Abstract
We aimed to investigate the clinicopathological features of pertussis in children admitted to a tertiary-care university hospital in Brazil.
This was a retrospective cohort study of all pediatric hospital admissions with pertussis from January 1, 2008 to December 31, 2014. We also reported the autopsy findings in children who died.
Fifty-five patients admitted to the hospital over the study period had laboratorial confirmation of Bordetella pertussis infection, 17 (30.9%) needed pediatric intensive care unit (PICU) admission and 6 (10.9%) died. All patients who died were younger than 60 days old and unvaccinated for pertussis; 50% of them had coinfection with respiratory syncytial virus. Leukocyte count ≥40,000/mm3 at hospital admission was an independent risk factor for PICU admission. Mean heart rate during hospitalization ≥160 bpm was an independent risk factor for death. A cut-off point of 41,200 leukocytes/mm3 at hospital admission had sensitivity of 64.7% and specificity of 89.5% to predict PICU admission (area under the curve 0.75) and sensitivity of 100% and specificity of 81.6% to predict death (area under the curve 0.93). Autopsy showed medial thickening of small pulmonary arteries in 80% of patients who had pulmonary hypertension; intravascular aggregates of leukocytes or pulmonary thrombosis were not observed. Immunohistochemical staining of tissue samples obtained at autopsy identified B pertussis and respiratory syncytial virus in pulmonary and extra-pulmonary sites.
Marked leukocytosis at presentation was associated with morbidity and mortality in children hospitalized with pertussis. Implementation of preventive strategies is crucial to diminish the incidence of the disease, especially in young unimmunized infants.
Keywords: autopsy, Bordetella pertussis, critical care, death, hospitalization
1. Introduction
Severe pertussis infection has resurged in industrialized and developing countries in the last 2 decades, despite extensive immunization coverage. According to the World Health Organization, 142,512 cases of pertussis were reported in 2015 worldwide, with an estimated 86% Diphtheria-Tetanus-Pertussis (DTP3) global immunization coverage.[1] There were 25,768 confirmed cases of pertussis in Brazil from 2008 to 2014, with 425 deaths.[2]
Health care costs of severe pertussis infection are high. In Australia and New Zealand, more than USD$1,000,000 per year were spent on direct hospitalization costs related with severe pertussis from 2002 to 2014.[3] In Brazil, more than USD$2,000,000 were spent in 2013 by the Public Health System on hospitalizations for pertussis in children.[4]
Data on the clinical course of severe pertussis requiring hospitalization and/or intensive care unit admission are scarce and a few studies have included only laboratory confirmed cases of Bordetella pertussis infection.[5–8] In addition, autopsy studies in children who died from pertussis are limited, and findings are not consistent.[9–13]
We aimed to study the epidemiology and clinical features of severe pertussis in children admitted to a tertiary-care university hospital in Brazil; to investigate the risk factors for pediatric intensive care unit (PICU) admission and death; and to describe the autopsy findings of children with pertussis who died. We hypothesized that knowledge on epidemiology, clinical features, and anatomopathological data could contribute to the implementation of preventive and treatment strategies aiming at improving the outcome of severe pertussis infection in children.
2. Methods
This was a retrospective cohort study of pediatric patients with pertussis admitted to Hospital das Clinicas of Ribeirão Preto Medical School, University of São Paulo, from January 1, 2008 to December 31, 2014. The study was approved by the Institutional Research Ethics Board (#11958/2014). The informed consent form was waived because of the retrospective nature of the study. All children 0 to 18 years of age hospitalized with laboratory confirmed pertussis were eligible for the study. Laboratory confirmation included isolation of B pertussis in nasopharyngeal aspirates by culture and/or detection of B. pertussis DNA by polymerase chain reaction (PCR). Patients with clinically suspected pertussis but without laboratory confirmation were excluded.
Demographic and clinical data were collected from patients’ health records. The weight z-score and the weight-for-age index were obtained from the World Health Organization Antro Software program available on the World Health Organization's website.[14] Need for PICU admission, laboratory data, treatment, and outcomes were also recorded. For patients who were admitted to the PICU, severity of illness was assessed by Pediatric Index of Mortality (PIM) score[15] and organ dysfunction was assessed by Pediatric Logistic Organ Dysfunction score.[16] Total inotropic support was estimated by a modified inotropic score, calculated as follows: doses of dopamine + dobutamine + milrinone × 10 + epinephrine × 100 + norepinephrine × 100.[17]
Autopsy findings of all children who died were reported. Permission to perform an autopsy was requested from the families of all patients who died in the PICU by attending physicians or residents. Six to 20 hours after death, complete autopsies were performed by a pathology resident under supervision of a senior pathologist (FSR). After “ex situ” inspection of all organs, tissue samples were collected and immersed in 10% buffered formalin solution for 36 hours, and subsequently embedded into paraffin blocks. Sections of 4 μm thickness were stained with hematoxylin and eosin, and sent for histopathological evaluation. Additionally, 4 μm thick tissue sections were deparaffinized, rehydrated, immersed in citrate buffer (10 mmol/L, pH 6.0), and submitted to heated water vapor for 45 minutes for antigen retrieval. Slides were then washed in phosphate buffer solution (PBS), immersed for 20 minutes in 3% hydrogen peroxide for blocking endogenous peroxidases and for 30 minutes in normal serum (Vectastain Elite ABC Universal Kit, Vector Laboratories Inc., Burlingame, CA) for blocking nonspecific proteins. Sections were then incubated with primary anti-B pertussis monoclonal antibodies (clone IgG3; LifeSpan BioSciences, Inc., Seattle, WA, dilution 1: 100) and antirespiratory syncytial virus (Clone 4 clone blend; LifeSpan BioSciences, Inc.: 1: 100 dilution) for 2 hours in a humid chamber at room temperature. After successive washes in PBS, universal biotinylated secondary antibody (Vectastain Elite ABC Universal Kit) was applied for 30 minutes at room temperature. In sequence, the slides were incubated for 5 minutes with the streptavidin–biotin complex (Vectastain Elite ABC Universal Kit) and stained for 5 minutes with diamino-benzidine. The samples were counterstained with Mayer hematoxylin and mounted with Permount (Fischer Scientific, Fairlawn, NJ). All slides were reviewed by a senior pathologist (FSR) and the lung tissue slides were also assessed by an expert pulmonary pathologist (ATF).
2.1. Statistical analysis
Analysis was made using SAS 9.4 (SAS Institute Inc., Cary, NC). Data were expressed as median (range) or absolute frequency (%). The sample size was defined based on the number of hospital admissions of pertussis with laboratory confirmation during the study period. Patients were divided into groups according to the need for PICU admission and outcome (death or survival). Continuous variables were compared by Mann–Whitney U test, and categorical variables were compared by Fisher exact test. Relative risks (RRs) and 95% confidence intervals (CIs) were obtained after adjusting log-binomial regression models. Initially, simple log-binomial regression models were fitted, resulting in crude RRs. Subsequently, the adjustment of multiple log-binomial regression models resulted in adjusted RRs. Receiver-operating characteristic curves were constructed to evaluate the ability of leukocyte count at hospital admission to predict need for PICU admission and death. A 5% significance level was considered in all analyses.
3. Results
Over the 7-year study period, 60 cases of pertussis were admitted to our university hospital. Fifty-five patients were included in the study; 5 were excluded because the diagnosis of pertussis was made based only on the clinico-epidemiological criteria, without laboratory confirmation. Forty-nine patients (89%) had B pertussis DNA detected by PCR in nasopharyngeal secretions and in 17 patients (30.9%) B pertussis was isolated by culture.
Demographic, clinical, and laboratory data of study patients are shown in Table 1. The underlying diseases included congenital heart malformation (n = 2), bronchopulmonary dysplasia (n = 1), end-stage renal disease (n = 1), gastroesophageal reflux disease (n = 1), glucose-6-phosphate dehydrogenase deficiency (n = 1), chronic encephalopathy (n = 1), genetic syndrome (n = 1), and allergic rhinitis (n = 1). Eleven patients (20%) had a viral coinfection detected by PCR in nasopharyngeal aspirates: 8 with respiratory syncytial virus, 2 with adenovirus, and 1 with influenza. All patients were treated with macrolide antibiotics (46 with erythromycin and 9 with clarithromycin). There was a progressive increase in the number of hospitalizations from 2010 to 2014, with 12 hospital admissions per year over the last 3 years (Fig. 1).
Table 1.
Demographic, clinical, and laboratory data of study patients.
Figure 1.
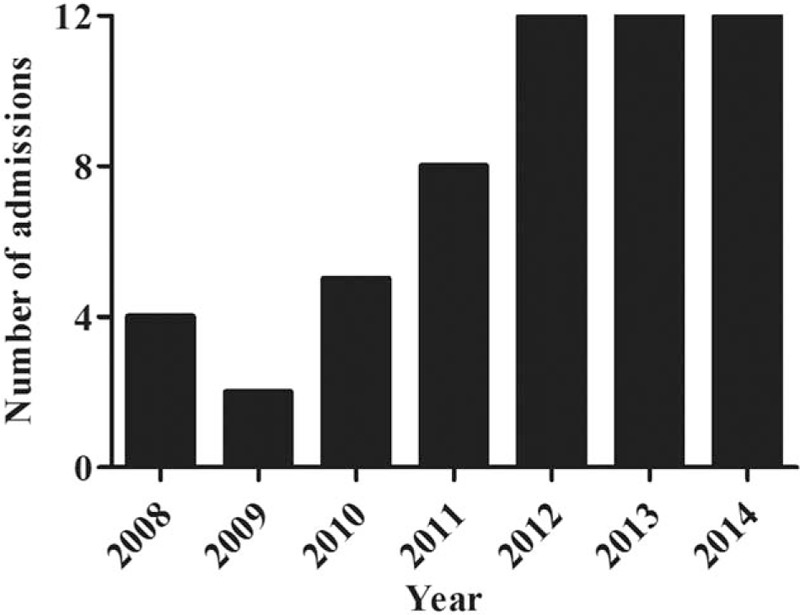
Number of admissions for pertussis per year.
3.1. Comparison between patients admitted to the pediatric ward versus those who needed PICU admission
Patients who needed PICU admission had lower weight, higher heart rate, and greater leukocyte and absolute lymphocyte count at hospital admission compared with those hospitalized in the pediatric ward. Length of hospital stay was also longer in the PICU group (Table 2).
Table 2.
Comparison between ward and pediatric intensive care unit (PICU) groups.

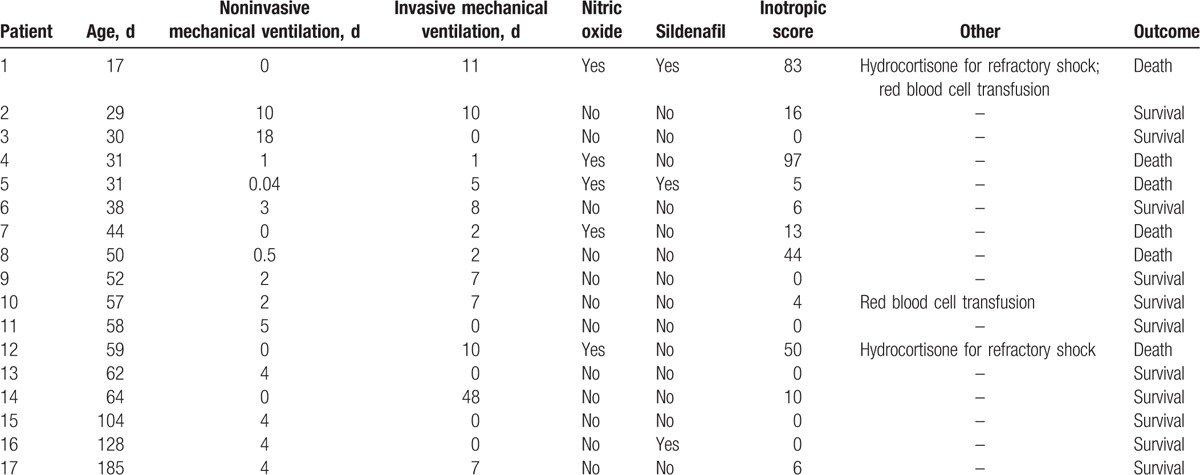
All patients (n = 17) admitted to the PICU needed respiratory support: 4 received only invasive mechanical ventilation, 5 received only noninvasive mechanical ventilation, and 8 patients received invasive and noninvasive ventilation. Duration of noninvasive mechanical ventilation ranged from 0.04 to 18 days (median 4 days) and duration of invasive mechanical ventilation ranged from 1 to 48 days (median 7 days). Eleven patients required treatment with vasoactive/inotropic drugs during PICU stay. Inotropic score ranged from 0 to 97 (median 6). Five patients received nitric oxide and 3 patients received sildenafil for the treatment of pulmonary hypertension. Two patients received stress doses of hydrocortisone for catecholamine-refractory shock and 2 patients received red blood cell transfusion (Table 3).
Table 3.
Characteristics of pediatric intensive care unit treatment and outcome.
3.2. Comparison between death and survival groups
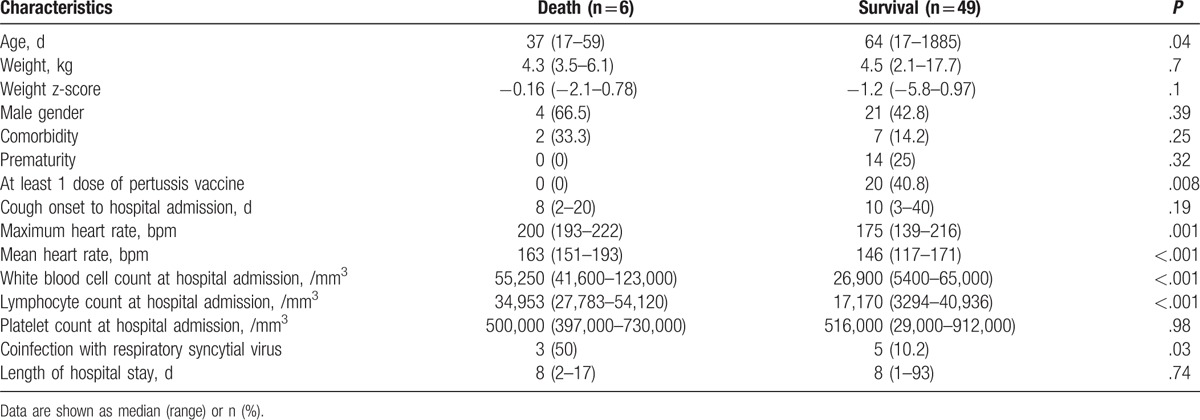
Patients who died were significantly younger and had higher heart rate, greater leukocyte and absolute lymphocyte count at hospital admission, and more coinfection with respiratory syncytial virus compared with those who survived. All nonsurvivors were unvaccinated for pertussis compared with 29 (59.2%) survivors (P = .008) (Table 4).
Table 4.
Comparison between death and survival groups.
All patients who died were admitted to the PICU and received invasive mechanical ventilation for a median time of 3.5 days (range 1–11 days). Leukocyte count was significantly greater in nonsurvivors compared to patients admitted to the PICU who survived, at PICU admission (median 62,050/mm3, range 43,700–117,700/mm3 vs median 24,700, range 5400–65,000/mm3; P = .01), in the middle of PICU stay (median 77,150/mm3, range 37,400–111,300/mm3 vs median 19,300/mm3, range 7800–80,000/mm3; P = .01), and at death/ PICU discharge (median 98,500/mm3, range 59,700–218,400/mm3 vs 21,900/mm3, range 6200–51,600/mm3; P < .001), respectively. Patients who died had higher inotropic score (median 47; range 5–97) compared with those admitted to the PICU who survived (median 0; range 0–16) (P = .002). In addition, Pediatric Logistic Organ Dysfunction score was higher in nonsurvivors compared with survivors (median 26, range 11–40 vs median 11, range 1–21, respectively; P = .002). However, PIM score was not significantly different in both groups (median 59, range 17–74 vs median 37, range 4–73, respectively; P = .59). The causes of death for all patients were respiratory failure, refractory shock, and multiple organ dysfunction. Five of 6 patients (83%) who died received inhaled nitric oxide for the treatment of pulmonary hypertensive crisis compared with none who survived (P = .001).
3.3. Risk factors for PICU admission and death
The results of multiple log-binomial regression analyses showed that leukocyte count ≥40,000/mm3 at hospital admission was an independent risk factor for PICU admission while mean heart rate during hospitalization ≥160 bpm was an independent risk factor for death (Table 5).
Table 5.
Risk factors for admission to the pediatric intensive care unit and death.
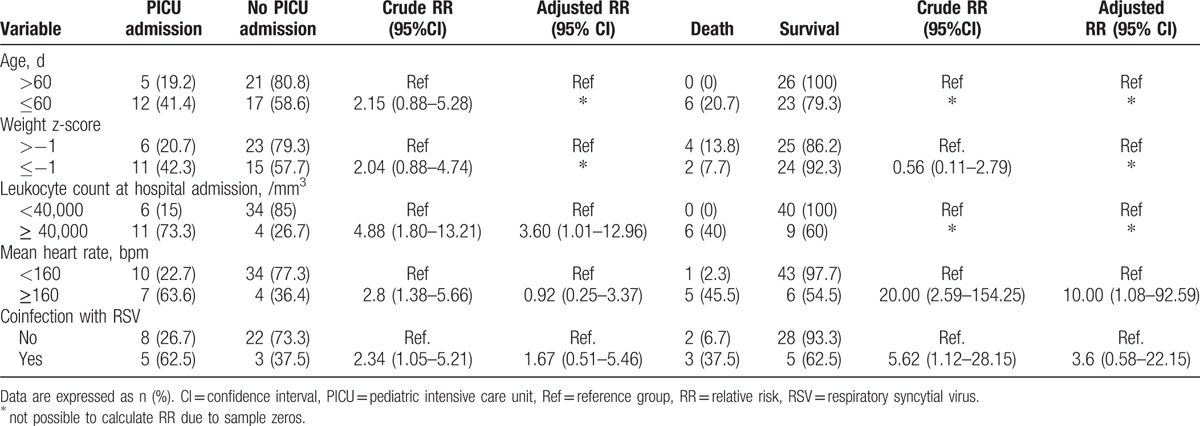
Receiver-operating characteristic curves of leukocyte count at hospital admission for the prediction of PICU admission and death are shown in Fig. 2. A cut-off point of 41,200 leukocytes/mm3 had a sensitivity of 64.7% and specificity of 89.5% to predict PICU admission (area under the curve 0.75, 95% CI 0.59–0.90) and a sensitivity of 100% and specificity of 81.6% to predict death (area under the curve 0.93, 95% CI 0.84–0.98).
Figure 2.
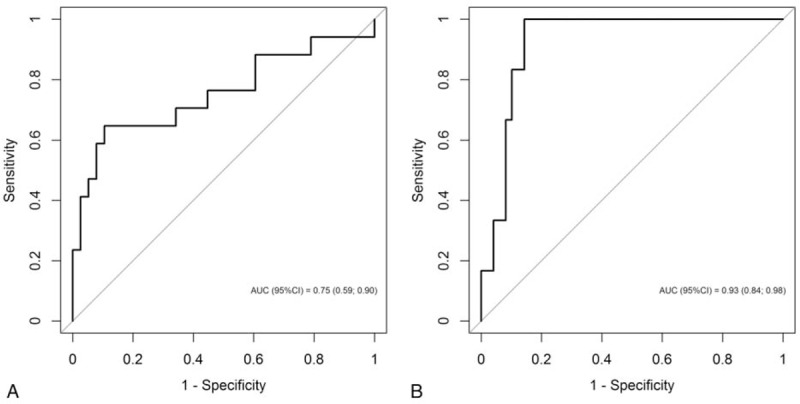
Receiver-operating characteristics (ROCs) curves of leukocyte count at hospital admission for prediction of pediatric intensive care unit admission (A) and death (B).
3.4. Autopsy findings
Bronchopneumonia was found in all patients (n = 6) and necrotizing bronchiolitis was observed in 3 patients. Hyaline membrane was seen in 4 cases, 3 of them had a coinfection with respiratory syncytial virus. Medial thickening of small pulmonary arteries was observed in 4 of 5 patients who had pulmonary hypertension. However, intravascular aggregates of leukocytes or pulmonary thrombosis were not seen in any case. Additional findings included pleural effusion (n = 4), splenic white pulp depletion (n = 4), hepatic steatosis (n = 4), renal vascular congestion (n = 2), and thymus cortical atrophy (n = 2). Immunohistochemistry identified B pertussis in lung tissue in 2 cases and kidney in 1 case. Respiratory syncytial virus was detected in lung tissue in 3 cases, pancreas in 2 cases, liver in 1 case, and brain in 1 case (Fig. 3).
Figure 3.
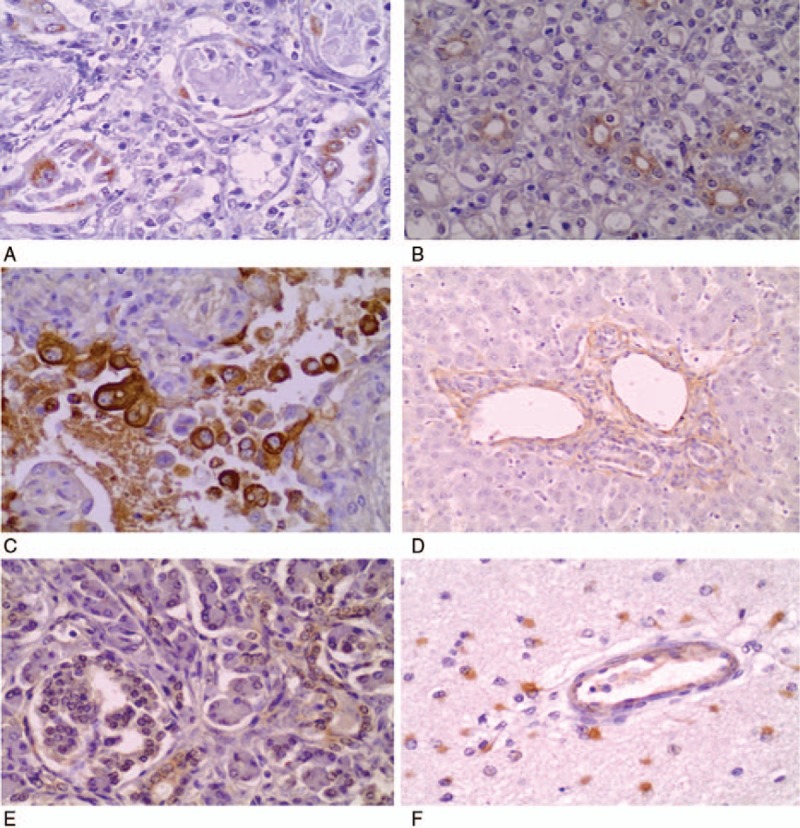
Immunohistochemical staining of Bordetella pertussis in type 1 pneumocytes (A; original magnification, ×400) and in the luminal surface of renal tubular epithelial cells (B; original magnification, ×200). Immunohistochemical staining of respiratory syncytial virus in type 1 pneumocytes (C; original magnification, ×400), periportal space (D; original magnification, ×40), pancreatic acinar cells (E; original magnification, ×200), and microglia cells (F; original magnification, ×400).
4. Discussion
In this study, we report the clinicopathological characteristics of 55 children with severe pertussis admitted to a tertiary-care university hospital in Brazil. We observed an increase in the number of hospitalizations for pertussis over the study period, which was more pronounced between 2012 and 2014. A significant increase in hospitalizations for pertussis from 2007 to 2011 has been reported in Seville, Spain.[18] Also, an increase in the number of PICU admissions for pertussis has been reported between 2009 and 2013 in Australia and New Zealand.[3] In Brazil, there was a marked increase in the number of reported cases of pertussis from 2012 to 2014, with the highest number of cases over the last 30 years being reported in 2014.[19] The increase in the incidence of pertussis may be attributed to several factors, including increased awareness of the disease, the cyclic nature of pertussis, with peaks every 3 to 5 years, and widespread use of PCR assays for laboratory confirmation.[20] This laboratory technique, introduced in Brazil in 2009, allowed improvement in laboratory diagnosis and triplicated laboratory confirmation of pertussis.[21]
We found that increased heart rate and greater leukocyte count at hospital admission were associated with need for PICU admission and death. Leucocyte count at hospital admission was higher than 40,000/mm3 in all patients who died. Moreover, a cut-off point of 41,200 leukocytes/mm3 had a good predictive ability for need for PICU admission and death. Furthermore, leukocyte counts were significantly higher over the course of the PICU stay in nonsurvivors compared with patients admitted to the PICU who survived. In agreement with these results, higher leukocyte count and increased heart rate have been associated with disease severity and death in children with B pertussis infection.[6–9,22] In addition, leukocyte counts greater than or equal to 30,000/mm3 have been associated with a more severe B pertussis infection[6] and a maximum leukocyte count greater than 50,000/mm3 has been associated with an RR of death of approximately 10.[7]
In our study, all patients who died were younger than 60 days and none had received any dose of pertussis vaccine. Our data are in accordance with previous studies showing that infants less than 2 months of age who were unvaccinated for pertussis had a more severe disease and higher mortality.[3,8] We also found that a coinfection with respiratory syncytial virus was more frequent in patients who died compared with survivors (50% vs 10%; P = .03). Indeed, there is evidence that a viral or bacterial coinfection is associated with disease severity in children hospitalized for pertussis.[8] In contrast with other studies showing that prematurity was associated with a more severe disease and higher mortality,[8,22] in our study, all patients with a history of prematurity survived.
We observed that severity of illness assessed by PIM score at PICU admission was similar in survivors and nonsurvivors. However, patients who died had more organ dysfunction and required higher doses of inotropes and vasopressors compared with those who survived. In addition, 5 of 6 patients who died received inhaled nitric oxide for the treatment of pulmonary hypertension. It has been previously shown that the use of nitric oxide is an independent predictor of death in children with pertussis.[3,22] Although the association between need for nitric oxide and death could be related to disease severity, experimental studies have shown that pertussis toxin causes damage to respiratory epithelial cells through the release of nitric oxide, which has cytopathologic effects.[22,23]
The pathogenesis of pulmonary hypertension in infants with pertussis is complex and multifactorial. It has been proposed that, in addition to the prominent muscular component of pulmonary vessels and the highly reactive pulmonary vasculature during the 1st months of life, circulating leukocytes may form aggregates in the lumen of small pulmonary vessels, that ultimately results in pulmonary thrombosis and reduces pulmonary blood flow.[6,11,22] In our study, intravascular aggregates of leukocytes or pulmonary thrombosis were not observed in any case. A study of 15 samples of respiratory tissue obtained at autopsy from children who died of pertussis showed partially occlusive fibrin thrombi in small pulmonary vessels in half of the samples.[11] Nevertheless, the presence of definitive pulmonary thrombosis has not been identified,[11,12] which suggests that physical leucostasis is not the only factor involved in the development of pulmonary hypertension in infants with pertussis.[12] In addition, leukoreduction therapy has not conferred a survival benefit in children with severe pertussis and pulmonary hypertension.[6,7] Although all patients who died in our study had refractory shock, no significant cardiac abnormalities were found. On the contrary, previous autopsy studies of children who died from pertussis have observed extensive myocardial necrosis and subendocardial infarcts affecting both ventricles.[12,24]
Immunohistochemistry identified B pertussis and respiratory syncytial virus in pulmonary and extra-pulmonary sites in our study. Interestingly, B pertussis was shown in renal tissue in 1 case, which suggests that the bacterium or its antigens may reach the bloodstream and disseminate to other organs by the hematogenous route. Indeed, there is evidence of B pertussis bacteremia in immunocompromised patients.[25]
Strategies to decrease the incidence of pertussis among the most vulnerable nonimmunized infants include vaccination of pregnant women during the 3rd trimester of gestation. At the end of this study (November 2014), the Brazilian Public Health System introduced the tetanus-diphtheria-acellular pertussis (Tdap) for pregnant women in the National Vaccination Calendar aiming at decreasing the disease incidence and mortality.
The limitations of our study include its retrospective nature and a relatively small sample size. As this is a single-center study, generalizability of our data may be limited. The strengths of the study are that we included only patients with laboratory confirmed B pertussis infection, and we presented data from complete autopsies of all patients who died over the study period.
In conclusion, marked leukocytosis at presentation was associated with morbidity and mortality in children hospitalized with pertussis. Implementation of preventive strategies is crucial to diminish the incidence of the disease, especially in young unimmunized infants.
Footnotes
Abbreviations: CI = confidence interval, PCR = polymerase chain reaction, PICU = pediatric intensive care unit, PIM = Pediatric Index of Mortality, RR = relative risk.
Funding/support: This study was supported by FAEPA (Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo).
The authors have no conflicts of interest to disclose.
References
- [1].World Health Organization. Immunization, Vaccines and Biologicals; Pertussis. Available at: http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/pertussis/en/. Accessed June 22, 2017. [Google Scholar]
- [2].Óbitos de Coqueluche. Brasil, Grandes Regiões e Unidades Federadas. 1998–2015. Available at: http://portalsaude.saude.gov.br. Accessed June 22, 2017. [Google Scholar]
- [3].Straney L, Schibler A, Ganeshalingham A, et al. Burden and outcomes of severe pertussis infection in critically ill infants. Pediatr Crit Care Med 2016;17:735–42. [DOI] [PubMed] [Google Scholar]
- [4].Mançaneira JF, Benedetti JR, Zhang L. Hospitalizations and deaths due to pertussis in children from 1996 to 2013. J Pediatr (Rio J) 2016;92:40–5. [DOI] [PubMed] [Google Scholar]
- [5].Rocha G, Flôr-de-Lima F, Soares P, et al. Severe pertussis in newborns and young vulnerable infants. Pediatr Infect Dis J 2013;10:1152–4. [DOI] [PubMed] [Google Scholar]
- [6].Murray EL, Nieves D, Bradley JS, et al. Characteristics of severe Bordetella pertussis infection among infants ≤90 days of age admitted to pediatric intensive care units – Southern California, September 2009-June 2011. J Pediatric Infect Dis Soc 2013;2:1–6. [DOI] [PubMed] [Google Scholar]
- [7].Berger JT, Carcillo JA, Shanley TP, et al. Critical pertussis illness in children: a multicenter prospective cohort study. Pediatr Crit Care Med 2013;14:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Marshall H, Clarke M, Rasiah K, et al. Predictors of disease severity in children hospitalized for pertussis during an epidemic. Pediatr Infect Dis J 2015;34:339–45. [DOI] [PubMed] [Google Scholar]
- [9].Mikelova LK, Halperin SA, Scheifele D, et al. Predictors of death in infants hospitalized with pertussis: a case-control study of 16 pertussis deaths in Canada. J Pediatr 2003;143:576–81. [DOI] [PubMed] [Google Scholar]
- [10].Donoso A, Léon J, Ramírez M, et al. Pertussis and fatal pulmonary hypertension: a discouraged entity. Scand J Infect Dis 2005;37:145–8. [DOI] [PubMed] [Google Scholar]
- [11].Paddock CD, Sanden GN, Cherry JD, et al. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis 2008;47:328–38. [DOI] [PubMed] [Google Scholar]
- [12].Sawal M, Cohen M, Irazuzta JE, et al. Fulminant pertussis: a multi-center study with new insights into the clinico-pathological mechanisms. Pediatr Pulmonol 2009;44:970–80. [DOI] [PubMed] [Google Scholar]
- [13].Torre JA, Benevides GN, de Melo AM, et al. The resurgence of a public health threat. Autops Case Rep 2015;5:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].World Health Organization. Child growth standards. Available at: www.who.int/childgrowth/software/en. Accessed June 22, 2017. [Google Scholar]
- [15].Shann F, Pearson G, Slater A, et al. Paediatric index of mortality (PIM): a mortality prediction model for children in intensive care. Intensive Care Med 1997;23:201–7. [DOI] [PubMed] [Google Scholar]
- [16].Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicenter study. Lancet 2003;362:192–7. [DOI] [PubMed] [Google Scholar]
- [17].Carmona F, Manso PH, Vicente WVA, et al. Risk stratification in neonates and infants submitted to cardiac surgery with cardiopulmonary bypass: a multimarker approach combining inflammatory mediators, N-terminal pro-B-type natriuretic peptide and troponin I. Cytokine 2008;42:317–24. [DOI] [PubMed] [Google Scholar]
- [18].Hurtado-Mingo A, Mayoral-Cortés JM, Falcón-Neyra D, et al. [Clinical and epidemiological features of pertussis among hospitalized infants in Seville during 2007-2011]. Enferm Infecc Microbiol Clin 2013;31:437–41. [DOI] [PubMed] [Google Scholar]
- [19].Guimarães LM, Carneiro EL, Carvalho-Costa FA. Increasing incidence of pertussis in Brazil: a retrospective study using surveillance data. BMC Infect Dis 2015;15:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chiappini E, Stival A, Galli L, et al. Pertussis re-emergence in the post-vaccination era. BMC Infect Dis 2013;13:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Coqueluche no Brasil: análise da situação epidemiológica de 2010 a 2014. Bol Epidemiol 2015;46:1–8. [Google Scholar]
- [22].Winter K, Zipprich J, Harriman K, et al. Risk factors associated with infant deaths from pertussis: a case-control study. Clin Infect Dis 2015;61:1099–106. [DOI] [PubMed] [Google Scholar]
- [23].Flak TA, Goldman WE. Autotoxicity of nitric oxide in airway disease. Am J Respir Crit Care Med 1996;154:S202–6. [DOI] [PubMed] [Google Scholar]
- [24].Serra A, Gutiérrez C, Menchaca A. [Tos convulsa grave y su correlación anatomopatológica]. Arch Pediatr Urug 2013;84:136–42. [Google Scholar]
- [25].Trøseid M, Jonassen TØ, Steinbakk M. Isolation of Bordetella pertussis in blood culture from a patient with multiple myeloma. J Infect 2006;52:e11–3. [DOI] [PubMed] [Google Scholar]


