Abstract
Background:
TAVR is a rapidly spreading treatment option for severe aortic valve stenosis. Significant coronary artery disease (CAD) is present in 40% to 75% of patients undergoing TAVR. However, when to treat the concomitant coronary artery lesions is controversial.
Methods:
This is a systematic review comparing concomitant PCI and TAVR versus staged PCI and TAVR. The OVID database was systematically searched for studies reporting PCI in patients undergoing TAVR. A random effects model was used to calculate the pooled odds ratio (OR) with 95% confidence intervals.
Results:
Four observational studies and a total of 209 patients were included in this analysis. Overall 30-day mortality was similar between concomitant PCI and TAVR versus staged PCI and TAVR [OR: 1.47 (0.47–4.62); P = .51], renal failure was not significantly different between both groups [OR: 3.22 (0.61–17.12); P = .17], periprocedural myocardial infarction was not different between the 2 groups [OR: 1.44 (0.12–16.94); P = .77], life-threatening bleeding did not differ between both groups [OR: 0.45 (0.11–1.87); P = .27], and major stroke also was not significantly different [OR: 3.41 (0.16–74.2); P = .44].
Conclusion:
These data did not show a significant difference in short-term outcomes between concomitant PCI and TAVR versus staged PCI and TAVR.
Keywords: aortic stenosis, coronary artery disease, PCI, TAVR
1. Introduction
Transcatheter aortic valve replacement (TAVR), also known as transcatheter aortic valve implantation (TAVI), being performed since 2002, has now emerged as a viable treatment option for intermediate to high-risk patients with severe AS who are not suitable candidates for surgical aortic valve replacement (SAVR).[1] The majority of patients currently evaluated for TAVR is older than 70 years. Furthermore, risk factors for aortic stenosis (AS) have been shown to be similar to atherosclerosis.[2] Not surprisingly, cardiac catheterization often reveals coexisting coronary artery disease (CAD).[3] In the FRANCE 2 (French Aortic National CoreValve and Edwards 2) registry, the largest published multicenter study of 3195 TAVR patients, 47.9% patients had CAD.[4] The percentage of CAD patients reach to 74.9% in TAVR group in the PARTNER trial.[5] Accumulating evidence shows that severe CAD is associated with adverse clinical outcome after TAVI.[6] Traditionally, SAVR combined with coronary artery bypass graft surgery (CABG) has been the standard treatment option for such patients.[7] In current practice, pre-existing severe and proximal coronary artery lesions are usually treated by staged PCI before TAVR or concomitant PCI during the TAVR procedure. PCI after TAVR is relatively rare because the prosthetic valve's commissures or stent frame may be positioned in close proximity to coronary ostia and might interfere with diagnostic or guiding catheters.[8] The staged approach may reduce contrast usage per session and hence theoretically decrease the risk of acute kidney injury (AKI). However, this occurs at the expense of reduced patient comfort and compliance. On the contrary, combined PCI and TAVR as a single session can decrease repeated puncture or incision, reduce the suffering of patients, shorten the duration of hospital stay, and save the cost and medical resources. If PCI is performed first, the patients remain at risk for decompensation, while aortic valve stenosis is left untreated. Otherwise, if TAVI is performed first, the risk of myocardial infarction may be elevated as a consequence of untreated CAD.[9] Berry et al[10] reported the first case of combined percutaneous aortic valve replacement and coronary artery revascularization in 2006. Thereafter, a series of successful case reports and retrospective studies about concomitant PCI and TAVR were reported.[11,12] In experienced centers, there is a tendency to proceed with PCI at the time of TAVR in the presence of significant coronary lesions if the risk of the procedure does not outweigh the potential benefits. However, is the combined single-staged approach as safe as staged PCI prior to TAVR? So, we made this meta-analysis that compared the major safety outcome of single-staged approach versus staged strategy in patients undergoing TAVR.
2. Methods
2.1. Study identification and selection
Systematic searches were performed on OVID, which selected 4 resources: EBM Reviews-Cochrane Central Register of Controlled Trials May 2017, Embase 1974 to May 1, 2017, Ovid MEDLINE(R) 1996 to Present with Daily Update, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations May 1, 2017. Using the terms “TAVI OR TAVR (Aortic Valve Stenosis, Aortic Valve, Transcatheter Aortic Valve Replacement) AND PCI (Percutaneous Coronary Intervention, Coronary Artery Disease) as keywords.
Eligible studies were those reporting clinical outcomes of patients undergoing TAVR combined with PCI, including articles and conference abstracts. No language restriction was applied. The exclusion criteria were editorials, guidelines, comments, and case series, studies without controls, and studies about the unplanned PCI during TAVR because of coronary obstruction or other complications. When institutions published duplicated studies, only the most complete reports were included. In the absence of any prospective randomized studies, only nonrandomized observational studies could be included.
3. Data extraction
Two of the investigators evaluated all studies and independently extracted relevant data from each study using a structured table. To resolve the dispute through consultation, if necessary, the third investigator was consulted. The following items were extracted from each study if available: first author's name, publication year, study design, concomitant PCI+ TAVR and staged PCI+TAVR case numbers, patients’ age, female proportion, BMI (body mass index), hypertension, DM (diabetes mellitus), New York Heart Association classification, ejection fraction, mean transvalvular gradient, logistic European System for Cardiac Operative Risk Evaluation (EuroScore), the Society of Thoracic Surgeons (STS) score, TAVR and PCI access, stent number, contrast volume, radiation time, 30-days all-cause mortality and renal failure (RIFLE Stage 3), periprocedural myocardial infarction, life threatening bleeding, and major stroke. The quality of included observational studies was assessed by the NOS scale (The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis).
4. Statistical analysis
Pre-determined primary endpoints were 30-day all-cause mortality and renal failure (RIFLE Stage 3). Secondary endpoints were life-threatening bleeding, periprocedural myocardial infarction, and major stroke. Continuous variables were expressed as mean ± SD. Dichotomous data were analyzed using odds ratio (OR) with effect size indicated by the 95% confidence intervals (95% CIs) and statistical heterogeneity was measured using the I2 statistic. Pooled values for clinical outcomes were computed using DerSimonian–Laird random effects model by Review Manager 5.3. We did not test for publication bias due to the small number of studies included in this analysis.
5. Results
5.1. Studies selection, baseline, and procedural characteristics
Our search strategy yielded 823 potentially relevant articles. On the basis of title and abstract, we excluded 787 articles for not fulfilling inclusion criteria. A total of 36 articles and conference abstracts were full text reviewed and 4 studies (3 articles and 1 conference abstract), including 209 patients, satisfied the predetermined inclusion criteria (Fig. 1).[3,13–15] These 4 studies contained synchronous PCI+TAVR and staged PCI+TAVR groups in the same period. Tables 1–3 summarize the baseline characteristics, procedural characteristics, and short-term safety endpoints in the different studies, respectively. Mean age of the subjects was more than 80 years in the 4 studies. Female patients accounted for 54.1% and average BMI was 25 in all the studies. The prevalence of hypertension and DM was reported by 2 studies. The proportion of hypertension was 64.4% and DM was 19.5%. Previous stroke patients accounted for 11.3% in 2 studies. Three studies reported the result of cardiac function evaluated by echocardiography pre-procedure, with the average LVEF between 45.6% and 52%. All the studies did not report the echocardiographic results after procedures (Table 1). Standardized valve academic research consortium (VARC)-2 endpoints were used by 1 study.[3] Another study examined all patients postoperatively according to the VARC.[14] The other 2 studies did not report which standards were used.[13,15] All TAVR procedures were performed under general anesthesia.
Figure 1.
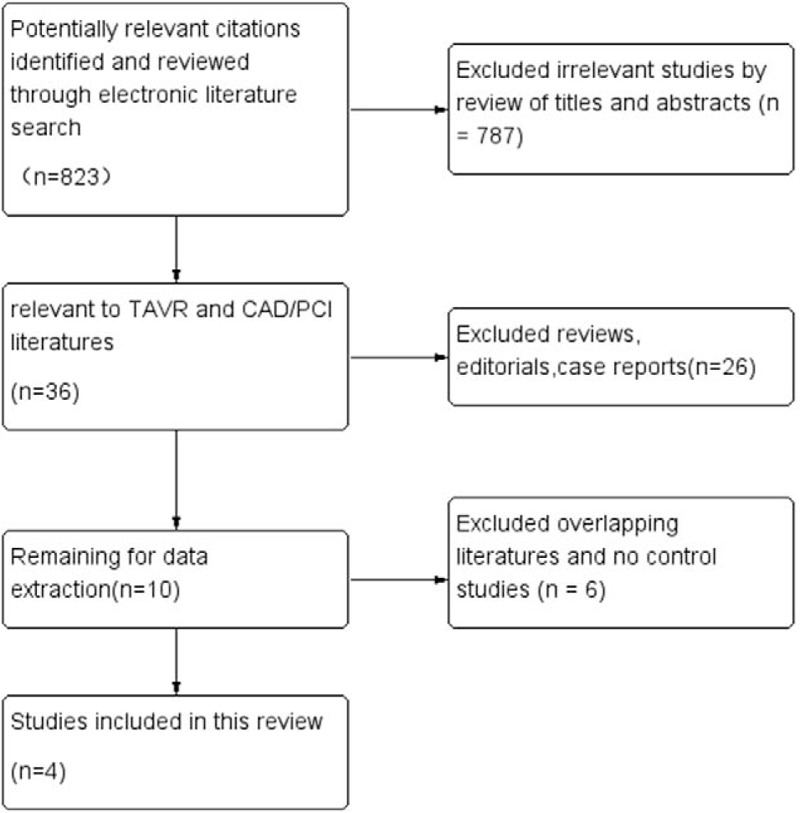
Flow chart of study selection process.
Table 1.
Baseline characteristics.
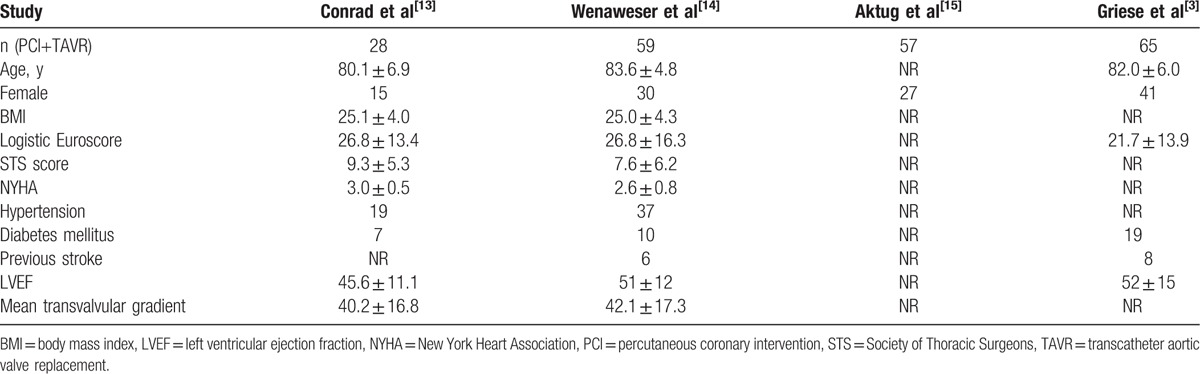
Table 3.
Safety endpoints.
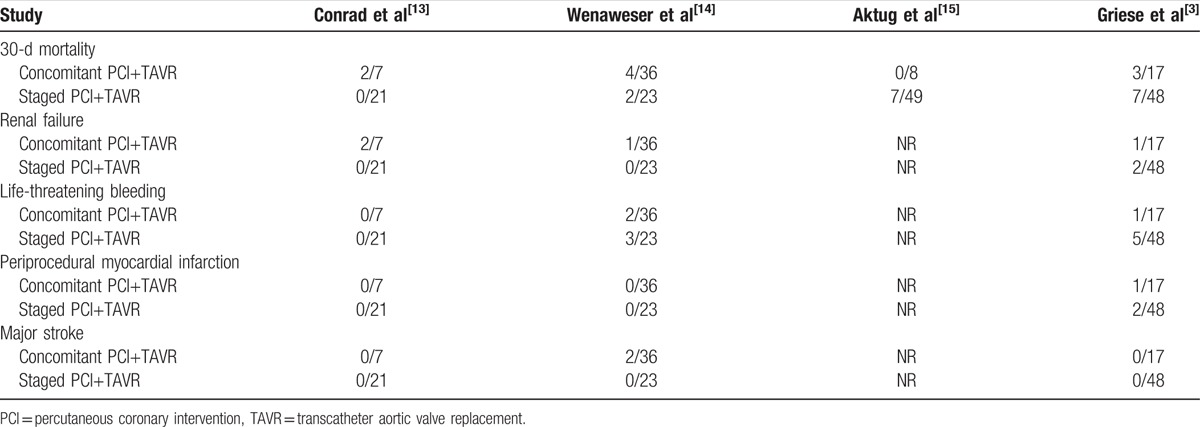
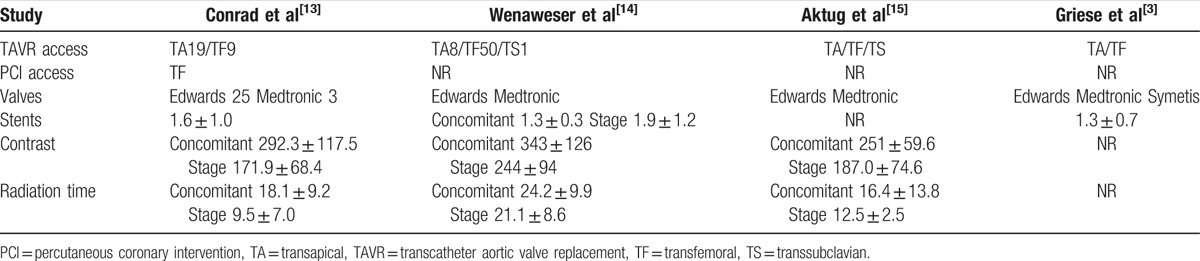
Table 2.
Procedural characteristics.
5.2. Thirty-day all-cause mortality and renal failure (RIFLE Stage 3)
The pooled average 30-day all-cause mortality rate in concomitant PCI+TAVR group was 13.2%, and in staged PCI+TAVR group was 11.3%. This difference was not statistically different between the groups [odds ratio (OR): 1.47, 95% CI 0.47–4.62; P = .51] (Fig. 2). Renal failure (RIFLE Stage 3) was observed in 3 out of 60 patients in concomitant PCI+TAVR group and 2 out of 92 patients in the staged PCI+TAVR group. However, the difference was not statistically significant (OR: 3.22, 95% CI 0.61–17.12; P = .17) (Fig. 3).
Figure 2.
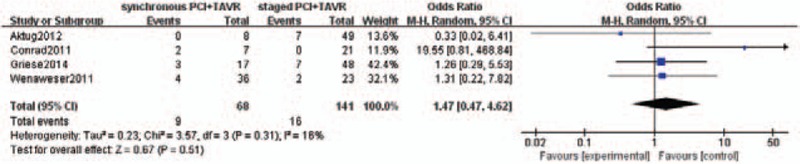
Thirty-day all-cause mortality.
Figure 3.
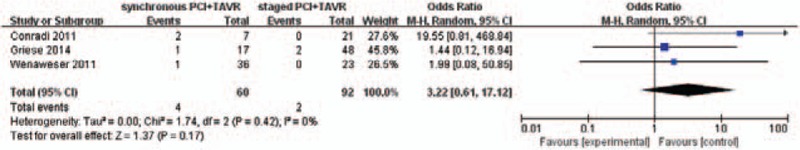
Renal failure (RIFLE Stage 3).
5.3. Periprocedural myocardial infarction, life-athreatening bleeding, and major stroke
The rate of pooled periprocedural myocardial infarction was not different between the 2 groups [1 of 60 patients in the concomitant PCI+TAVR group and 2 in 92 patients in the staged PCI+TAVR group; OR: 1.44, 95% CI 0.12–16.94; P = .77) (Fig. 4).
Figure 4.
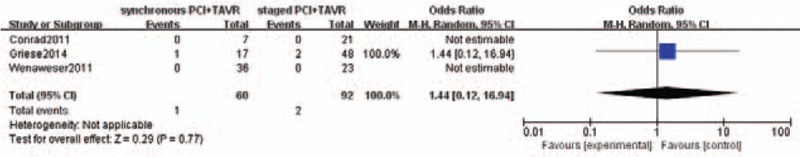
Periprocedural myocardial infarction.
Pooled incidences of life-threatening bleeding did not differ between the 2 groups [3 of 60 patients in the concomitant PCI+TAVR group and 8 of 92 patients in the staged PCI+TAVR group; OR was 0.45 (0.11– 1.87); P = .27] (Fig. 5). Major stroke occurred in 2 out of 60 patients in concomitant PCI+TAVR group and 0 out of 92 patients in staged PCI+TAVR group. It was not statistically significant, too (OR: 3.41, 95% CI 0.16–74.2, P = .44) (Fig. 6).
Figure 5.
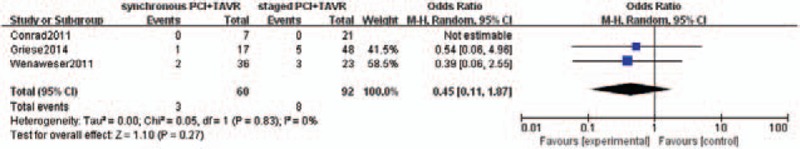
Life-threatening bleeding.
Figure 6.
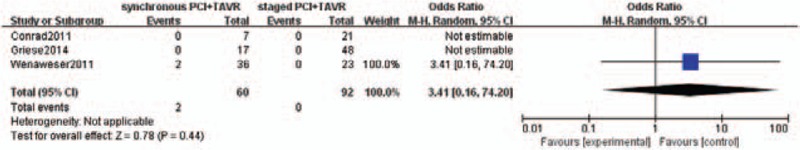
Major stroke.
6. Discussion
There is no validated method to assess myocardial ischemia caused by coronary artery stenosis among patients undergoing TAVR. The optimal management of CAD in patients undergoing TAVR remains uncertain. It might be that only medical treatment or incomplete revascularization of CAD is enough when TAVR eliminates severe aortic valve stenosis.[9,16] If the coronary artery lesions put a large myocardial area at risk, the lesions should be considered for PCI before TAVR. This meta-analysis, based on nonrandomized data, compared the short-term safe outcome of concomitant versus staged PCI with TAVR in severe AS patients, demonstrates that the 30-day all-cause mortality and other major safety endpoints did not significantly differ between the 2 approaches. Noteworthy, the pooled rate of renal failure (RIFLE Stage 3) was not statistically different between the 2 groups, although the incidence was relatively higher in synchronous PCI+TAVR group (5% vs 2.2%). There are following possible reasons. On the one hand, the impact of contrast agent utilization on AKI (after TAVR remains controversial, although contrast agent may result in CIN (contrast-induced nephropathy).[1] More reports have not demonstrated an association between contrast media and higher AKI incidence following TAVR. Goebel et al[17] analyzed data of 270 patients who underwent transapical aortic valve implantation between September 2008 and March 2012. They found that the amount of contrast agent (83.7 ± 32.4 mL) applied intraprocedurally had no impact on the development of AKI.[17] Another trial that used contrast medium ranging from 136.4 to 142.5 mL also demonstrates no association between AKI and the use of contrast media in TAVR patients.[18] In the balloon aortic valvuloplasty (BAV) era, Ben-Dor et al[19] found that PCI done concomitantly with BAV does not affect safety and efficacy, although used significantly greater amount of contrast (95.1 ± 45.5 vs 36.7 ± 38.4 mL). In accordance with this systematic review, Penkalla et al[20] reported that patients (n = 76) with highly significant CAD undergoing TAVI and PCI as single-staged procedure had similar 30-day all-cause mortality rate and 3 years survival as patients (n = 285) without CAD received TAVI only. Although more contrast agent (162.7 ± 87.5 vs 105.8 ± 49.8 mL) was applied during the combined treatment, the rate of AKI was not higher.[20] The usage of contrast media reached to 343 ± 126 mL in TAVI and concomitant PCI group also showed no significant difference of renal failure (RIFLE stage 3) according to the study by Dr Wenaweser et al.[14] On the other hand, the total contrast volume used by staged approach was not less than the concomitant strategy; Dr Wenaweser et al[14] reported total contrast agent usage of 330 ± 140 mL in staged versus 343 ± 126 mL in concomitant group. In spite of the interval between PCI and TAVR, in other words, the time of repeated exposures to contrast agents varied widely. Nevertheless, repeated exposure to contrast media within 72 hours is a risk factor related to CIN.[21] In addition, another probable explanation is that inadequate statistical power due to the small number of cases.
In fact, the pathogenesis of TAVR-related AKI is multifactorial, including perioperative renal hypoperfusion related to a combination of pre-, intra-, and postoperative factors.[1] A series of researches investigated the risk or predictive factors of TAVR-related AKI in recent years.[17,18,22,23] Interestingly, although these results are not completely consistent, they all exclude contrast media and discovered a same risk factor: blood transfusion. Preserved RBCs (red blood cells) undergo progressive functional and structural changes leading to a reduction in RBC function and viability, and accumulate proinflammatory molecules, free iron, and hemoglobin, and all these changes might favor renal dysfunction, particularly in older patients with impairment of kidney autoregulation.[22] The staged PCI+TAVR group shows a rising trend of life-threatening bleeding compared with the single-staged group (8.7% vs 5.0%), despite it is not statistically different between the 2 groups. According to a recent systematic review, with a pooled incidence of 16.3%, the risk of major bleeding may be higher in patients undergoing pre-TAVI PCI owing to the necessity of dual anti-platelet therapy.[7] Another study reported that as many as 37% patients received a blood transfusion in severe symptomatic AS who underwent TAVI through the transfemoral route.[24] Therefore, we speculate that the benefit achieved by reducing contrast agent maybe offset by rising incidence of major bleeding in staged PCI and TAVR strategy. The factors that affect stroke in TAVR may include temporary circulatory disruptions during balloon valvuloplasty or rapid ventricular pacing, embolism because of calcium deposits detachment, and increased thrombogenicity caused by release of tissue factor. There was no major stroke reported in other studies except Wenaweser et al[14] reported 2 cases of major stroke in concomitant group. They used the Edwards and Medtronic valves similar to the other 3 studies. The difference of major stroke was not statistically significant between concomitant and staged PCI+TAVR group. But this conclusion needs to be verified by large sample research.
In addition, the average number of stents is 1.3 to 1.9 per patient reported in 3 studies.[3,13,14] SYNTAX scores are 13.0 ± 8.7 in staged group and 11.4 ± 8.2 in concomitant group reported by 1 study.[14] These data may indicate that the coronary lesions included in this meta-analysis are relatively simple. However, TAVR combined with complex coronary intervention is also feasible using percutaneous left ventricular assist device.[12] Another case report demonstrates that a single-stage combined PCI and TAVR approach is reasonable in severe AS patients presented with acute coronary syndrome.[25,26] Left main revascularization during TAVR is well reported recently.[27] But further data and experience are needed to evaluate this single-stage strategy in these higher risk patients.
In fact, after the first pioneering experiences, research in the TAVI field has shifted quickly exploring concepts such as “feasibility” at the very early stage, moving toward “effectiveness,” and lately “simplification” and “optimization.”[28] CAD screening is required before TAVI. Although invasive coronary angiography (CA) remains the gold standard for this assessment, Chieffo et al[29] recently demonstrated that CT CA can be performed safely and effectively as a routine noninvasive imaging tool in TAVI patients. Sixteen patients had concomitant PCI at the same time of TAVR procedure after coronary vasculature evaluation by means of invasive CA.[29] Recently, a systematic review compared TAVR versus TAVR+PCI for significant CAD in patients undergoing TAVR and found no significant differences in major safety endpoints.[30] While a staged approach may represent a preferable strategy in selected patients, concomitant treatment of combined cardiac diseases represents an appealing option in a majority of patients.[31]
7. Limitations
This meta-analysis is based on 4 nonrandomized studies due to lack of randomized controlled trials. The results are therefore subject to confounding factors, mainly based on a learning curve effect, and the assignment to staged TAVR following PCI or simultaneous PCI and TAVR was often based on the heart team's decision. All studies are relatively small and heterogeneous, which may generate false-negative results. There was also considerable heterogeneity between studies with regard to the duration of time between TAVI and PCI, the severity of multivessel CAD, choice of stent, and TAVI access route.
8. Conclusion
The results of this meta-analysis demonstrate that there is no significant difference in short-term safety outcomes using either staged PCI and TAVR or synchronous PCI and TAVR procedures. Concomitant PCI+TAVR could be considered in severe AS patients with relative simple coronary lesion and without renal insufficiency. However, randomized controlled trials are needed to guide patient selection and further investigate the optimal percutaneous treatment strategies for patients with severe AS concomitant CAD.
Footnotes
Abbreviations: AKI = acute kidney injury, AS = aortic stenosis, BMI = body mass index, CABG = coronary artery bypass graft surgery, CAD = coronary artery disease, CI = confidence intervals, CIN = contrast-induced nephropathy, DM = diabetes mellitus, EuroScore = European System for Cardiac Operative Risk Evaluation, NOS = Newcastle–Ottawa Scale, OR = odds ratio, PCI = percutaneous coronary intervention, RBC = red blood cell, RIFLE = risk, injury, failure, loss of kidney function, and end-stage renal disease, SAVR = surgical aortic valve replacement, STS = Society of Thoracic Surgeons, TAVI = transcatheter aortic valve implantation, TAVR = transcatheter aortic valve replacement, VARC = valve academic research consortium.
Funding/support: This study was supported by the National High-tech Research and Development Program of China (grant number: 2012AA02A510), and MC was responsible for enabling the project completion.
The authors report no conflicts of interest.
References
- [1].Cheungpasitporn W, Thongprayoon C, Kashani K. Transcatheter aortic valve replacement; a kidney's perspective. J Renal Inj Prev 2016;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Goel SS, Ige M, Tuzcu EM, et al. Severe aortic stenosis and coronary artery disease—implications for management in the transcatheter aortic valve replacement era. J Am Coll Cardiol 2013;62:1–0. [DOI] [PubMed] [Google Scholar]
- [3].Griese DP, Reents W, Tóth A, et al. Concomitant coronary intervention is associated with poorer early and late clinical outcomes in selected elderly patients receiving transcatheter aortic valve implantation. Eur J Cardiothorac Surg 2014;46:e1–7. [DOI] [PubMed] [Google Scholar]
- [4].Gilard M, Eltchaninoff H, Lung B. Registry of transcatheter aortic-valve implantation in high-risk patients. J Vasc Surg 2012;56:1180–1. [DOI] [PubMed] [Google Scholar]
- [5].Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–98. [DOI] [PubMed] [Google Scholar]
- [6].van Rosendael PJ, van der Kley F, Kamperidis V, et al. Timing of staged percutaneous coronary intervention before transcatheter aortic valve implantation. Am J Cardiol 2015;115:1726–32. [DOI] [PubMed] [Google Scholar]
- [7].Virk SA, Tian DH, Liou K, et al. Systematic review of percutaneous coronary intervention and transcatheter aortic valve implantation for concomitant aortic stenosis and coronary artery disease. Int J Cardiol 2015;187:453–5. [DOI] [PubMed] [Google Scholar]
- [8].Blumenstein J, Kim W, Liebetrau C, et al. Challenges of coronary angiography and intervention in patients previously treated by TAVI. Clin Res Cardiol 2015;104:632–9. [DOI] [PubMed] [Google Scholar]
- [9].Pasic M, Dreysse S, Unbehaun A, et al. Combined elective percutaneous coronary intervention and transapical transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg 2012;14:463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Berry C, Lamarche Y, Laborde JC, et al. First case of combined percutaneous aortic valve replacement and coronary artery revascularisation. EuroIntervention 2006;2:257–61. [PubMed] [Google Scholar]
- [11].Salhab KF, Al Kindi AH, Lane JH, et al. Concomitant percutaneous coronary intervention and transcatheter aortic valve replacement: safe and feasible replacement alternative approaches in high-risk patients with severe aortic stenosis and coronary artery disease. J Card Surg 2013;28:481–3. [DOI] [PubMed] [Google Scholar]
- [12].Piazza N, Serruys PW, de Jaegere P. Feasibility of complex coronary intervention in combination with percutaneous aortic valve implantation in patients with aortic stenosis using percutaneous left ventricular assist device (TandemHeart(®). Catheter Cardio Interv 2009;73:161–6. [DOI] [PubMed] [Google Scholar]
- [13].Conradi L, Seiffert M, Franzen O, et al. First experience with transcatheter aortic valve implantation and concomitant percutaneous coronary intervention. Clin Res Cardiol 2011;100:311–6. [DOI] [PubMed] [Google Scholar]
- [14].Wenaweser P, Pilgrim T, Guerios E, et al. Impact of coronary artery disease and percutaneous coronary intervention on outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention 2011;7:541–8. [DOI] [PubMed] [Google Scholar]
- [15].Aktug O, Herpertz R, Brehmer K. Transcatheter aortic valve implantation and concomitant percutaneous coronary intervention in high-risk patients with severe aortic stenosis. 28th National Congress of Cardiology; Antalya Turkey 2012;40:49. [Google Scholar]
- [16].van Mieghem NM, van der Boon RM, Faqiri E, et al. Complete revascularization is not a prerequisite for success in current transcatheter aortic valve implantation practice. JACC Cardiovasc Interv 2013;6:867–75. [DOI] [PubMed] [Google Scholar]
- [17].Goebel N, Baumbach H, Ahad S, et al. Transcatheter aortic valve replacement: does kidney function affect outcome? Ann Thorac Surg 2013;96:507–12. [DOI] [PubMed] [Google Scholar]
- [18].Elhmidi Y, Bleiziffer S, Piazza N, et al. Incidence and predictors of acute kidney injury in patients undergoing transcatheter aortic valve implantation. Am Heart J 2011;161:735–9. [DOI] [PubMed] [Google Scholar]
- [19].Ben-Dor I, Maluenda G, Looser PM, et al. Outcomes of concomitant percutaneous coronary intervention and balloon aortic valvuloplasty. Catheter Cardiovasc Interv 2013;82:E835–41. [DOI] [PubMed] [Google Scholar]
- [20].Penkalla A, Pasic M, Drews T, et al. Transcatheter aortic valve implantation combined with elective coronary artery stenting: a simultaneous approach. Eur J Cardiothorac 2015;47:1083–9. [DOI] [PubMed] [Google Scholar]
- [21].Mohammed NA, Rafie I, Mahfouz A, et al. Contrast-induced nephropathy. Heart Views 2013;14:106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010;31:865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aregger F, Wenaweser P, Hellige GJ, et al. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant 2009;24:2175–9. [DOI] [PubMed] [Google Scholar]
- [24].Escárcega RO, Lipinski MJ, Magalhaes MA, et al. Impact of blood transfusions on short- and long-term mortality in patients who underwent transcatheter aortic valve implantation. Am J Cardiol 2015;115:93–9. [DOI] [PubMed] [Google Scholar]
- [25].Steude J, Abdel-Wahab S, Schubring M M. Combined percutaneous coronary intervention and transcatheter aortic valve implantation in cardiogenic shock. J Heart Valve Dis 2013;6:824–7. [PubMed] [Google Scholar]
- [26].Mostafa AE, Geist V, Abdel-Wahab M, et al. Ad-hoc percutaneous coronary intervention and transcatheter aortic valve implantation as a combined transfemoral procedure. J Invasive Cardiol 2011;5:E102–5. [PubMed] [Google Scholar]
- [27].Chakravarty T, Sharma R, Abramowitz Y, et al. Outcomes in patients with transcatheter aortic valve replacement and left main stenting. J Am Coll Cardiol 2016;67:951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Barbanti M, Tamburino C. Optimisation of TAVI: is it mature enough to be defined as a PCI-like procedure? Eurointervention 2015;14:W110–3. [DOI] [PubMed] [Google Scholar]
- [29].Chieffo A, Giustino G, Spagnolo P, et al. Routine screening of coronary artery disease with computed tomographic coronary angiography in place of invasive coronary angiography in patients undergoing transcatheter aortic valve replacement. Circ Cardiovasc Interv 2015;8:e002025. [DOI] [PubMed] [Google Scholar]
- [30].Bajaj A, Pancholy S, Sethi A, et al. Safety and feasibility of PCI in patients undergoing TAVR: a systematic review and meta-analysis. Heart Lung 2017;46:92–9. [DOI] [PubMed] [Google Scholar]
- [31].Taramasso M, Maisano F, Nietlispac F. TAVI and concomitant procedures: from PCI to LAA closure. Eurointervention 2015;14:W96–100. [DOI] [PubMed] [Google Scholar]


