Abstract
The aim of this study was to retrospectively analyze the clinical data of resected adenosquamous lung cancer (ASLC) and to explore the influencing factors and clinicopathological characteristics of the metastasis lymph nodes. A total of 1156 consecutive patients with surgically resected lung cancer from January 2009 to June 2014 were studied. Fifty-four previously diagnosed ASLC patients were re-evaluated by experienced pathologists. IHC and H&E staining were employed to examine the primary focus and metastasis lymph nodes. The relationship between lymph node metastasis and clinicopathological characteristics of ASLC patients was then analyzed and the pathological type of metastasis lymph node was also determined. Forty-nine cases of typical ASLC were included in the study. Of the 49 ASLC patients, 26 cases presented lymph node metastasis. Lymph node metastasis was not associated with gender, smoking, tumor distribution, histological type of primary focus, and preoperative CEA level, but was associated with age ≥ 65 (P < .05) and tumor size ≥ 3 cm (P < .05). Lymph node metastasis adenocarcinoma was the main type in ASLC patients, and was related to the age and tumor size of the primary focus. Further large sample studies are necessary to identify influencing factors and clinicopathological characteristics of metastasis lymph nodes.
Keywords: adenosquamous carcinoma, immunohistochemical staining, lymph node metastasis
1. Introduction
As a subtype of nonsmall cell lung cancer, adenosquamous lung cancer (ASLC) has been detected in 1.1% to 4.5% of patients with surgically resected primary lung cancer.[1–3] Anecdotally, ASLC has a worse prognosis compared with single histology adenocarcinoma (AD) and squamous cell carcinoma (SCC).[1,4,5] Recently, the incidence of ASLC has increased with a tendency toward presenting in younger patients.[6,7] According to 2015 World Health Organization (WHO) histological criteria, ASLC is typed as a carcinoma that shows the components of both SCC and AD, with each comprising at least 10% of the tumor.[8]
Lymph node metastasis is considered the most common marker of malignant tumors that are highly invasive and have a poor prognosis. However, no evidence has proven that the lymph node metastasis rate for ASLC is more aggressive than single histology AD and SCC. Nakagawa et al[9] showed no significant difference between the lymph node metastasis rate for ASLC and that for AD and SCC. However, Maeda et al[5] reported that the lymph node metastasis rate for ASLC was more aggressive than single histology AD, while there was no significant difference when compared with single histology SCC. Due to the heterogeneity of properties for ASLCs, in 1992, Ishida et al[10] proposed a classification system according to the ratio of AD to SCC, and defined predominantly AD with > 60% AD components, predominantly SCC type with > 60% SCC components, and mixed type. In the mixed type, there is no overt evidence of squamous or glandular differentiation demonstrated by light microscopic examination. Therefore, we presumed that ASLCs have 5 different types in metastasis lymph node: AD, SCC, predominantly AD type, predominantly SCC type, and mixed type. Even with a full resection of primary focus and metastatic lymph nodes, a relative high frequency of relapse and distant metastasis still presented in many of the NSCLC patients.[11,12] It is reasonable to choose treatment targeting micro-metastasis focus based on different clinicopathological characteristics between AD and SCC, which might be more effective after resection of the primary focus.
In this study, we retrospectively analyzed the clinical data of 49 cases of surgically resected ASLC patients to compare the metastasis rate between ASLC and single histology type of NSCLC and determine influencing factors. Moreover, H&E staining and IHC were performed to clarify the histological type of metastatic lymph node in ASLC patients.
2. Materials and methods
2.1. Ethics statement
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University and Taizhou Hospital of Zhejiang Province.
2.2. Patient selection
Clinicopathological data were obtained and retrospectively reviewed from January 2009 to June 2014 for 1156 consecutive patients who underwent lung cancer resection surgery at the First Affiliated Hospital of Guangzhou Medical University and Taizhou Hospital of Zhejiang Province. Among these, 49 cases were typical ASLC. The patients underwent resection surgery and lymphadenectomy if the lung cancer was confirmed by intraoperative cryosection. All the patients were free of chemoradiotherapy and target therapy before tumor sampling. Immunohistochemical analysis was performed after obtaining written informed consent.
2.3. Pathological diagnosis and immunohistochemical analysis
All surgical specimens were fixed with 10% formalin and embedded in paraffin. Four to six paraffin embedded samples were selected according to the tumor size and involved range, and stained with H&E. ASLC diagnostic criteria were defined as the presence of components of both AD and SCC, with at least 10% of each component. All the specimens were re-evaluated by two senior pathologists. When H&E staining alone failed to diagnose ASLC, the presence of SCC (CK5/6, p40, and p63) and AD [CK7, Thyroid Transcription Factor-1 (TTF-1), and NapsinA] markers was examined using IHC in the adenosquamous region and metastatic lymph nodes by the Super Vision two-step method. The monoclonal antibodies including CK5/6, p63, CK7, and TTF1 were purchased from Dako, Denmark. Meanwhile, p40 and NapsinA were from Jinqiao Biological Company, China. The process of IHC was carried out according to manufacturer instructions.
2.4. Statistical analysis
All the statistical analyses were performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL). The enumeration data were analyzed with χ2 test or the Fisher exact test, as appropriate, to compare the difference between groups. Two-tailed P < .05 was considered as statistically significant.
3. Results
3.1. Patient demographic data
Among 1156 patients, 738 (63.8%) were AD, 213 (18.4%) were SCC, 32 (2.8%) were small cell lung cancer (SCLC), 46 (4.0%) were neuroendocrine lung tumor, 73 (6.3%) were other types, and 54 (4.7%) were primary diagnosed ASLC. After excluding five cases of atypical ASLC, analysis was done on the clinical data of 49 cases of typical ASLCs.
We compared clinicopathological characteristics among ASLC, AD, and SCC (Table 1). Mean age of SCC was significantly higher than ASLC and AC (63.1, 59.2 and 59.4 years, respectively, P < .05). SCC patients were more frequently male when compared to ASLC patients. The ratio of performance score (PS) I in ASLC patients was significantly higher than that in AC patients (30.6% vs. 13.3%, P < .01), while no significant difference was found compared to SCC patients. A significantly higher ratio of smoking was found in SCC patients than in ASLC patients (P < .05), whereas a significantly lower ratio of smoking was found in AC patients than in ASLC (P < .01). A significantly lower ratio of CEA elevation was found in SC patients than in ASLC patients (P < .05). The frequency of T2a and T2b was higher in ASLC patients than in AD patients (T2a: 36.7% vs. 29.8%; T2b: 12.2% vs. 3.5%). A significantly higher lymph node metastasis rate was found in ASLC patients than in AD patients (P < .01), while no significant difference was found between ASLC and SCC patients. N2 stage lymph node metastasis was higher in ASLC patients (40.5%) than that in AD (18.7%) and SCC patients (21.6%). Therefore, a higher IIIa pathological stage was found in ASLC (40.5%) than in AD (17.9%) and SCC (18.3%) patients.
Table 1.
Characteristics of patients with adenosquamous carcinoma, adenocarcinoma, and squamous cell carcinoma, and comparisons among adenosquamous carcinoma and single histologic carcinoma types.
3.2. Histopathological type of primary focus
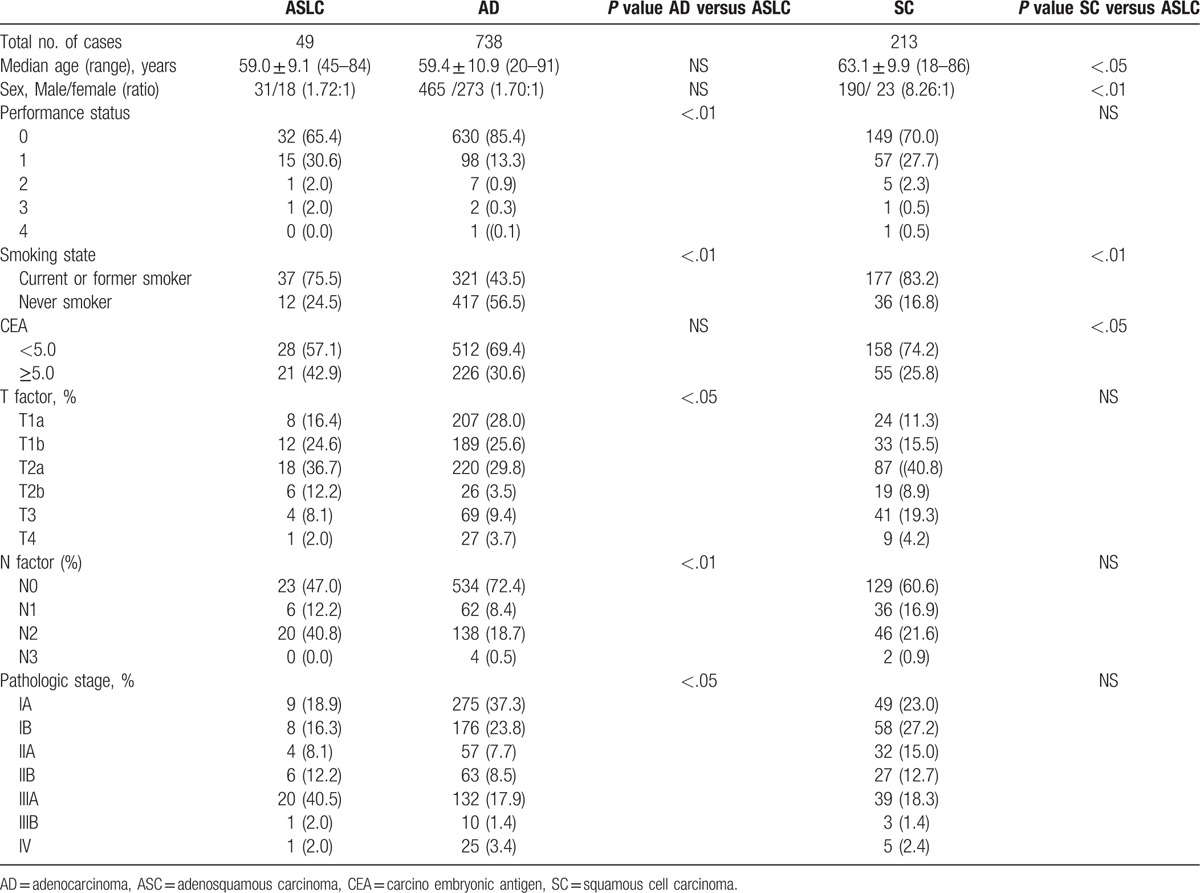
Based on heterogenic characteristics, ASLC could be divided into three different types: predominantly AD, predominantly SCC, and mixed type.[10] After microscope examination, all 49 cases of specimens were confirmed with bi-direction differentiation. Among these 49 cases, 35 patients, 4 patients, and 10 patients presented with predominantly AD, predominantly SCC, and mixed type, respectively. Around 6 cases and 5 cases of ASLC were found with keratinization and intercellular bridge. An acinar, tubule, or papillary structure could be found in the AD component. Separated, combined, and mixed adeno- and squamous components were found (Fig. 1).
Figure 1.
H&E staining of adenosquamous lung cancer (ASLC). Both components of adenocarcinoma (left side) and SCC (right side) could be found in the tissue (200×). ASLC = adenosquamous lung cancer, SCC = squamous cell carcinoma.
3.3. Immunohistochemical results of primary focus
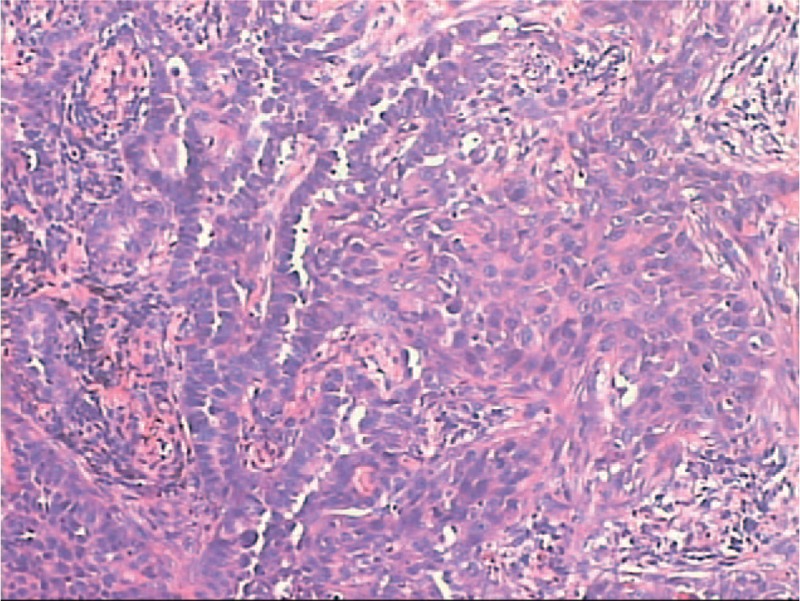
Immunohistological analysis of CK7, TTF-1, CK5/6, p40, and p63 were employed to differentiate the components of AD or SCC (Fig. 2). However, weak expression of TTF-1 was found in SCC, whereas the expression of p63 was also found in some of the AD cases. All 49 cases of tumor specimens were analyzed with immunohistochemical staining of CK7, TTF-1, CK5/6 p40, and p63. Among these specimens, TTF-1 was expressed in 46 cases (93.8%) of the AD components and only three cases (6.2%) of SCC components. Moreover, positive expression of CK7 was found in all the AD components and 12 cases (24.5%) of SCC components. Furthermore, CK5/6 and p40 were expressed in all squamous carcinoma components, whereas weak expression of p63 was also found in four cases (8.2%) of solid AD component. In addition, six cases of specimens presented with solid mucinous structure, in which TTF-1 was weakly expressed in two cases while Napsin A was positive in all the cases.
Figure 2.
Immunohistochemical analyses of adenosquamous lung cancer (ASLC). (A) CK7 staining located in cytoplasm with strong positive expression on adenocarcinoma and weak positive expression of SCC (200×); (B) TTF-1 staining located in cell nucleus with positive expression on adenocarcinoma and without expression on SCC (200×); (C) CK5/6 staining located in cytoplasm with strong positive expression on SCC and without expression on adenocarcinoma (200×); (D) P63 located in cell nucleus with positive expression on SCC and without expression on adenocarcinoma (200×). ASLC = adenosquamous lung cancer, SCC = squamous cell carcinoma, TTF-1 = thyroid transcription factor-1.
3.4. The relationship between lymph node metastasis and clinicopathological characteristics
Lymph node metastasis presented in 26 cases (57%). Of these, 6 (23%) were N1 and 20 (77%) were N2. We further compared the relationship between lymph node metastasis and clinicopathological characteristics (Table 2). Lymph node metastasis was not associated with gender, smoking, tumor distribution, histological type of primary focus, or preoperative CEA level. However, lymph node metastasis was more frequently found in patients aged < 65 years. (88% vs 53%, P < .05). Moreover, lymph node metastasis presented more frequently in patients with tumors ≥ 3 cm tumor than in patients with tumors < 3 cm (74% vs 35%, P < .05).
Table 2.
Relationship among age, gender, smoking, location, tumor size, histological type, CEA and lymph node status (N = 49).
3.5. Histopathological type of the metastatic lymph node
Moreover, 24 cases were diagnosed as AD according to the histopathological type of the metastatic lymph node, whereas only two cases presented with lymph node metastasis adenosquamous carcinoma. In addition, TTF-1 was expressed in all 24 cases of AD, whereas TTF-1 and p40 expression was found in the case of lymph node metastasis adenosquamous carcinoma.
4. Discussion
ASLC is a rare nonsmall cell lung cancer, comprising 0.4% to 4% of lung cancers,[8] and the incidence of ASLC has increased gradually in recent years. In this study, we found a 4.2% incidence of surgically resected lung cancer, which was consistent with previous reports. However, a similar study from China reported a 9.7% (680/6990) incidence of ASLC,[8] which was significantly higher than previous descriptions. However, IHC was only performed in 55 cases of ASLCs, but not in the other 625 cases; therefore, further analysis should be performed to clarify the incidence of the disease. Furthermore, the different reported incidences might also be associated with different sample scope and diagnosis criteria.
ASLC is typed as a carcinoma that shows (routinely by H&E staining) the components of both SCC and AD in at least 10% of the tumor. Even for experienced pathologists, a single histological analysis of the tumor sometimes could not differentiate the range of the AD and SCC components, especially in poorly differentiated AD and SCC. Even when the SCC showed alveolar or bronchial acinar by H&E staining, misdiagnosis of adeno-differentiated carcinoma could occur. A similar misdiagnosis could also be made in SCC components. Moreover, the diagnosis of adenosquamous carcinoma was difficult when the AD is restricted to a solid mucinous structure.
With the introduction of immunohistochemical markers, it has become routine to employ a panel of markers to aid in histological diagnosis.[13] Among these markers, cytology application of p40, p63, and TTF-1 are used for differential diagnosis of AD and SCC.[14] However, previous studies also showed a weak expression of TTF-1 in SCC and focal staining of p63 in AD. We also confirmed this phenomenon here. Therefore, multiple immunohistochemical markers are necessary for differential diagnosis in AD and SCC. In this study, we employed CK7, TTF-1, CK5/6, p40, and P63 alone or in combination to differentiate the histology subtype in ASLC with lower differentiation.
Anecdotally, ASLC has a worse prognosis compared with single histology AD and SCC.[1,4,5] Lymph node metastasis is considered the most common marker of malignant tumors that are highly invasive and have a poor prognosis. The results of this study are similar to findings by Maeda where a higher incidence of lymph node metastasis could be found in ASLC than in single AD and SCC.
Until now, no report has confirmed that a higher incidence of lymph node metastasis could be found in ASLC than single AD and SCC. In this study, we found 26 cases of lymph node metastasis, and further analysis on the influencing factors of lymph node metastasis revealed that lymph node metastasis was not associated with gender, smoking, tumor distribution, histological type of primary focus, or preoperative CEA level, but was associated with age and tumor size of the ASLC patients. We found a greater frequency of lymph node metastasis in patients aged < 65 and with tumors ≥ 3 cm.
Both AD and SCC components could be presented in the primary focus of ASLC. However, studies on the lymph node histological type have not yet been reported. This study is the first to report AD as the main component in metastatic lymph node in ASLC. We attributed this feature to the dominant proportion of AD in ASLC patients, and further studies with larger sample sizes are necessary to clarify the difference in invasive and metastasis properties between AD and SCC.
To further analyzed influencing factors on lymph node metastasis in ASLC patients, and 49 ASLC patients were divided into a lymph node metastasis group and a nonmetastasis group. We found a greater frequency of lymph node metastasis in patients aged < 65 and with ≥ 3 cm. The primary focus of ASLC contains both components of AD and SCC, which could be classified into three subtypes according to histology components (defined predominantly AD with > 60% AD components, predominantly SCC type with > 60% SCC components, and mix type). We presumed five different types in metastasis lymph node: AD, SCC, predominantly AD type, predominantly SCC type, and mixed type (Fig. 3). However, only two cases presented with lymph node metastasis adenosquamous carcinoma. We attributed these results to the following reasons: (1) the frequency of predominantly AD is higher in ASLC patients with lymph node metastasis; (2) the higher risk of lymph node metastasis presents in the AD component of ASLC patients; (3) the study sample was too small and no other case of lymph node metastasis was found; and (4) AD and SCC components may be derived from the same tumor stem cell.
Figure 3.
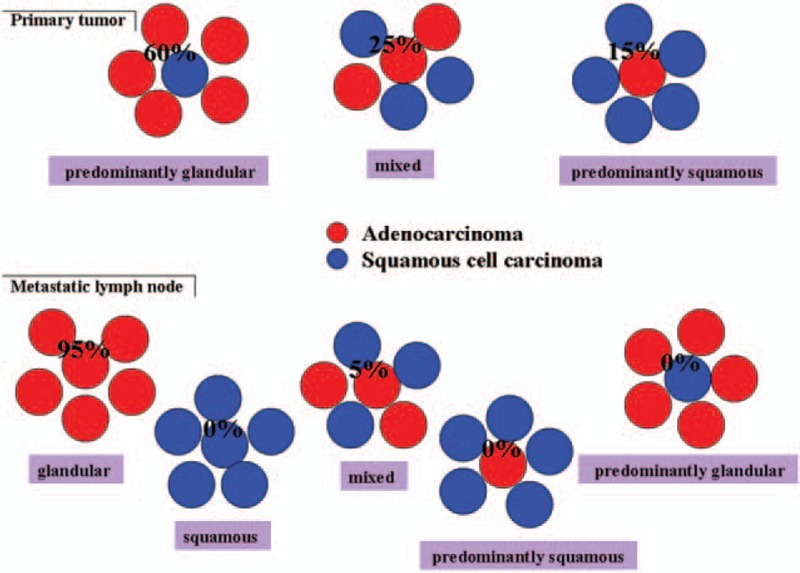
Schematic figure of ASLC histological type. AD and SCC were found in ASLCs. We defined predominantly AD with >60% AD components, predominantly SCC type with >60% SCC components, and mixed type and five different types in metastasis lymph node: AD, SCC, predominantly AD type, predominantly SCC type, and mixed type. AD = adenocarcinoma, ASLC = adenosquamous lung cancer, SCC = squamous cell carcinoma.
In conclusion, this study is the first to report on lymph node metastasis histological type in ASLC patients and found that AD was the dominant component of lymph node metastasis. This paper also found that the age of the patient and tumor size could affect lymph node metastasis in ASLC. Further larger sample studies should be performed to verify influencing factors and histopathological type in ASLC.
Footnotes
Abbreviations: AD = adenocarcinoma, ASLC = adenosquamous lung cancer, CEA = carcino embryonic antigen, HE = hematoxylin-eosin, LL = left lower, LU = left upper, NSCLC = nonsmall lung cancer, RL = right lower, RM = right middle, RU = right upper, SCC = squamous cell carcinoma, SCLC = small cell lung cancer, WHO = World Health Organization.
MK, JJ, and XC contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Filosso PL, Ruffini E, Asioli S, et al. Adenosquamous lung carcinomas: a histologic subtype with poor prognosis. Lung Cancer 2011;74:25–9. [DOI] [PubMed] [Google Scholar]
- [2].Uramoto H, Yamada S, Hanagiri T. Clinicopathological characteristics of resected adenosquamous cell carcinoma of the lung: risk of coexistent double cancer. J Cardiothorac Surg 2010;5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gawrychowski J, Brulinski K, Malinowski E, et al. Prognosis and survival after radical resection of primary adenosquamous lung carcinoma. Eur J Cardiothorac Surg 2005;27:686–92. [DOI] [PubMed] [Google Scholar]
- [4].Cooke DT, Nguyen DV, Yang Y, et al. Survival comparison of adenosquamous, squamous cell, and adenocarcinoma of the lung after lobectomy. Ann Thorac Surg 2010;90:943–8. [DOI] [PubMed] [Google Scholar]
- [5].Maeda H, Matsumura A, Kawabata T, et al. Adenosquamous carcinoma of the lung: surgical results as compared with squamous cell and adenocarcinoma cases. Eur J Cardiothorac Surg 2012;41:357–61. [DOI] [PubMed] [Google Scholar]
- [6].Riquet M, Perrotin C, Lang-Lazdunski L, et al. Do patients with adenosquamous carcinoma of the lung need a more aggressive approach? J Thorac Cardiovasc Surg 2001;122:618–9. [DOI] [PubMed] [Google Scholar]
- [7].Jia XL, Chen G. EGFR and KRAS mutations in Chinese patients with adenosquamous carcinoma of the lung. Lung Cancer 2011;74:396–400. [DOI] [PubMed] [Google Scholar]
- [8].Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th edn.2015;Lyon: International Agency for Research on Cancer, 9–96. [DOI] [PubMed] [Google Scholar]
- [9].Nakagawa K, Yasumitu T, Fukuhara K, et al. Poor prognosis after lung resection for patients with adenosquamous carcinoma of the lung. Ann Thorac Surg 2003;75:1740–4. [DOI] [PubMed] [Google Scholar]
- [10].Ishida T, Kaneko S, Yokoyama H, et al. Adenosquamous carcinoma of the lung. Clinicopathologic and immunohistochemical features. Am J Clin Pathol 1992;97:678–85. [DOI] [PubMed] [Google Scholar]
- [11].Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer 2005;50:227–34. [DOI] [PubMed] [Google Scholar]
- [12].Kang CH, Ra YJ, Kim YT, et al. The impact of multiple metastatic nodal stations on survival in patients with resectable N1 and N2 nonsmall-cell lung cancer. Ann Thorac Surg 2008;86:1092–7. [DOI] [PubMed] [Google Scholar]
- [13].Rao N. Adenosquamous carcinoma. Seminars in diagnostic pathology 2014;31:271–7. [DOI] [PubMed] [Google Scholar]
- [14].Wu M, Szporn AH, Zhang D, et al. Cytology applications of p63 and TTF-1 immunostaining in differential diagnosis of lung cancers. Diagn Cytopathol 2005;33:223–7. [DOI] [PubMed] [Google Scholar]


