Supplemental Digital Content is available in the text
Keywords: administrative claims, healthcare costs, healthcare resource utilization, herpes zoster, solid tumor malignancy
Abstract
Immunosuppressed patients with solid tumor malignancies (STMs) are particularly vulnerable to herpes zoster (HZ). This study estimated the incidence of HZ and evaluated healthcare resource utilization and costs for persons with STM receiving chemotherapy with and without incident HZ.
We conducted a retrospective claims study of adults with STM receiving chemotherapy between January 1, 2010 and June 30, 2014. Patients were followed from their first chemotherapy date through development of HZ, health plan disenrollment, the study end date, or 24 months. HZ incidence was calculated and stratified by patient characteristics. Adjusted HZ incidence was estimated using Poisson regression. Healthcare resource utilization and costs were compared between patients with HZ (cases) and propensity score–matched controls without HZ during a variable follow-up period. Adjusted healthcare costs were estimated using Lin regression to control for informative censoring.
Of 155,480 patients with STM receiving chemotherapy, 3100 (2.0%) developed HZ, yielding an adjusted HZ incidence rate of 13.8/1000 person-years (PY). HZ cases (n = 3004) had significantly higher healthcare resource utilization than matched controls (n = 15,020). Adjusted annual costs were $48,077 for cases vs $41,645 for matched controls, corresponding to a differential cost of $6432 annually.
After adjustment for potential confounders, patients with STM receiving chemotherapy had an HZ incidence of 13.8/1000 PY; those who developed HZ used more healthcare resources and incurred higher costs than those who did not. These findings suggest that HZ prevention by vaccination could improve outcomes and reduce costs in this population.
1. Introduction
Although advances in chemotherapy have helped improve disease-free and overall survival among patients with solid tumor malignancy (STM), the management of treatment complications remains a challenge.[1] Herpes zoster (HZ), the reactivation of latent varicella-zoster virus in sensory ganglia, is a known complication of immunosuppressive chemotherapy.[1,2] Estimates of HZ incidence in the general population range from 3 to 5 per 1000 person-years (PY)[3] but are substantially higher among immunosuppressed patients with STM,[4–6] ranging from 6/1000 to 40/1000 PY depending on the site and tumor type.[5]
HZ generally manifests as a dermatomal vesicular rash accompanied by pain. HZ is often a self-limited condition, but HZ-related pain may interfere with daily activities in a minority of patients, leading to weight loss, fatigue, depression, social withdrawal, and diminished quality of life.[7–10] Approximately 10% of patients with HZ will develop postherpetic neuralgia (PHN), commonly defined as pain lasting >90 days after rash onset.[11,12] The risk of PHN and other complications of HZ, including ophthalmologic and neurologic complications, disseminated skin disease, and visceral involvement, is increased among immunosuppressed persons.[2,10]
Despite the fact that immunosuppressed persons are particularly vulnerable to both HZ and its complications, there are few data regarding the effect of HZ on healthcare resource utilization in this population. To address this gap, we estimated the incidence of HZ and examined healthcare resource utilization and costs for persons with STM receiving chemotherapy with and without incident HZ.
2. Materials and methods
2.1. Data sources
This was a retrospective study conducted using administrative claims data from January 1, 2008 through June 30, 2015. Data sources were the Optum Research Database, which contained approximately 13.9 million commercial enrollees and 3.2 million Medicare Advantage enrollees with medical and pharmacy coverage in 2014, and the Impact National Benchmark Database, which contains multiple-payer administrative claims data representing >20 million lives from 2004 to the present. These databases include enrollment information and medical and pharmacy claims for commercial and Medicare Advantage health plan members, and are geographically diverse across the United States. Medical claims included diagnosis codes and procedure codes from the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM); Current Procedural Terminology, Version 4 procedure codes; Health Care Common Procedure Coding System codes; revenue codes; and site of service codes. Outpatient pharmacy claims included National Drug Codes for dispensed medications, quantity dispensed, dose, and days’ supply. Because no identifiable protected health information was extracted or accessed during the course of the study, Institutional Review Board approval or waiver of authorization was not required.
2.2. Study sample selection
The study sample consisted of adult patients (18 years or older) with STM who were enrolled in a commercial or Medicare Advantage health plan with both medical and pharmacy coverage and had at least 1 pharmacy or medical claim for chemotherapy (Supplement 1) between January 1, 2010 and June 30, 2014 (identification period). Patients were identified as having incident STM if they had no evidence of receiving chemotherapy for at least 18 months before the cohort-entry date, and no claims with an STM diagnosis in any position during the period from 24 months before the cohort-entry date through 6 months before the cohort-entry date. The presence of at least 1 medical claim with an ICD-9-CM diagnosis code for STM in either the primary or the secondary position (141.x-165.x, 170.x-176.x, 179, 180.x, 181, 182.x-184.x, 185, 186.x-192.x, 193, 184.x-197.x, 198.1–198.7, 198.8x, 190.x, or 209.xx) during the identification period was considered as evidence of STM, and the date of first chemotherapy was designated as the cohort-entry date. Patients were required to have at least 18 months of continuous health plan enrollment with both medical and pharmacy coverage before the cohort-entry date and were excluded from the study if they had incomplete demographic data, any claim for hematological malignancy (ICD-9-CM codes 200.xx-208.xx, 238.4, 238.6, 238.7x, 273.3, or 289.83 in any position) during the study period, Zostavax use during the study period, or any claim for HZ or HZ-associated complications (ICD-9-CM code 053.xx) from the beginning of the study period to the cohort-entry date.
2.3. Study measures
2.3.1. Patient characteristics
Demographic characteristics were assessed during the baseline period, which was defined as the 12 months before the index date (the first date of HZ diagnosis for patients who developed HZ, or for those that did not develop HZ, a date assigned such that the post-index duration was comparable to that of the corresponding case; i.e., within 60 days of each other). The modified Quan-Charlson comorbidity score excluded malignancy and was assessed in the 12 months before the cohort-entry date. Tumor type was assessed in the 18 months before the cohort-entry date and was not mutually exclusive; for patients with multiple cancers, each tumor type was counted. Chemotherapy immunosuppression level was assessed during each patient's time at risk for HZ (risk period), which began on the cohort-entry date and ended at the first occurrence of development of HZ, health plan disenrollment, the study end date, or 24 months after the cohort-entry date. Chemotherapy immunosuppression level (none, low, moderate, high, or very high; Supplement 1) was defined as the highest immunosuppression level observed during the risk period among patients whose chemotherapy type was known. Antiviral medication use was assessed from 21 days before the cohort-entry date through the end of the risk period.
2.3.2. Herpes zoster incidence rates
Each patient was followed from the cohort-entry date through the end of the risk period (Supplement 2A). HZ cases were defined by the presence of a medical claim with an ICD-9-CM diagnosis code for HZ (053.xx) in any position during the risk period. HZ incidence rates (the number of HZ cases divided by PY accrued by all patients) were calculated for the full population and stratified by age group, sex, modified Quan-Charlson score, tumor type, chemotherapy immunosuppression level, and antiviral medication use. Accrued person-time for patients who developed HZ was calculated as the total number of years accrued between cohort entry and the date of first HZ diagnosis. Accrued person-time for patients who did not develop HZ was calculated as the total number of years accrued between cohort entry and the end of the risk period.
2.3.3. Healthcare resource utilization and costs
Per-patient-per-month (PPPM) healthcare resource utilization and costs were assessed during a variable follow-up period extending from 21 days before the index date through the earliest of the following events: death, health plan disenrollment, the study-end date, or 12 months after the index date (Supplement 2B). Percentages and counts of office visits, outpatient visits, emergency room (ER) visits, and inpatient stays were captured to reflect healthcare utilization. Healthcare costs were calculated as combined health plan–paid and patient-paid amounts, and then adjusted to 2014 US dollars using the annual medical care component of the Consumer Price Index[13] to account for inflation. Total healthcare costs were subdivided into medical and pharmacy costs, and the medical category was further subdivided into office, outpatient, ER, inpatient, and other medical costs.
2.4. Statistical analysis
2.4.1. Adjusted herpes zoster incidence rates
Adjusted HZ incidence was estimated using a Poisson regression model to control for possible confounding of the outcome by patient demographic and clinical characteristics. Covariates in the model included age, sex, modified Quan-Charlson score, tumor type, chemotherapy immunosuppression level, and antiviral medication use (Supplement 3).
2.4.2. Healthcare resource utilization and costs
For the HZ disease burden analysis, propensity score matching was undertaken to reduce the bias due to confounding variables that may have resulted from simply comparing healthcare resource utilization and cost outcomes between those with and without HZ. Patients with evidence of HZ during the risk period (cases) were 1:5 nearest-neighbor propensity score matched to patients with no evidence of HZ during the risk period (controls) on the following covariates: age, geographic region, tumor type, modified Quan-Charlson comorbidity score,[14] presence of incident STM, sex, insurance type, data source, and chemotherapy immunosuppression level (Supplement 1). Standardized differences were calculated to assess similarities in the means and prevalence of baseline characteristics between the propensity-matched cohorts.[15] Descriptive analyses were conducted for all outcome study variables, and results were compared between HZ cases and controls using Rao-Scott chi-square tests and robust variance estimators for categorical variables and continuous variables, respectively.[16,17]
Adjusted total all-cause healthcare costs were estimated using Lin regression[18] to control for informative censoring, as patients with HZ may have been sicker and had a greater risk of dying during follow-up. In this method, the follow-up period was divided into 12 one-month intervals, and an ordinary least squares regression model, weighted according to the subjects’ Kaplan-Meier probability of not being included in the data (i.e., of being censored), was fit to the cost data within each interval. The regression coefficients for each covariate for each interval were then summed over the entire follow-up period to estimate the overall cost effect of a unit change in the covariate. Covariates in the model included age, sex, modified Quan-Charlson score, tumor type, chemotherapy immunosuppression level, and antiviral medication use.
Statistical significance was defined as P < .05. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Study sample
A total of 155,480 patients with STM on chemotherapy were included in the study of HZ incidence. Of these, 3100 patients (2.0%) had HZ during the risk period. After 1:5 matching of persons with STM on chemotherapy with HZ to persons with STM on chemotherapy without HZ, a total of 3004 cases and 15,020 controls were included for the study of HZ-associated healthcare resource utilization and costs (Fig. 1).
Figure 1.

Sample selection and attrition flow diagram. HZ = herpes zoster, ID = identification, STM = solid tumor malignancy.
3.2. Antiviral use
Among the total study population, 8053 patients (5.2%) had at least 1 claim for an antiviral medication during the risk period, with 5105 (3.3%), 1140 (0.7%), 439 (0.3%), and 1369 (0.9%) of patients using antiviral medications for <30 days, 30 to 59 days, 60 to 89 days, and ≥90 days, respectively. Of the 2755 patients with an antiviral use duration of 7 days or less, antiviral prescription duration was truncated in 221/2755 (8.0%) patients by the end of the patient's time at risk. Of these patients, the time at risk ended due to an HZ diagnosis for 162/221 (73.3%), and due to the end of the study period for 59/221 (26.7%). Among the 1.8% (2755/155,480) of patients who had antiviral prescriptions for 7 days or less, 5.6% (162/2,755) had evidence of an HZ diagnosis near the time of antiviral therapy initiation.
3.3. Herpes zoster incidence rates
Unadjusted HZ incidence rates by patient characteristics are presented in Table 1. The unadjusted incidence rate was 14.9/1000 PY [95% confidence interval (CI) 14.4–15.4/1000 PY] among patients with STM receiving chemotherapy. Unadjusted HZ incidence increased with age (16.2/1000 PY for ages ≥70 vs 10.5/1000 PY for ages 18–39), modified Quan-Charlson comorbidity score (18.4/1000 for scores ≥5 vs 11.4/1000 for a score of 0), and chemotherapy immunosuppression level (17.1/1000 for moderate/high/very high vs 11.8/1000 for none/low). Incidence was greater among women vs men (15.6/1000 PY vs 13.8/1000 PY) and was highest among patients with lung or bronchus cancer (24.8/1000 PY) compared with other tumor types. The HZ incidence rate among patients who used antiviral medications for ≥30 days was 12.4/1000 PY. In contrast, the HZ incidence among patients with antiviral use of <30 days was 56.3/1000 PY (likely reflecting antiviral use as a treatment regimen). The HZ rate among those who did not use antiviral agents was 13.4/1000 PY. After adjusting for demographic characteristics, modified Quan-Charlson comorbidity score, tumor type, chemotherapy immunosuppression level, and antiviral medication use, the overall HZ incidence rate was 13.8/1000 PY (95% CI 13.3–14.3), similar to the overall unadjusted rate (Supplement 3). Adjusted HZ incidence rates by patient characteristics suggested increasing linear trends with age and chemotherapy immunosuppression level.
Table 1.
Unadjusted herpes zoster incidence rates by patient characteristics.

3.4. Healthcare resource utilization
Baseline demographic and clinical characteristics were similar between the propensity score–matched cohorts of patients with and without HZ, as evidenced by standardized differences of ≤10% (Table 2). HZ cases had significantly more office visits, outpatient visits, ER visits, and inpatient stays than controls (P < .001 for all; Fig. 2A). On average, HZ cases had more PPPM visits for all 4 healthcare settings than controls (P < .001 for all; Fig. 2B).
Table 2.
Baseline demographic and clinical characteristics among matched cohorts.
Figure 2.
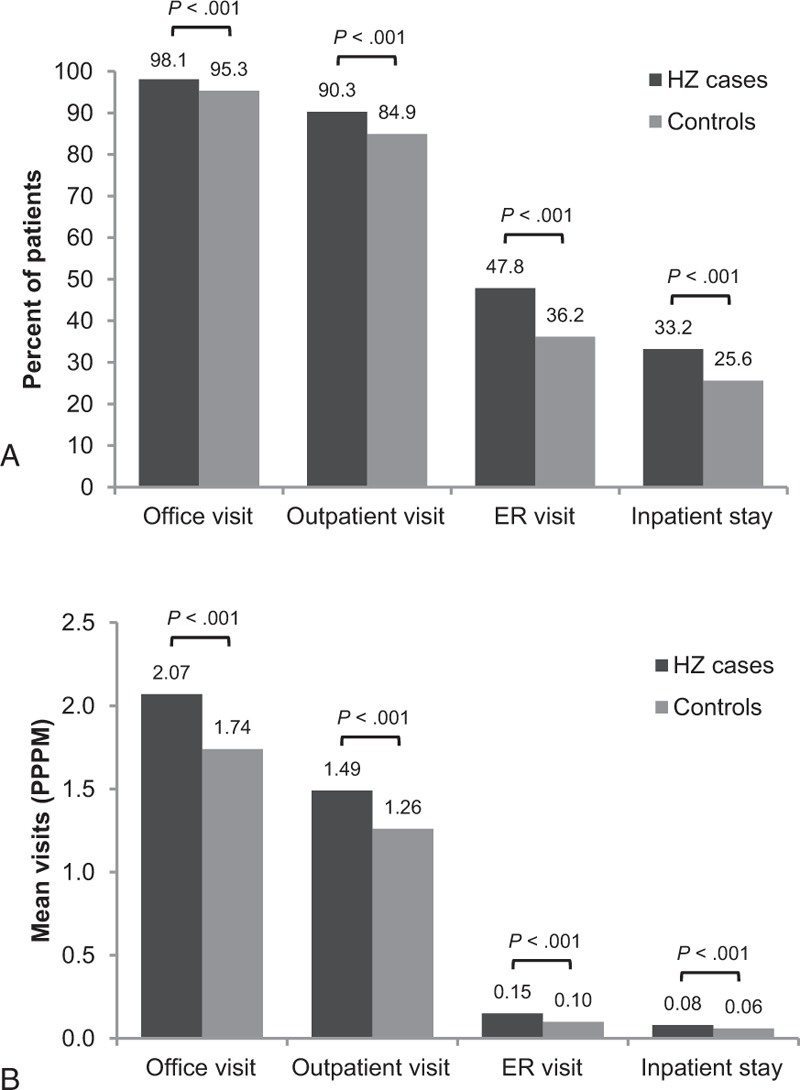
Follow-up healthcare resource utilization among matched cohorts. A, Percent of patients with at least one visit. B, Mean per-patient-per-month (PPPM) visits. ER = emergency room, HZ = herpes zoster.
3.5. Healthcare costs
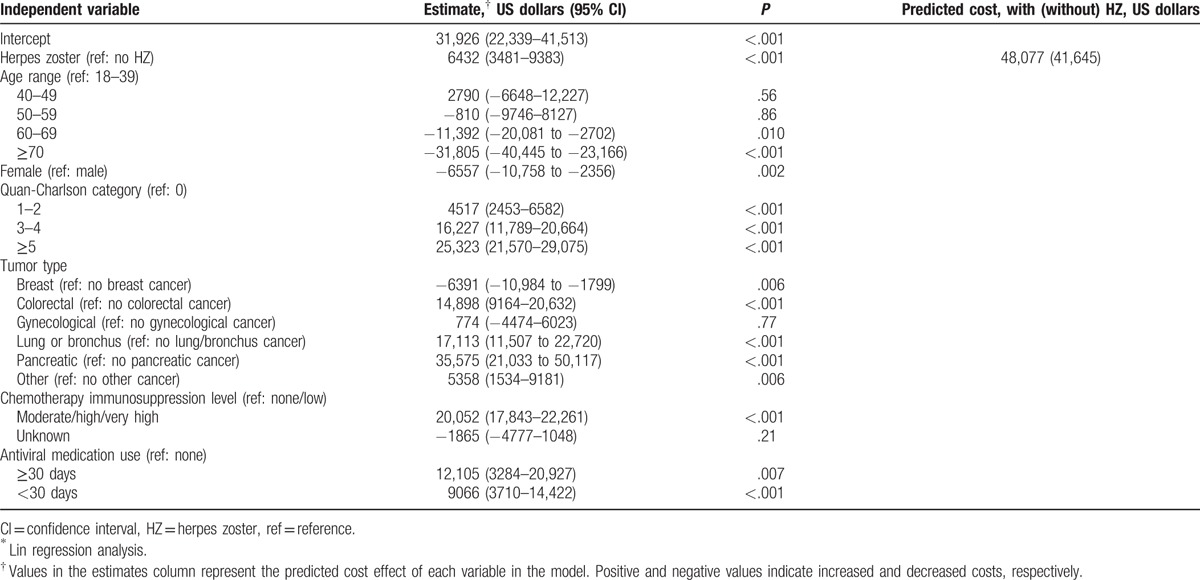
HZ cases incurred an average of $1055 in additional healthcare costs per month than their matched controls; mean total monthly healthcare costs for cases were $5258 vs $4203 for controls (P < .001; Fig. 3A). Both medical and pharmacy costs were higher for HZ cases than for controls ($4758 vs $3766, P < .001 and $500 vs $438, P = .03, respectively; Fig. 3A). Medical costs were dominated by outpatient costs ($1699 for cases, $1518 for controls, P = .006) and inpatient costs ($1822 for cases, $1177 for controls, P < .001) compared with other categories (Fig. 3B). After adjustment for age, sex, comorbidity burden, tumor type, chemotherapy immunosuppression level, and antiviral medication use, costs remained higher for HZ cases: annual costs among HZ cases were $48,077 compared with $41,645 among controls (Table 3), corresponding to a differential cost of $6432 annually or $536 monthly. Other significant cost drivers included modified Quan-Charlson score 5 or higher ($25,323 additional annual cost compared with score 0) and moderate/high/very high chemotherapy immunosuppression level ($20,052 additional annual cost compared with none/low) (Table 3; P < .001 for both).
Figure 3.

Unadjusted follow-up per-patient-per-month (PPPM) healthcare costs among matched cohorts, in 2014 US dollars. A, Total, medical, and pharmacy costs. B, Medical costs subdivided into office, outpatient, emergency room (ER), and other medical costs.
Table 3.
Adjusted∗ annual healthcare costs among matched cohorts.
4. Discussion
This is the first study using real-world data to evaluate healthcare resource utilization and costs associated with herpes zoster among patients with STM receiving chemotherapy. Patients with STM receiving chemotherapy had increased rates of HZ compared to rates in the general population, and those who developed HZ had higher healthcare resource utilization and incurred higher healthcare costs than those who did not develop HZ. Although there are no existing guidelines for the use of antiviral chemoprophylaxis to prevent HZ in patients receiving chemotherapy for STM, this study provides insight into antiviral use patterns among these patients.
The adjusted incidence of HZ in this study was 13.8/1000 PY, similar to the rate found in previous studies of patients with solid tumors[4,5] and approximately 3 times the rate of 3/1000 to 5/1000 PY estimated for the general population.[3] Consistent with the literature, HZ incidence increased with age and comorbidity burden,[20–23] was greater among women than among men,[20,21,24] and was highest among patients with lung or bronchus cancer,[4] which may reflect differences in the patient population (e.g., age and advanced cancer stage at diagnosis) or immunosuppression level of chemotherapy used for lung/bronchus cancers compared with other cancer types.
Healthcare utilization and costs are greater in our study than those in studies done among patients with HZ in the general population.[25,26] Using claims data from 1998 to 2003, White et al[26] found that annual healthcare costs for patients with HZ were $983 higher (in 2008 USD) than those of matched controls without HZ after adjustment for demographic characteristics, insurance status, comorbidities, and baseline medical expenditures; however, only 7.4% of patients in the study were immunocompromised. In a more recent claims-based analysis that excluded immunocompromised patients, Johnson et al[25] found mean unadjusted total annual healthcare costs to be $1308 higher for patients with HZ compared to those without HZ. Costs in our study were more similar to those in a recent study by Li et al,[27] who conducted a claims-based analysis of direct medical costs attributed to HZ in selected immunocompromised populations and found that healthcare costs for cancer patients with HZ were $4297 higher for ages 18 to 64 years (based on the first and second quarters after diagnosis) and $3108 higher for ages 64 and older (based on the first quarter after diagnosis) compared with those without HZ. Findings of higher costs in immunocompromised patients were also demonstrated in a subanalysis conducted within the White et al[26] study that found incremental costs to be higher in immunocompromised patients compared with matched immunocompetent patients with HZ ($1745 vs $983 in 2008 USD). Several methodological differences could have contributed to the differences in costs between our study and prior studies, such as variation in study populations (our study was limited to immunocompromised patients with STM receiving chemotherapy) and the adjustment for informative censoring included in our statistical analysis, which may have reduced the bias in estimates of cost outcomes.[25–27]
There are currently no existing guidelines for the use of antiviral chemoprophylaxis to prevent HZ in patients with STM, with the exception of a risk-adapted approach to patients treated with the proteasome inhibitor, bortezomib.[28] Overall receipt of antiviral prescriptions was low in our study; only 5.2% (8053/155,480) of patients had claims for antiviral medications from 21 days before the cohort-entry date through the end of the risk period. Among the 1.8% (2755/155,480) of patients who had antiviral prescriptions for 7 days or less, 5.6% (162/2755) had evidence of an HZ diagnosis near the time of antiviral therapy initiation, indicating that antivirals may have been prescribed for HZ treatment rather than prophylaxis. Patients who had no antiviral medications prescribed had similar rates of HZ to patients that were treated for ≥30 days, suggesting that there could have been a bias toward prescription of antivirals to patients who were considered to be at high risk of infection and no prescription to patients who were considered to be at lower risk of infection.
The statistical methods used in our study were uniquely designed to avoid biases common to retrospective claims-based analyses of healthcare costs. Informative censoring can occur in studies for which the outcome (in this case, HZ) is associated with being sicker and being more likely to die during follow-up; subjects may enter the health plan shortly before the end of the study period, or die or disenroll from the health plan before the study is completed. Traditional methods for analyzing cost data involve excluding subjects with incomplete data or follow-up, and can produce erroneous results by failing to account for censoring. For example, excluding or censoring patients with the outcome of interest who are sicker and whose follow-up times are shorter due to death from more severe disease may bias cost data. To address this issue, we used propensity score matching to balance the distribution of measured baseline covariates between patients with and without HZ, and then modeled total annual healthcare costs using Lin regression.[18] In this method, costs were estimated over 1-month intervals of the follow-up period using a regression model weighted to account for the likelihood of censoring, and then summed over the entire follow-up period; thus, censoring and the effects of potential confounders were controlled for simultaneously, resulting in an unbiased estimate of cost.
4.1. Limitations
Because this study was conducted in a US managed care population, the results may not be generalizable other populations (e.g., patients who are uninsured). This study is also subject to certain limitations inherent to claims-based analyses. The presence of an HZ diagnosis code on a medical claim does not prove the presence of disease, as it may be coded incorrectly (e.g., in the case of misclassification between HZ and herpes simplex virus) or intended as a rule-out diagnosis. The potential for erroneous identification of HZ was mitigated in this study by the requirement that patients have at least 1 nondiagnostic claim indicating HZ infection. Although HZ cases cannot be definitively confirmed using claims data, a previous general-population study found that approximately 85% of HZ cases identified from administrative billing codes are true positives.[29] Similarly, the potential for misidentification of patients with STM was minimized by the requirement of an STM diagnosis code from a nonlaboratory claim in addition to at least 1 claim for chemotherapy. Finally, patients with mild HZ who forego treatment or self-treat with over-the-counter medications are not captured in the claims data; this could lead to an underestimation of HZ incidence.
5. Conclusion
In this real-world study, the HZ incidence rate among patients receiving chemotherapy for STM was approximately 3 times that of the general population; moreover, patients with STM who developed HZ while receiving chemotherapy used more healthcare resources and incurred higher costs than those who did not develop HZ. Together with existing evidence that HZ substantially reduces quality of life, our findings suggest that prevention of HZ through vaccination could be an effective strategy for improving outcomes and reducing costs in this population. Current HZ vaccines based on attenuated live virus are contraindicated in patients receiving immunosuppressive chemotherapy[2]; however, the development of new vaccine formulations that may prove safer for individuals receiving immunosuppressive chemotherapy could expand opportunities for HZ prevention.[30,31]
Supplementary Material
Acknowledgments
This study was funded by Merck & Co., Inc. (Kenilworth, NJ). Medical writing assistance was provided by Yvette Edmonds, PhD, an employee of Optum (Eden Prairie, MN). Yiyu Fang and Lynn Wacha, employees of Optum, created the study dataset; Laura Becker, Djibril Liassou, and Ryan Wolbeck, all employees of Optum, helped with analysis and table creation.
Footnotes
Abbreviations: CI = confidence interval, ER = emergency room, HZ = herpes zoster, ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification, PHN = postherpetic neuralgia, PPPM = per-patient-per-month, PY = person-years, STM = solid tumor malignancy.
This study was funded by Merck & Co., Inc., Kenilworth, NJ.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Morrison VA. Immunosuppression associated with novel chemotherapy agents and monoclonal antibodies. Clin Infect Dis 2014;59(suppl 5):S360–4. [DOI] [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention. Shingles (herpes zoster). 2015; Available at: http://www.cdc.gov/shingles/index.html. Accessed October 19, 2016. [Google Scholar]
- [3].Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014;4:e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yenikomshian MA, Guignard AP, Haguinet F, et al. The epidemiology of herpes zoster and its complications in Medicare cancer patients. BMC Infect Dis 2015;15:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Habel LA, Ray GT, Silverberg MJ, et al. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomarkers Prev 2013;22:82–90. [DOI] [PubMed] [Google Scholar]
- [6].Masci G, Magagnoli M, Gullo G, et al. Herpes infections in breast cancer patients treated with adjuvant chemotherapy. Oncology 2006;71:164–7. [DOI] [PubMed] [Google Scholar]
- [7].Gater A, Abetz-Webb L, Carroll S, et al. Burden of herpes zoster in the UK: findings from the zoster quality of life (ZQOL) study. BMC Infect Dis 2014;14:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Song H, Lee J, Lee M, et al. Burden of illness, quality of life, and healthcare utilization among patients with herpes zoster in South Korea: a prospective clinical-epidemiological study. Int J Infect Dis 2014;20:23–30. [DOI] [PubMed] [Google Scholar]
- [9].Drolet M, Brisson M, Schmader KE, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ 2010;182:1731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cohen JI. Herpes zoster. N Engl J Med 2013;369:1766–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oxman MN, Levin MJ. Shingles Prevention Study G. Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis 2008;197(suppl 2):S228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yawn BP, Itzler RF, Wollan PC, et al. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc 2009;84:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].US Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Medical Care. Series ID: SUUR0000SAM. 2012; Available at: http://data.bls.gov/cgi-bin/surveymost?su. Accessed January 20, 2016. [Google Scholar]
- [14].Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. [DOI] [PubMed] [Google Scholar]
- [15].Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rao JNK, Scott AJ. The analysis of categorical data from complex sample surveys: chi-squared tests for goodness-of-fit and independence in two-way tables. J Am Stat Assoc 1981;76:221–30. [Google Scholar]
- [17].Rao JNK. On simple adjustments to chi-squared tests with survey data. Ann Stat 1987;15:385–97. [Google Scholar]
- [18].Lin DY. Regression analysis of incomplete medical cost data. Stat Med 2003;22:1181–200. [DOI] [PubMed] [Google Scholar]
- [19].Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008;27:2037–49. [DOI] [PubMed] [Google Scholar]
- [20].Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection 2014;42:325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Johnson BH, Palmer L, Gatwood J, et al. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis 2015;15:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McDonald JR, Zeringue AL, Caplan L, et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis 2009;48:1364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Joesoef RM, Harpaz R, Leung J, et al. Chronic medical conditions as risk factors for herpes zoster. Mayo Clin Proc 2012;87:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Leung J, Harpaz R, Molinari NA, et al. Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clin Infect Dis 2011;52:332–40. [DOI] [PubMed] [Google Scholar]
- [25].Johnson BH, Palmer L, Gatwood J, et al. Healthcare resource utilization and costs associated with herpes zoster in the US. J Med Econ 2016;19:928–35. [DOI] [PubMed] [Google Scholar]
- [26].White RR, Lenhart G, Singhal PK, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics 2009;27:781–92. [DOI] [PubMed] [Google Scholar]
- [27].Li Q, Chen SY, Burstin SJ, et al. Cost of herpes zoster in patients with selected immune compromised conditions in the United States. Open Forum Infect Dis 2016;3:ofw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sandherr M, Hentrich M, Von Lilienfeld-Toal M, et al. Antiviral prophylaxis in patients with solid tumours and haematological malignancies—update of the Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO). Ann Hematol 2015;94:1441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yawn BP, Wollan P, St Sauver J. Comparing shingles incidence and complication rates from medical record review and administrative database estimates: how close are they? Am J Epidemiol 2011;174:1054–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mullane KM, Winston DJ, Wertheim MS, et al. Safety and immunogenicity of heat-treated zoster vaccine (ZVHT) in immunocompromised adults. J Infect Dis 2013;208:1375–85. [DOI] [PubMed] [Google Scholar]
- [31].Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372:2087–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


