Absract
Rationale:
Although still relatively rare, multiple primary malignant neoplasms (MPMNs) have been increasingly reported in recent years.
Patient concerns and diagnoses:
A 65-year-old man was referred to our hospital for a painless, incidental left axillary lump. Ultrasound showed enlarged left axillary lymph nodes. An excisional biopsy was conducted on 3 lymph nodes. The pathological diagnosis was determined to be metastatic adenocarcinoma and mantle cell lymphoma (MCL) in the lymph nodes. Further physical examination of the patient yielded a 1.5-cm hard, left subareolar mass.
Interventions and outcomes:
The patient underwent modified radical mastectomy. The diagnosis was grade II invasive ductal carcinoma (stage IIA). The axillary lymph node showed MCL (stage I, group A), but not metastatic ductal carcinoma. The patient received chemotherapy, including 6 courses of CHOP (A chemotherapy protocol consists of cyclophosphamide 1.2 g day 1, doxorubicin 80 mg day 1, vindesine 4 mg day1, and prednisone 90 mg from day 1 to 5) for lymphoma and breast cancer. The patient was also administered endocrine therapy. After a 54-month follow-up, the patient was well with no evidence of disease.
Lessons:
MPMNs are easily misdiagnosed as a primary and metastatic tumor, leading to delayed or erroneous treatment. Male breast cancer in a patient with MCL is rare. Early diagnosis and proper therapy are necessary for an optimal prognosis. Further studies are required to define the mechanisms and risk factors of MPMNs.
Keywords: male breast cancer, mantle cell lymphoma, multiple primary malignant neoplasms
1. Introduction
Billroth was the first to report a case of dual malignancies in 1889.[1] Multiple primary malignant neoplasms (MPMNs) are rarely reported and are defined as the diagnosis of ≥2 independent, primary malignancies of different histologies/origins in a single individual. The prevalence of MPMNs varies from 0.73% to 11.70% of all patients diagnosed with carcinomas in western countries.[1] With improved health care provision, an increasing number of patients survive cancer for longer intervals, which in turn increases the risk of developing a second tumor. In this study, we report a patient with male breast cancer (MBC) and coexisting mantle cell lymphoma (MCL).
2. Patient information
A 65-year-old man was referred to our hospital for a painless, incidental left axillary lump.
2.1. Clinical findings
Examination by palpation revealed a well-defined, rounded 4-cm mass of soft texture and distinct boundary under the left armpit. There was no fixation to the skin.
2.2. Diagnostic assessment
Ultrasound showed enlarged left axillary lymph nodes. An excisional biopsy was conducted on 3 lymph nodes, measuring 5.0, 4.0, and 0.8 cm, respectively. These nodes were white to tan and fleshy with areas of hemorrhage and necrosis. The resection specimens were fixed in formalin, routinely processed, and 3-μm sections were stained with hematoxylin and eosin. Immunohistochemistry (IHC) was also performed.
Microscopic examination of 2 of the 3 lymph nodes showed effaced architecture, being replaced by small- to medium-sized atypical lymphocytes (Fig. 1AA), with a mantle zone and partly nodular growth. On immunohistochemical examination, the neoplastic lymphoid cells were found to be positive for CD5 (Fig. 1B), CD20 (Fig. 1C), cyclin D1 (Fig. 1D), CD79α, Bcl-2, p53, and Ki-67 (5%), and negative for CD3, CD45RO, CD10, Bcl-6, Mum-1, CD21, and CD23. Therefore, a diagnosis of MCL was rendered.
Figure 1.
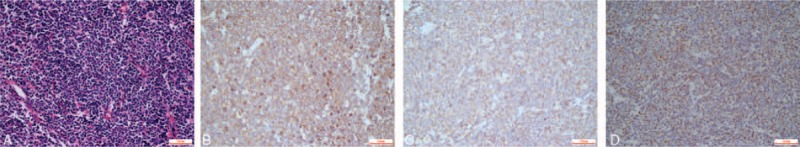
(A) A biopsy from the armpit masses showed small- to medium-sized atypical lymphocytes with mantle zone growth and partial nodular growth (hematoxylin and eosin stain, magnification ×400). Immunohistochemistry analysis showed that the tumor cells were positive for (B) CD5, (C) CD20, and (D) cyclin D1 (all, magnification ×400).
Examination of 1 of the 3 lymph nodes revealed the presence of malignant cells, with enlarged hyperchromatic nuclei containing duct/tubule formations. IHC showed that the malignant cells were positive for CK7, CK19, CA153, CEA, cyclooxygenase-2, and synaptophysin, and negative for thyroglobulin, thyroid transcription factor-1, prostate-specific antigen, chromogranin A, CA19-9, CK20, AFP, and CD117. The pathological diagnosis was determined to be metastatic adenocarcinoma.
Further physical examination of the patient yielded a 1.5-cm hard, left subareolar mass with a smooth surface and ill-defined margin that was fixed to the underlying tissue. A computed tomography scan showed the patient's subareolar mass with axillary lymph node enlargement (Fig. 2). A lumpectomy was performed and frozen sections showed adenocarcinoma with duct/tubule formations.
Figure 2.
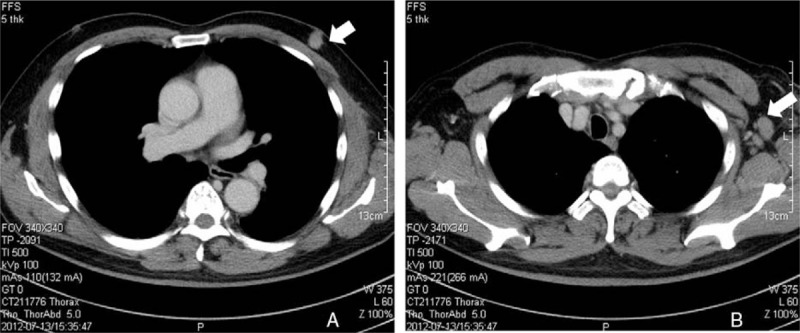
(A) CT scan showing the patient's left subareolar mass (arrow). (B) CT scan showing an enlarged axillary lymph node (arrow). CT = computed tomography.
The patient underwent modified radical mastectomy. The diagnosis was invasive ductal carcinoma (grade II) (Fig. 3AA). IHC showed that the malignant cells were strongly positive for estrogen receptor (ER) (Fig. 3B) and progesterone receptor (PR) (Fig. 3C), positive for E-cadherin, p53, and Ki-67 (50%), and negative for human epidermalgrowth factor receptor 2 (HER2) (Fig. 3D), gross cystic disease fluid protein-15, synaptophysin, chromogranin A, and high-molecular-weight cytokeratin. The resected margins were negative. A total of 30 axillary lymph nodes showed no metastatic ductal carcinoma, but 28 of 30 axillary lymph nodes showed MCL.
Figure 3.
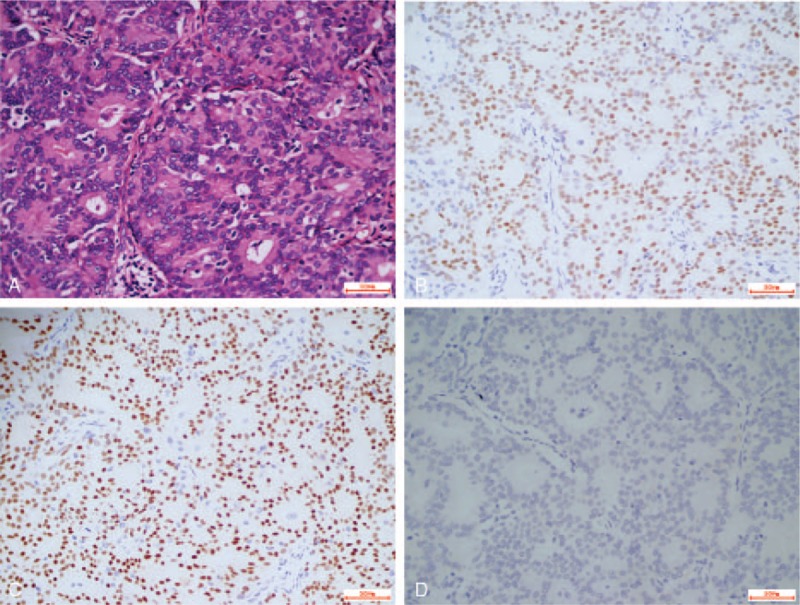
(A) Histological appearance of invasive ductal carcinoma (hematoxylin and eosin stain, magnification ×400). Immunohistochemistry analysis showed that the tumor cells were positive for (B) estrogen receptor and (C) progesterone receptor, and (D) tumor cells were negative for HER2 (all, magnification × 400). HER2 = human epidermalgrowth factor receptor 2.
The final diagnosis was grade II infiltrating ductal carcinoma of the left breast (stage IIA) and MCL of the left axillary lymph node (stage I, group A).
2.3. Therapeutic intervention
The patient received chemotherapy, including 6 courses of CHOP (cyclophosphamide 1.2 g day 1, doxorubicin 80 mg day 1, vindesine 4 mg day 1, and prednisone 90 mg from day 1 to 5) for lymphoma and breast cancer. The patient was also administered endocrine therapy.
2.4. Follow-up and outcomes
The patient underwent ultrasonography every 6 months after the operation. He was well with no evidence of recurrence or distant metastasis after 54 months of follow-up.
3. Discussion
The diagnosis of multiple primary malignancies in this study was made according to the criteria developed by Warren and Gates[2]: each tumor must be malignant; each tumor must have its own unique pathological features; and metastasis or recurrence must be excluded. Depending on the time of diagnosis, the dual malignancies can be synchronous or metachronous. This case showed a combination of MBC and MCL, 2 completely different cancer types, diagnosed at the same time, and therefore met the criteria for simultaneous MPMNs.
The pathogenesis of MPMNs remains unclear. Risk factors include age, genetic predisposition, alcohol, smoking, and chemoradiotherapy.[3] The association between MBC and MCL is unknown and should be further investigated. This patient denied smoking or alcohol intake, had no history of exposure to radioactive substances and had no family history of cancer. One possible mechanism of the formation of the MPMNs in this patient may be immune defects. In addition, the polycentric theory of tumorigenesis[4] could also be a possible mechanism. Based on this theory, there are multiple tumor-susceptible centers in the human body. Eventual tumor formation is subject to different carcinogenic factors, locations, and types, resulting in multiple primary tumors. The incidence rates of both MBC and MCL are very low, underpinning the rarity of the current case.
MBC is rare, accounting for ∼0.6% of all breast cancers and <1% of all cancers in men.[5] The pathogenesis of MBC is not clear. Known risk factors include genetic defects (BRCA2), estrogen–androgen imbalance, radioactive injury, and testicular disease (cryptorchidism, orchitis, or orchiectomy).[6] There is no direct evidence of an association between gynecomastia and MBC. MBC frequently displays skin, nipple, and chest involvement at the early stages, with the periareolar lymphatic network facilitating tumor metastasis. Moreover, MBC has a poor prognosis. Based on 2537 cases and 38,316 cases of breast cancer in men and women, respectively, Giordano et al retrospectively found that, when compared to female breast cancer, MBC had a later age of onset, demonstrated a higher rate of lymph node metastasis, was diagnosed at later clinical stages, and had a higher tendency to be ER/PR-positive.[5] The median age of onset has been reported to be 68 years, with half of these patients being diagnosed with stage III or IV disease.[7] All pathological types of breast cancer found in women have been reported in men, with invasive ductal carcinoma accounting for the majority of cases (93.7%), followed by papillary carcinoma (2.6%), medullary carcinoma, tubular carcinoma, mucinous carcinoma, metaplastic carcinoma, inflammatory breast cancer, and Paget disease.[5,8] The current case showed tubular structures and was compatible with infiltrating ductal carcinoma. Most MBCs have been reported to be hormone receptor positive and HER2 negative,[9] which is similar to the findings in this case (Table 1).
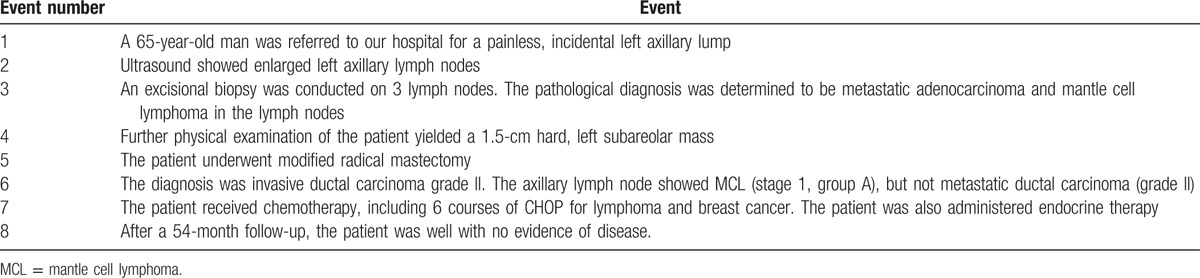
Table 1.
Timeline of the patient's history.
MCL is a subtype of B-cell non-Hodgkin's lymphoma with a unique immune phenotype, genetic features, and clinical characteristics. MCL is relatively rare, accounting for <10% of all lymphomas.[10] In addition, this disease is more common in older men, often involving the gastrointestinal tract, liver, spleen, bone marrow, and peripheral blood, with diagnosis at later clinical stages (III or IV).[11] The patient in this report had lymphadenopathy, but not splenomegaly, and the bone marrow was spared. The MCL originated from the mantle zone, and cytogenetically showed the characteristic t (11;14) (q13;q32) translocation involving the BCL-1 and immunoglobulin heavy chain genes; the enhanced BCL-1 gene expression resulted in cyclin D1 overexpression.[12] In this patient's case, the lymph node structure was effaced by single small- and medium-sized lymphocytes, with a partial mantle zone and nodular-like growths and atrophy of the non-neoplastic follicular centers. Immunotyping showed a cell profile that confirmed the diagnosis of MCL. Because of different tumor pathological types and clinical stages, MPMNs have different treatment principles. Therefore, the appropriate treatment should be based on the specific characteristics of the primary tumors. MPMNs are easily misdiagnosed as a primary and metastatic tumor, or the second primary tumor is undetected altogether, leading to delayed or erroneous treatment. Although MBC is rare, the incidence of this tumor is increasing.[5] Current treatment principles are based on protocols for postmenopausal female breast cancer patients. However, whether this represents the optimal therapy for male patients is uncertain, as biological and epidemiological differences exist. MCL runs an aggressive clinical course and is not sensitive to radiotherapy or chemotherapy. The prognosis is poor, with an average survival period of 2 to 5 years.
To the best of our knowledge, this is the first report of MBC in a patient with MCL. This diagnosis is consistent with MPMNs, which have an increasing incidence and an unknown underlying mechanism. Early diagnosis and treatment are needed for optimal prognosis. Further studies are required to define the mechanisms and risk factors of MPMNs. Molecular genetics could be used in the early detection and prevention of MPMNs, potentially increasing patient survival.
Footnotes
Abbreviations: IHC = immunohistochemistry, MBC = male breast cancer, MCL = mantle cell lymphoma, MPMNs = multiple primary malignant neoplasms.
Ethics approval and consent to participate: The ethical approval and documentation for a case report was waived with approval of the TongDe Hospital of Zhejiang Province.
Patient consent: The patient gave verbal consent to the use of his patient history and all the related images and information for scientific purposes, as well as for the publication of this case.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Billroth T. Die Allgemeine Chirurgische Pathologie and Therapie. In: Reimer G. 51 Vorlesungen-Ein Handbuch fur Studierende and Artze, 14. Berlin: Auflage, 1889. [Google Scholar]
- [2].Warren S, Gates O. Multiple primary malignant tumours: a survey of the literature and a statistical study. Am J Cancer 1932;16:1358–414. [Google Scholar]
- [3].Hu NC, Hsieh SC, Chen TJ, et al. Multiple primary malignancies including colon, stomach, lung, breast, and liver cancer: a case report and literature review. Chin Med J 2009;122:3091–3. [PubMed] [Google Scholar]
- [4].Wen D, Wang S, Zhang L, et al. Early onset, multiple primary malignancies, and poor prognosis are indicative of an inherited predisposition to esophageal squamous cell carcinoma for the familial as opposed to the sporadic cases-an update on over 14-year survival. Eur Med Genet 2009;52:381–5. [DOI] [PubMed] [Google Scholar]
- [5].Giordano SH, Cohen DS, Buzdar AU, et al. Breast carcinoma in men: a population based study. Cancer 2004;101:51–7. [DOI] [PubMed] [Google Scholar]
- [6].Leinung S, Horn LC, Backe J. Male breast cancer: history, epidemiology, genetic and histopathology. Zentralbl Chir 2007;132:379–85. [DOI] [PubMed] [Google Scholar]
- [7].Teo JY, Tan PH, Yong WS. Male breast cancer in Singapore: 15 years of experience at a single tertiary institution. Ann Acad Med Singapore 2012;41:247–51. [PubMed] [Google Scholar]
- [8].Aqrawal A, Avantunde AA, Rampaul R, et al. Male breast cancer: a review of clinical management. Breast Cancer Res Treat 2007;103:11–21. [DOI] [PubMed] [Google Scholar]
- [9].Bloom KJ, Govil H, Gattuso P, et al. Status of HER-2 in male and female breast carcinoma. Am J Surg 2001;182:389–92. [DOI] [PubMed] [Google Scholar]
- [10].Klapper W. Histopathology of mantle cell lymphoma. Semin Hematol 2011;48:148–54. [DOI] [PubMed] [Google Scholar]
- [11].Goy A, Kahl B. Mantle cell lymphoma: the promise of new treatment options. Crit Rev Oncol Hematol 2011;80:69–86. [DOI] [PubMed] [Google Scholar]
- [12].Kallen ME, Kim Y, Yang L, et al. A cryptic t (11;14) translocation in mantle cell lymphoma highlights the importance of FISH. J Assoc Genet Technol 2015;41:13–6. [PubMed] [Google Scholar]


