is known as B lymphocyte chemoattractant (BLC), had been reported playing key roles in the pathogenesis of SLE by interacting with specific chemokine receptor C-X-C chemokine receptor type 5 (CXCR5).[1] Not only the correlation between serum CXCL13 levels and SLE Disease Activity Index (SLEDAI), but the association between CXCL13 and certain organ affection such as lupus nephritis and SLE-related autoimmune hemolytic anemia had been demonstrated in previous studies.[2–5] The expression of chemokine receptors varies in different subgroup of B-cell lineage.[6] Thus, CXCL13 may have different effects on these B-cells subsets.
In this study, we investigated for the first time whether serum CXCL13 levels correlate with the circulating plasmablasts and memory B-cells disfunction in SLE patients, which has vital pathological significance. The diagnostic use of CXCL13 concentrations in active lupus was also analyzed.
2. Materials and methods
2.1. Patients and controls
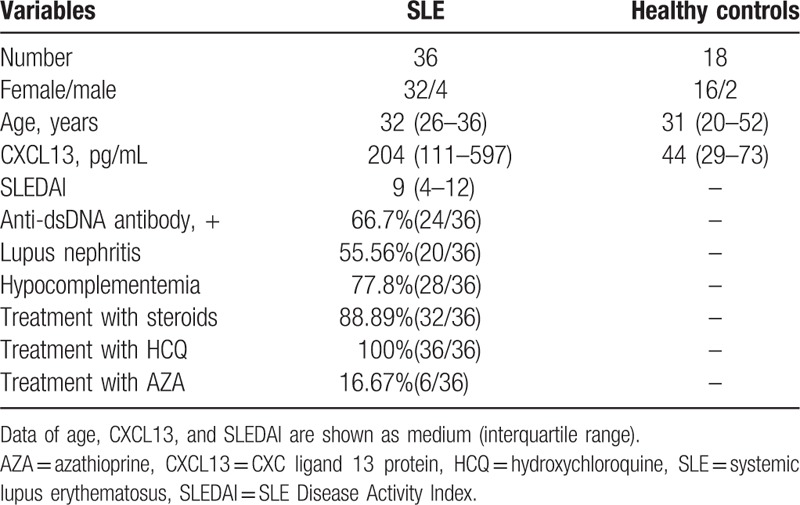
Patients and healthy controls were consecutively recruited at Second Affiliated Hospital of Fujian Medical University from September 2015 to November 2015. Informed consent forms were obtained from all individuals included. The study was carried out in accordance with the Declaration of Helsinki. In total, 36 SLE patients who fulfilled the 2009 American College of Rheumatology (ACR)/Systemic Lupus International Collaborating Clinics (SLICC) criteria for SLE[7] were recruited from the Department of Rheumatology, Second Affiliated Hospital of Fujian Medical University. Disease activity was assessed by SLEDAI. An active disease was define as SLEDAI≥5. In this way, the conditions of 25 patients were classified as active lupus and others were regarded as inactive disease. Individuals having infection as comorbidity were excluded, according to the research by Schiffer et al.[8] Blood samples were also obtained from 18 age, sex-matched healthy individuals without known history of malignancies or recent infections. Negativity of antinuclear antibodies was essential for healthy controls included. The characteristics of patients and controls were listed in Table 1. The study was approved by the Research Ethics Committee of the Hospital.
Table 1.
Characteristics of patients and controls.
2.2. Flow cytometry
Immunophenotyping of lymphocytes was performed using multi-color flow cytometry. Heparinized whole blood samples were immediately stained for 20 min with the following antibodies: PE-conjugated anti-human CD19 antibodies, FITC-conjugated anti-human CD27 antibodies, Percp-Cy5.5-conjugated Anti-Human CD38 antibodies, and isotype controls (all purchased from Biolegend). In addition, 500 μL of red blood cell lysis buffer (Beckman Coulter) was added and the sample was incubated for 10 min at room temperature. Phosphate-buffered saline (500 μL) was then added, followed by Counting Beads (Beckman Coulter) to quantify the B-cell subsets absolute count. The sample in its entirety was processed by flow cytometry on a Beckman Coulter FC500. Plasmablasts and memory B cells were defined as CD19+CD27+CD38+ cells and CD19+CD27+CD38- cells, respectively.
2.3. Enzyme-linked immunosorbent assay
Peripheral blood samples for quantification of circulating CXCL13 levels were centrifuged. Serums of all individuals were separated and stored at –80°C until use. Serum concentrations of CXCL13 were quantified according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). All samples were measured in duplicate. Color intensity was measured by a standard enzyme-linked immunosorbent assay (ELISA) microplate reader (Tecan, Germany).
2.4. Statistical analysis
Differences between patients and controls and correlationship were analyzed using the t test and Pearson's correlation analysis, respectively, if data are normally distributed. If not, the nonparametric Mann–Whitney rank sum test and Spearman's correlation analysis were applied. Statistical significance was defined as P < .05. Receiver operating characteristic curves (ROC curves) were created to judge the utility of serum CXCL13 level and circulating plasmablasts frequency as diagnostic tests for active SLE from overall SLE patients. All statistical analyses were performed with the GraphPad Prism software.
3. Results
3.1. Elevation of serum CXCL13 levels, higher plasmablasts frequency, and reduction of memory B-cells count were observed in SLE patients, compared with healthy controls
Serum CXCL13 levels and plasmablasts frequency in SLE patients were significantly increased when compared with the healthy controls (the median CXCL13 levels were 203.97 pg/mL vs 43.60 pg/mL, P < .001; the median plasmablasts frequencies were 5.45% vs 1.35%, P < .001) (Fig. 1A and B). In contrast, lower memory B-cells count was found in SLE patients (the median memory B cells counts were 35,565/mL vs 144,216/mL, P < .001) (Fig. 1C). Additionally, patients with active SLE had even higher CXCL13 levels, plasmablasts frequency, and the tendency of lower memory B-cells count than those categorized as inactive SLE patients (the median CXCL13 levels were 273.93 pg/mL vs 84.08 pg/mL, P < .01; the median plasmablasts frequencies were 6.80% vs 2.60%, P < .01; the median counts were 26,742/mL vs 39,292/mL, P = .18, respectively). The differences of CXCL13 levels, plasmablasts frequency, and memory B-cells count between inactive SLE patients and healthy controls also had statistical significance (P < .01; P < .001; P < .001, respectively).
Figure 1.
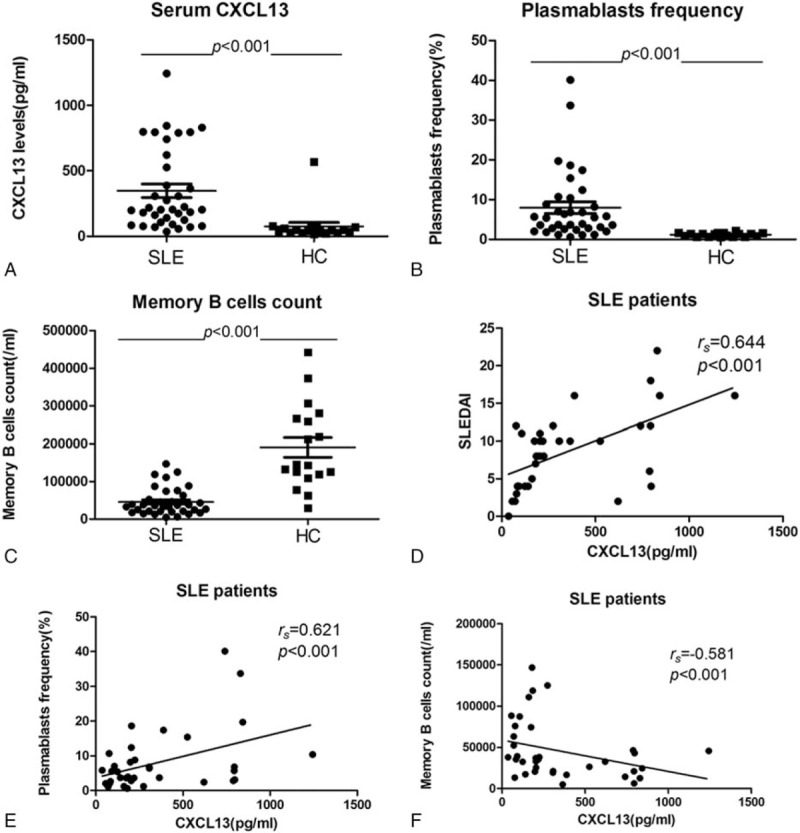
Elevation of serum CXCL13 levels (A), higher plasmablasts frequency (B), and reduction of memory B cells count (C) were observed in SLE patients. SLEDAI (D) and the alteration of plasmablasts frequency (E) and memory B cells (F) count correlated with CXCL13 levels. CXCL13 = CXC ligand 13 protein, SLE = systemic lupus erythematosus, SLEDAI = SLE Disease Activity Index.
Patients with lupus nephritis (LN) had significantly higher CXCL13 levels and plasmablasts frequency than those without LN (the median of CXCL13 levels: 376.21 pg/mL vs 115.73 pg/mL, P < .001; the median of plasmablasts frequency: 6.80% vs 3.35%, P < .05). Serum concentrations of CXCL13 and circulating plasmablasts frequency were significantly higher in SLE patients positive in anti-dsDNA antibodies than those who did not present with anti-dsDNA antibodies (the median of CXCL13 levels: 248.93 pg/mL vs 95.32 pg/mL, P < .01; the median of plasmablasts frequency: 5.75% vs 2.50%, P < .05).
3.2. Correlationship of CXCL13 levels and certain alteration of plasmablasts or memory B cells in SLE patients
The correlational analyses showed not only the significantly positive association of CXCL13 levels and SLEDAI, plasmablasts frequency, but the inverse correlation of CXCL13 concentrations and memory B-cell count in SLE patients (rs = 0.644, P < .001; rs = 0.621, P < .001; rs = −0.581, P < .001, respectively) (Fig. 1D–F). Additionally, similar tendency was observed when active SLE and inactive SLE patients were separately analyzed, even though partly having no statistical significance (rs = 0.553, P < .01; rs = 0.389, P = .055; rs = –0.437, P < .05, respectively vs rs = 0.690, P < .05; rs = 0.241, P = .474; rs = –0.564, P = .071, respectively), but not in healthy controls (rs = 0.006, P = .980; rs = 0.284, P = .254, respectively).
3.3. ROC curves showed the utility of CXCL13 levels and plasmablasts frequency in the diagnosis of active SLE from overall SLE patients
ROC curves demonstrated that CXCL13 levels and plasmablasts frequency were practical in identifying active disease from overall SLE patients, with considerable accuracy (area under the ROC curve (AUC) of 0.829; P < .01; 95% CI 0.624, 1.000; vs AUC of 0.795; P < .01; 95% CI 0.650, 0.939, respectively) (Fig. 2A and B).
Figure 2.
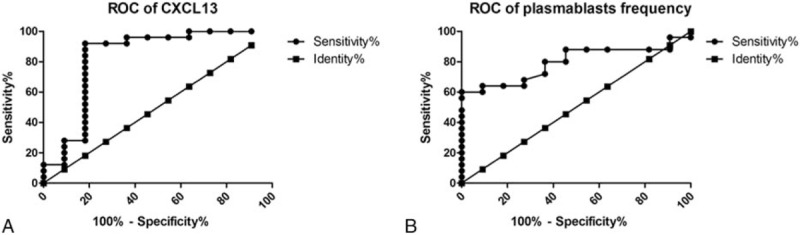
ROC curves showed the utility of CXCL13 levels (A) and plasmablasts frequency (B) in identifying active disease from overall SLE patients. AUCs were 0.829 versus 0.795 with statistical significance, respectively. AUC = area under the ROC curve, CXCL13 = CXC ligand 13 protein, ROC curves = receiver operating characteristic curves.
4. Discussion
In this study, first of all, we confirmed that elevation of serum CXCL13 levels and higher plasmablasts frequency were observed in our SLE cohort, especially in individuals with active SLE, when compared with healthy controls. We also observed that serum CXCL13 levels were in parallel with SLEDAI. These findings had been well documented in previous studies, as mentioned above.
CXCL13 plays a key role in chemotaxis of B cells. Nevertheless, there was no data published about phenotypic change of B cells associated with CXCL13 in SLE. This is crucial to clarify the pathogenic mechanism of CXCL13 in SLE. Elevation of plasmablasts frequency and reduction of memory B-cells count were found in our SLE cohort. Of interest, we observed that CXCL13 levels correlated with these alterations in overall SLE patients but not in healthy controls. Even the data were analyzed separately in different subgroups (active SLE and inactive SLE patients), the correlationship still existed. This result suggested that the association was specific in SLE patients.
Plasmablasts, comprise a stage intermediate between antigen-activated B cells and plasma cells, are characterized as CD19+CD27+CD38+. Clinical data suggested that in patients with active SLE, plasmablasts frequency in the blood correlates with disease activity.[9] Our result supported this finding. In addition, plasmablasts are the main resources of anti-dsDNA antibodies,[10] which is a core factor involved in active disease and the development of LN. Our result showed that SLE patients with positive result of anti-dsDNA antibodies or renal damage had even higher CXCL13 levels and plasmablasts frequency, when compared with those with negative result of anti-dsDNA antibodies or without evidence of LN, respectively. This observation conformed with the study by Lee et al. A further interesting finding in our cohort was that serum CXCL13 levels correlated with plasmablasts frequency, which could be explained by the theory that antigen-activated B cells or memory B cells, under the guidance of CXCL13, can migrate to the lightzone of germinal center, where the interaction between B cells and CXCR5+ follicular helper T cells producing interleukin-21 is formed. Then, activated B cells or memory B cells differentiate into plasmablasts or plasma cells and produce autoantibodies.
Memory B cells make up a subgroup with high heterogenicity which was marked with various differentiation antigens in different studies. CD19+ CD27+ CD38– cells were defined as memory B cells in our research. We observed that the memory B cells count significantly decreased in SLE patients than healthy controls. This result was consistent with the study by Szabó et al. They hypothesized that it was due to the switch from memory B cells to plasmablasts/plasma cells.[11] CXCR5-CXCL13 pair mediates the chemotaxis process of memory B cells. In our research, memory B cells count inversely correlated with serum CXCL13 levels. As described above, CXCL13 concentrations correlated with the plasmablasts frequency in our cohort. Thus, our result suggested that CXCL13 may participate in the above switch process. The chemotaxis effect mediated by CXCL13 is beneficial for the entrance and “blockade” of memory B cells or antigen-activated B cells to germinal center, further promoting maintaining the structure of germinal center and the production of pathogenetic autoantibodies which exacerbates the tissue destruction. Additionally, the difference in correlationship between CXCL13 and the 2 B cells subgroups might be partially attributed to the differential expression of chemokine receptors on peripheral blood B cells lineage: in patients with SLE, CXCR5 is mostly expressed on antigen-activated B cells, but it disappears in plasmablasts and plasma cells.[12] Thus, the migrational behavior of B cells can be influenced.
Due to the low cell proliferation and less dependence on B-cell activating factor of the tumor necrosis factor family (BAFF), memory B cells have little sensitivity to conventional medication of SLE such as mycophenolate mofetil, cyclophosphamide, and belimumab.[13–15] Several researches had pointed out the possibility of CXCL13-CXCR5 as a target of autoimmune disease: Wu et al[16] found that CXCL13 blockade attenuates lupus nephritis of MRL/lpr mice. Wiener et al[17] also reported that the substantial improvement was observed after CXCR5 knockout in lpr mice. Based on these findings and our results, whether therapies targeting CXCL13 can reduce the chemotaxis of memory B cells and plasmablasts frequency, which is important in the relapse in autoimmune disease, is worthy of in-depth investigation further.
As we know, CXCL13 and plasmablasts frequency were tightly associated with disease activity in SLE patients. We generated ROC curves to test and compare the diagnostic utility of CXCL13 and plasmablasts frequency in active SLE. ROC curves showed that both serum CXCL13 levels and plasmablasts frequency have considerable accuracy in distinguishing active disease from overall SLE patients. CXCL13 levels even had a bit higher AUC than plasmablasts frequency. The previous study showed that increased plasmablasts have a predictive value of disease relapse in SLE patients treated with rituximab.[18] Recently, the association between plasmablasts levels and lupus activity has also been reported in a large cohort study by Kubo et al.[19] Our research, therefore, corroborated these observations. Our result revealed that serum CXCL13 might have similar or even higher value in recognizing active SLE, probably because CXCL13 was mostly produced by dendritic cells activated in the stage of antigen presenting before the humoral immune response. Moreover, CXCL13 was 1 of only 2 chemokines (out of 61 inflammatory chemo- and cytokines) upregulated at the mRNA–level in the animal model for SLE before inflammatory infiltration in renal was evident.[20]
The main weakness of our research is the limited number of patients included. Our hypothesis should be confirmed in larger cohorts in the future. Functional assays to clarify the role of CXCL13 in B-cell trafficking and antibodies production are also needed further.
In summary, our study showed that elevation of serum CXCL13 levels, higher plasmablasts frequency, and reduction of memory B cells count could be observed in SLE patients, compared with healthy controls. Our results first revealed that serum CXCL13 levels correlated with the dysfunction of circulating plasmablasts and memory B cells in SLE, which had pathological significance. ROC curves also showed CXCL13 might be used as a practical tool in judgment of active SLE from overall SLE patients.
Acknowledgments
The authors thank Xianjing Huang and Hongzhi Gao for the excellent technical assistance.
Footnotes
Abbreviations: ACR = American College of Rheumatology, AUC = area under the ROC curve, BAFF = B-cell activating factor of the tumor necrosis factor family, BLC = B lymphocyte chemoattractant, CXCL13 = CXC ligand 13 protein, CXCR5 = C-X-C chemokine receptor type 5, ELISA = enzyme-linked immunosorbent assay, LN = lupus nephritis, ROC curves = receiver operating characteristic curves, SLE = systemic lupus erythematosus, SLEDAI = SLE Disease Activity Index, SLICC = Systemic Lupus International Collaborating Clinics.
Funding: This work was supported by the Construct Foundation of the Key Clinical Discipline of Fujian Medical University (grant no. XKPY201102) and the High-level Talent Development Foundation of Fujian Province (grant no. 2109901).
The authors have no conflicts of interest to disclose.
References
- [1].Ishikawa S, Sato T, Abe M, et al. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J Exp Med 2001;193:1393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lee HT, Shiao YM, Wu TH, et al. Serum BLC/CXCL13 concentrations and renal expression of CXCL13/CXCR5 in patients with systemic lupus erythematosus and lupus nephritis. J Rheumatol 2010;37:45–52. [DOI] [PubMed] [Google Scholar]
- [3].Wong CK, Wong PT, Tam LS, et al. Elevated production of B cell chemokine CXCL13 is correlated with systemic lupus erythematosus disease activity. J Clin Immunol 2010;30:45–52. [DOI] [PubMed] [Google Scholar]
- [4].Wu B, Wang W, Zhan Y, et al. CXCL13, CCL4, and sTNFR as circulating inflammatory cytokine markers in primary and SLE-related autoimmune hemolytic anemia. J Transl Med 2015;13:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ezzat M, El-Gammasy T, Shaheen K, et al. Elevated production of serum B-cell-attracting chemokine-1 (BCA-1/CXCL13) is correlated with childhood-onset lupus disease activity, severity, and renal involvement. Lupus 2011;20:845–54. [DOI] [PubMed] [Google Scholar]
- [6].Henneken M, Dörner T, Burmester GR, et al. Differential expression of chemokine receptors on peripheral blood B cells from patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther 2005;7:R1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Petri M. SLICC revision of the ACR classification criteria for SLE. Arthritis Rheum 2009;60:895. [Google Scholar]
- [8].Schiffer L, Kielstein JT, Haubitz M, et al. Elevation of serum CXCL13 in SLE as well as in sepsis. Lupus 2011;20:507–11. [DOI] [PubMed] [Google Scholar]
- [9].Jacobi AM, Mei H, Hoyer BF, et al. HLA-DRhigh/CD27high plasmablasts indicate active disease in patients with systemic lupus erythematosus. Ann Rheum Dis 2010;69:305–8. [DOI] [PubMed] [Google Scholar]
- [10].Dörner T, Jacobi AM, Lipsky PE. B cells in autoimmunity. Arthritis Res Ther 2009;11:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Szabó K, Papp G, Szántó A, et al. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjögren's syndrome and systemic lupus erythematosus. Clin Exp Immunol 2016;183:76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Koarada S, Tashiro S, Tokuda Y, et al. Persistent expression of CXCR5 on plasmablasts in IgG4-related disease. Ann Rheum Dis 2015;74:e32. [DOI] [PubMed] [Google Scholar]
- [13].Fassbinder T, Saunders U, Mickholz E, et al. Differential effects of cyclophosphamide and mycophenolate mofetil on cellular and serological parameters in patients with systemic lupus erythematosus. Arthritis Res Ther 2015;17:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stohl W, Hiepe F, Latinis KM, et al. Belimumab reduces autoantibodies, normalizes low complement, and reduces select B-cell populations in patients with systemic lupus erythematosus. Arthritis Rheum 2012;64:2328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Badr G, Borhis G, Lefevre EA, et al. BAFF enhances chemotaxis of primary human B cells: a particular synergy between BAFF and CXCL13 on memory B cells. Blood 2008;111:2744–54. [DOI] [PubMed] [Google Scholar]
- [16].Wu X, Guo J, Ding R, et al. CXCL13 blockade attenuates lupus nephritis of MRL/lpr mice. Acta Histochem 2015;117:732–7. [DOI] [PubMed] [Google Scholar]
- [17].Wiener A, Schippers A, Wagner N, et al. CXCR5 is critically involved in progression of lupus through regulation of B cell and double-negative T cell trafficking. Clin Exp Immunol 2016;185:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vital EM, Dass S, Buch MH, et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum 2011;63:3038–47. [DOI] [PubMed] [Google Scholar]
- [19].Kubo S, Nakayamada S, Yoshikawa M, et al. Peripheral immunophenotyping identifies three subgroups based on T cell heterogeneity in lupus patients. Arthritis Rheumatol 2017;69:2029–37. [DOI] [PubMed] [Google Scholar]
- [20].Schiffer L, Bethunaickan R, Ramanujam M, et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol 2008;180:1938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]


