Abstract
Rationale:
Anatomical variations of the celiac trunk and the hepatic artery are of considerable importance in hepatopancreatobiliary surgery, liver transplants, and radiological abdominal interventions.
Patient concerns:
Here, we report a 57-year-old man with 2 weeks of painless progressive jaundice. Preoperative imaging and cytology brush results suggested an ampullary tumor and common hepatic artery anomaly (CTA) was reported. The patient underwent pancreaticoduodenectomy (PD). Intraoperatively, the CHA and gastroduodenal artery (GDA) were abnormal. The CHA emerged from the superior mesenteric artery (SMA). Computer tomography angiography (CTA) was performed postoperatively; surprisingly, the left gastric artery (LGA) and splenic artery (SA) arising from the anterior wall of the abdominal aorta replaced the normal structure of the celiac trunk, and an accessory left hepatic artery (LHA) emerged from the LGA.
Diagnoses:
The patient was diagnosed with cholangiocarcinoma and accompanying extremely rare variation of celiac trunk and hepatic artery.
Interventions and outcomes:
The patient underwent PD and had an uneventful postoperative evolution. There was no recurrence of the tumor and with normal liver function during the 10-month follow-up.
Interventions:
The patient underwent PD and had an uneventful postoperative evolution.
Outcomes:
There was no recurrence of the tumor and with normal liver function during the 10-month follow-up.
Lessons:
Surgeons must keep in mind that arterial variation may be present in the vascular structures intraoperatively, even if it was not revealed in preoperative imaging. The preoperative identification of arterial variation and its relationship with the tumor is necessary to avoid intraoperative vascular injury and complications after surgery.
Keywords: celiac trunk, hepatic artery, hepatomesenteric trunk, pancreaticoduodenectomy, variation
1. Introduction
Pancreaticoduodenectomy (PD) is the most effective treatment for an ampullary tumor, which is associated with high morbidity and mortality rates, even if the complex procedure is performed in tertiary centers.[1,2] Anatomical variations of the hepatic artery and celiac trunk put an individual at a high risk of injury to the arterial supply and, subsequently, to severe hepatic ischemia, liver abscesses, biliary fistula, or hemorrhage.[1] Therefore, the accurate identification of these arterial variations would enhance the probability of successful surgery and decrease the rate of complications after the complex PD procedure. We describe a rare anomalous origin of the common hepatic artery (CHA) from the hepatomesenteric trunk (HMT). Moreover, the left gastric artery (LGA) and splenic artery (SA) arising from the anterior wall of the abdominal aorta and an accessory left hepatic artery (LHA) from the LGA supplying the left liver made this case extraordinary.
2. Case presentation
The study was approved by the Ethical Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, and written informed consent was obtained.
A 57-year-old man was admitted to our department with a chief complaint of painless progressive jaundice for 2 weeks. The patient was a lifelong nonsmoker who did not consume alcohol and had no history of inherited diseases. There was no significant history of biliary or liver disease. Physical examination was unremarkable apart from icterus, and a Murphy sign test was negative. Hemogram, electrolytes, and amylase were within the normal limits. Liver function tests revealed the following: albumin 37.5 g/L, alanine aminotransferase 45 U/L, aspartate transaminase 30 U/L, gamma-glutamyltranspeptidase 1517 U/L, total bilirubin 201 μmol/L, and direct bilirubin 143 μmol/L. The following tumor markers were normal: carcinoembryonic antigen (11.2 ng/mL), alpha-fetoprotein carbohydrate antigen (CA) 19–9, and CA125. An abdominal computed tomography showed dilation of the intrahepatic and extrahepatic bile duct with obstruction at the level of the distal common bile duct, and the bile duct wall was slightly enhanced. An anomalous origin of the CHA was also revealed (Fig. 1). Magnetic resonance cholangiopancreatography (MRCP) demonstrated a rat tail shaped stricture of the distal common bile duct, and biliary tract malignancy was considered (Fig. 2). Endoscopic retrograde cholangiopancreatography revealed irregular stenosis of the pancreatic biliary duct and brush cytology was performed; a heterocyst was confirmed.
Figure 1.
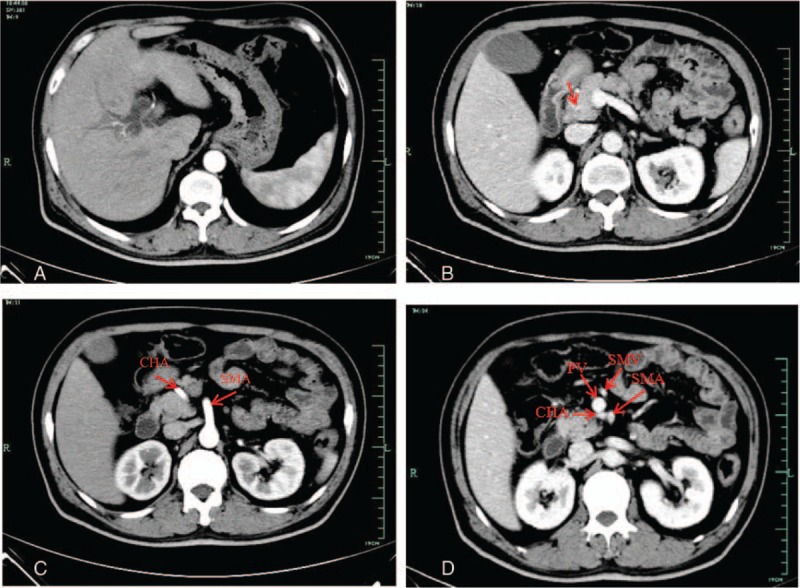
Contrast-enhanced computed tomography. (A) Dilation of intrahepatic and extrahepatic bile duct. (B) The distal common bile duct was slightly enhanced in portal venous phase (arrow). (C) In arterial phase: the common hepatic artery originated from the superior mesenteric artery and cross between the pancreas head and the uncinate process. (D) In portal phase, CHA running posterior to the portal vein. CHA = common hepatic artery, PV = portal vein, SMA = superior mesenteric artery, SMV = superior mesenteric vein.
Figure 2.
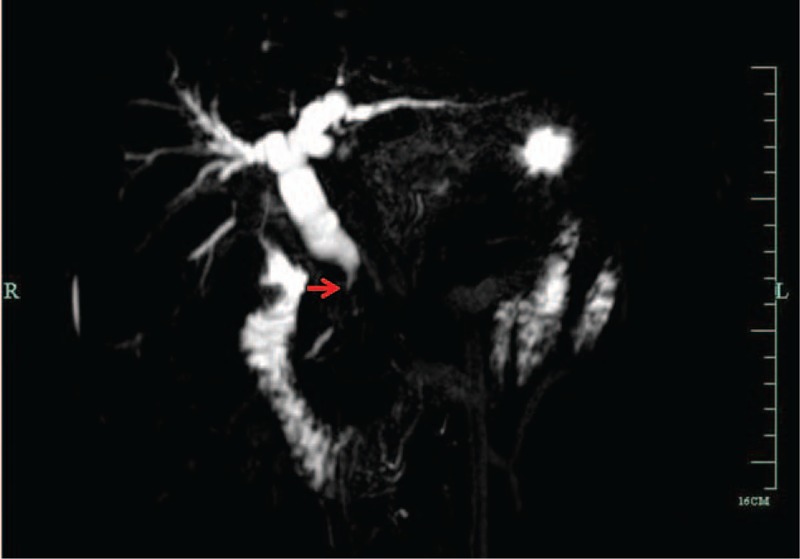
MRCP demonstrates rat-tail shaped stricture of the distal common bile duct (arrow). MRCP = magnetic resonance cholangiopancreatography.
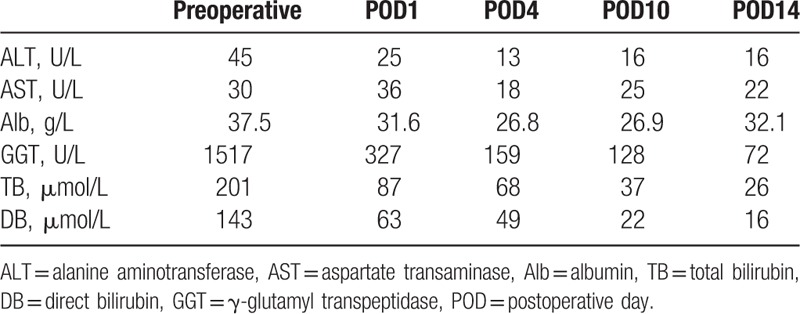
The diagnosis of ampullary tumor was suggested based on imaging findings and cytology results. PD was performed not only to release biliary obstruction but also to cure the disease. Intraoperatively, a rare variation of the hepatic artery was observed after kocherization and hilar dissection. The CHA and gastroduodenal artery (GDA) were abnormal, with the CHA arising from the superior mesenteric artery (SMA) and crossing between the pancreas head and the uncinate process, giving off a few pancreatic branches and then dividing into the right gastric artery and GDA before giving off the proper hepatic artery at the upper margin of the pancreas. The pancreas was transected at the neck anterior to the portal vein (PV) and the CHA was preserved (Fig. 3). Ultimately, PD was successfully performed and a definitive diagnosis of cholangiocarcinoma was made. Computer tomography angiography (CTA) was performed on postoperative day 10. The complexity of the variant artery was beyond what was found during the operation (Fig. 4). The classical celiac trunk was absent, with the LGA and SA arising from the anterior wall of the abdominal aorta and an accessory LHA arising from the LGA. The CHA arose from the SMA and the common origin was termed the “hepatomesenteric trunk” (HMT). Although local stenosis of the proper hepatic artery was observed postoperatively (due to the successful solving the problem of obstructive jaundice as well as the accessory LHA and an intact portal blood supply), liver function gradually improved (Table 1). Fortunately, the postoperative course was favorable and the patient was discharged on postoperative day 14. There was no recurrence of the tumor and with normal liver function during the 10-month follow-up.
Figure 3.
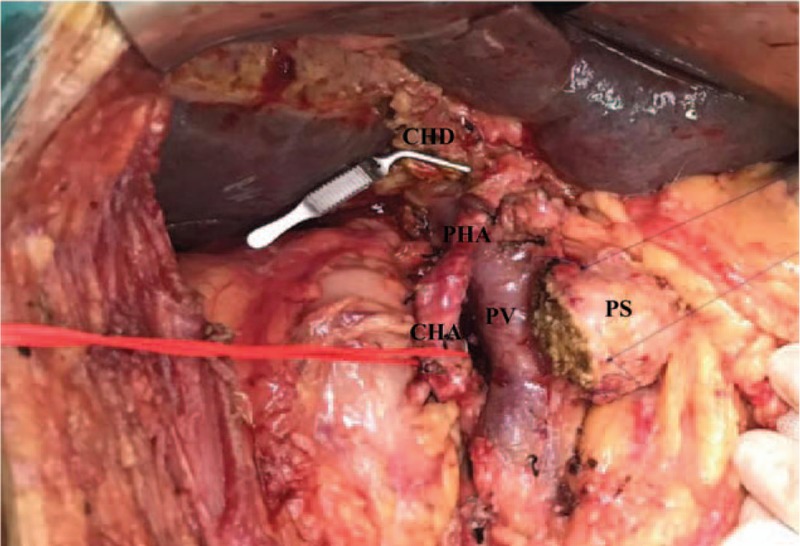
View of operating field after the neck of pancreas was transected. CHA = common hepatic artery, CHD = common hepatic duct, PHA = proper hepatic artery, PS = pancreatic stump, PV = portal vein.
Figure 4.
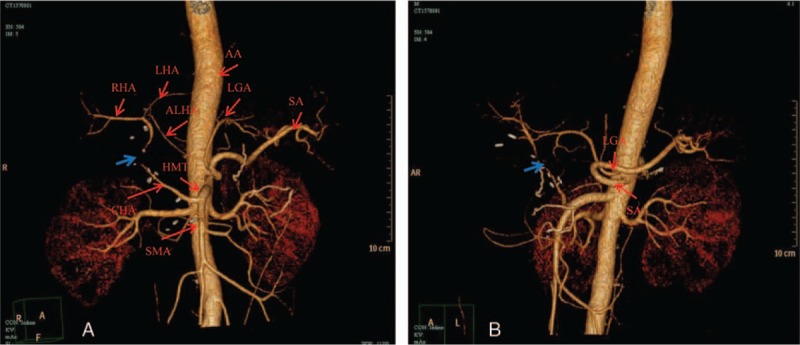
Computer tomography angiography postoperative. (A) The CHA and SMA arising from the HMT (Michels, type IX) and part of the proper hepatic artery is not clear, local stenosis is founded (blue arrow). In addition, an accessory LHA arising from LGA (Michels, type V) supplied part of the left hepatic blood flow. (B) In this view, LGA and SA arising from the anterior wall of the abdominal aorta respectively (red arrow) could be shown clearly. AA = abdominal aorta, ALHA = accessory left hepatic artery, CHA = common hepatic artery, HMT = hepatomesenteric trunk, LGA = left gastric artery, LHA = left hepatic artery, RHA = right hepatic artery, SA = splenic artery, SMA = superior mesenteric artery.
Table 1.
The changes of liver function in the perioperative period.
3. Discussion
The classical trifurcation of the celiac trunk into the common hepatic, left gastric, and splenic arteries was first reported by Haller[3] in 1756 at a frequency of 72% to 90% in the normal population,[3–6] while normal hepatic arterial anatomy is reported in 52% to 80% of operative cases.[7,8] Information on variations in the celiac trunk and hepatic artery are important in open and laparoscopic hepatopancreatobiliary surgeries, liver transplants, and radiological abdominal interventions.[9,10]
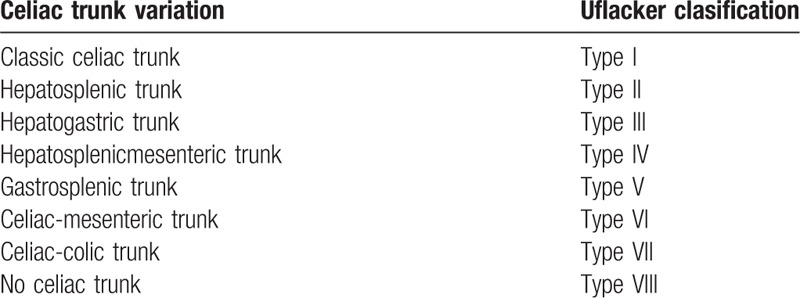
In this case, trifurcation of the celiac trunk was absent, with the CHA and SMA originating from a common trunk termed the “hepatomesenteric trunk,” and the LGA and SA originating directly from the anterior wall of the abdominal aorta. This variation of “no celiac trunk” was classified as Type VIII according to Uflacker classification[11] (Table 2). Celiac trunk bifurcation as a common anatomical variation has been reported at a rate of approximately 8% to 12% in the literature[6]; gastrosplenic trunk (Type V) and hepatosplenic trunk (Type II) were the most prevalent variation. However, the average rate of an absent celiac trunk was only 0.4% in the study by Bergman et al.[4] In addition, the rare variation termed “splenomesenteric trunk” has not described in any classification and has also been reported.[6,12]
Table 2.
Celiac trunk variations according to Uflacker classification[11].
The types of hepatic artery variation have been detailed described in Michel's classification[8] and other studies,[7,13,14] as well as anatomical monographs.[15] Nowadays, Michel's classification (Table 3) is still the most commonly used in clinic, as it established the difference between “replaced” and “accessory ” hepatic artery, which was critical for hepatopancreatobiliary surgeries and liver transplants. There are 10 variant subtypes of the hepatic arterial system in Michel's classification and the replaced right hepatic artery from the SMA (Type III) and the replaced LHA from the LGA (Type II) are regarded as the most common types of hepatic arterial variation.[16–18] Moreover, López-Andújar et al[19] reported 2 new types not included in Michel's classification (Fig. 5). In the case described herein, the CHA originated from the SMA (Type IX) and accompanied an accessory LHA originating from the LGA (Type V). The anatomical variations of the hepatic artery (Type IX + Type V) that occurred in this patient are extremely rare. To the best of our knowledge, the unclassified variation of the hepatic artery accompanying an absence of the celiac trunk has been very rarely reported previously.
Table 3.
Hepatic artery variations: Michel classification.
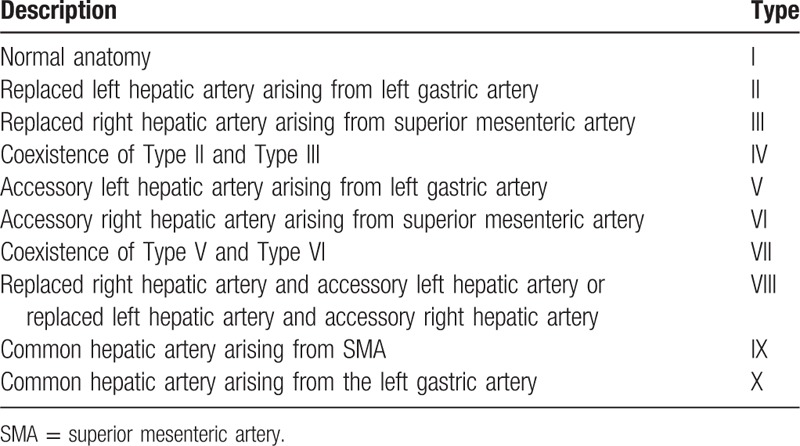
Figure 5.
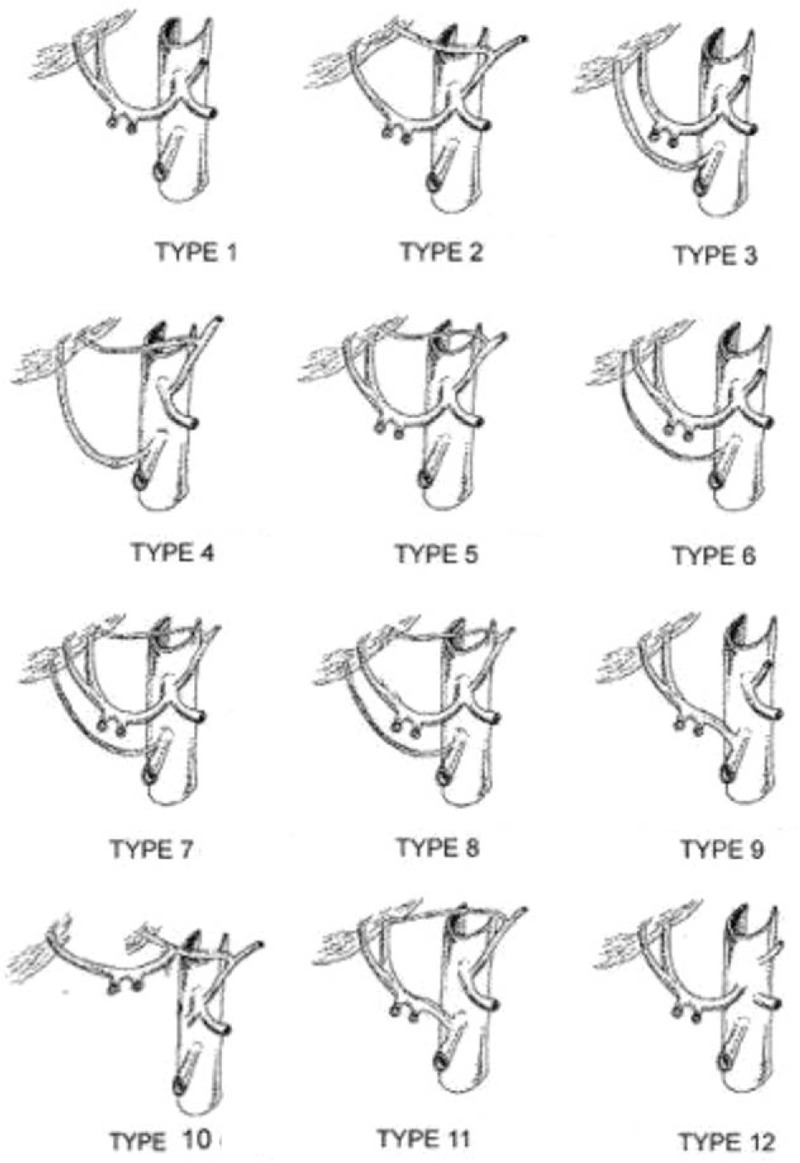
Ten variant subtypes of the hepatic arterial variations according to Michel's classification and 2 new types (type 11,12) not included in this classification.[19]
Aberrant arterial anatomy increases the surgical complexity and potential risk of injury to the arterial supply that could lead to ischemia, biliary fistula, bleeding, and liver abscess.[20,21] Although we were careful to perform an intraoperative dissection as far as possible and the CHA was preserved, the local stricture of the proper hepatic artery was still observed in the postoperative imaging. However, mainly due to the successful solving the problem of obstructive jaundice through the operation as well as the accessory LHA and an intact portal blood supply, liver function gradually improved and the patient recovered and was discharged. Therefore, clear recognition of these arterial variations both preoperatively and intraoperatively enhances the probability of a successful operation and limits harmful outcomes of complex hepatopancreatobiliary surgical procedures such as PD. Digital subtraction angiography was previously regarded as the gold standard in the evaluation of vascular structures but has now been replaced by CTA[22] and gadolinium-enhanced magnetic resonance angiography,[23,24] which could not only visualization of normal anatomy as well as anatomical variants, but also reduces the associated morbidity of angiography and the risk of introgenic injuries in complex surgical procedures, especially in hepatopancreatobiliary surgeries and liver transplants.[25] However, Yang et al[26] suggested that aberrant hepatic artery could be usually well demonstrated with routine MDCT once radiologists and surgeons paid more attention to the arterial variants, but had limitation in evaluating celiac trunk artery. Similarly, preoperative CT scan have also found hepatic artery variation in the present study, but we did not aware of the specific variation of celiac trunk until the CTA examination postoperative and also surprised to find an accessory LHA originating from the LGA.
4. Conclusion
The current study reports a case of PD with extremely rare celiac trunk and hepatic artery variation for cholangiocarcinoma. As a whole, the preoperative identification of arterial variation and its relationship with the tumor is necessary to avoid intraoperative vascular injury and complications after surgery.
Acknowledgment
The authors would like to thank our patient for allowing for his case to be presented.
Footnotes
Abbreviations: AA = abdominal aorta, CHA = common hepatic artery, CHD = common hepatic duct, CTA = computer tomography angiography, GDA = gastroduodenal artery, HMT = hepatomesenteric trunk, LGA = the left gastric artery, LHA = left hepatic artery, MRCP = magnetic resonance cholangiopancreatography, PD = pancreaticoduodenectomy, PHA= proper hepatic artery, PS = pancreatic stump, PV = portal vein, RHA = right hepatic artery, SA = the splenic artery, SMA = superior mesenteric artery.
The authors declare no conflicts of interest.
References
- [1].Feng J, Chen YL, Dong JH, et al. Post-pancreaticoduodenectomy hemorrhage: risk factors, managements and outcomes. Hepatobiliary Pancreat Dis Int 2014;13:513–22. [DOI] [PubMed] [Google Scholar]
- [2].Cusworth BM, Krasnick BA, Nywening TM, et al. Whipple-specific complications result in prolonged length of stay not accounted for in ACS-NSQIP Surgical Risk Calculator. HPB (Oxford) 2016 Dec 8. pii: S1365-182X(16)31963-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vandamme JP, Bonte J. The branches of the coeliac trunk. Acta Anat (Basel) 1985;122:110–4. [DOI] [PubMed] [Google Scholar]
- [4].Bergman RA, Afifi AK, Miyauchi R. Anatomy Atlases An anatomy digital library-Curated by Ronald A. Bergman, Ph.D.; Accessed January 15, 2017. [Google Scholar]
- [5].Matoba M, Tonami H, Kuginuki M, et al. Comparison of high-resolution contrast-enhanced 3D MRA with digital subtraction angiography in the evaluation of hepatic arterial anatomy. Clin Radiol 2003;58:463–8. [DOI] [PubMed] [Google Scholar]
- [6].Ugurel MS, Battal B, Bozlar U, et al. Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. Br J Radiol 2010;83:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 1994;220:50–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 1966;112:337–47. [DOI] [PubMed] [Google Scholar]
- [9].Lee SW, Shinohara H, Matsuki M, et al. Preoperative simulation of vascular anatomy by three-dimensional computed tomography imaging in laparoscopic gastric cancer surgery. J Am Coll Surg 2003;197:927–36. [DOI] [PubMed] [Google Scholar]
- [10].Ishigami K, Zhang Y, Rayhill S, et al. Does variant hepatic artery anatomy in a liver transplant recipient increase the risk of hepatic artery complications after transplantation? AJR Am J Roentgenol 2004;183:1577–84. [DOI] [PubMed] [Google Scholar]
- [11].Uflacker R. Atlas of Vascular Anatomy: an Angiographic Approach. 1997;Baltimore, MD: Williams & Wilkins, 203–206. [Google Scholar]
- [12].Oran I, Yesildag A, Memis A. Aortic origin of right hepatic artery and superior mesenteric origin of splenic artery: two rare variations demonstrated angiographically. Surg Radiol Anat 2001;23:349–52. [DOI] [PubMed] [Google Scholar]
- [13].Daly JM, Kemeny N, Oderman P, et al. Long-term hepatic arterial infusion chemotherapy. Arch Surg 1984;119:936–41. [DOI] [PubMed] [Google Scholar]
- [14].Suzuki T, Nakayasu A, Kawabe K, et al. Surgical significance of anatomic variations of the hepatic artery. Am J Surg 1971;122:505–12. [DOI] [PubMed] [Google Scholar]
- [15].Lanz VT, Wachsmuth W. Lanz/Wachsmuth Practical Anatomy. Berlin: Springer-Verlag; 2003. [Google Scholar]
- [16].Covey AM, Brody LA, Maluccio MA, et al. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology 2002;224:524–7. [DOI] [PubMed] [Google Scholar]
- [17].De Santis M, Ariosi P, Calo GF, et al. Anatomia vascolare arteriosa epatica e sue varianti [Hepatic arterial vascular anatomy and its variants]. Radiol Med 2000;100:145–51. [PubMed] [Google Scholar]
- [18].Koops A, Wojciechowski B, Broering DC, et al. Anatomic variations of the hepatic arteries in 604 selective coeliac and superior mesenteric angiographies. Surg Radiol Anat 2004;26:239–44. [DOI] [PubMed] [Google Scholar]
- [19].López-Andújar R, Moya A, Montalvá E, et al. Lessons learned from anatomic variants of the hepatic artery in 1,081 transplanted livers. Liver Transpl 2007;13:1401–2140. [DOI] [PubMed] [Google Scholar]
- [20].Shukla PJ, Barreto SG, Kulkarni A, et al. Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol 2010;17:186–93. [DOI] [PubMed] [Google Scholar]
- [21].Chamberlain RS, El-Sedfy A, Rajkumar D. Aberrant hepatic arterial anatomy and the Whipple procedure: lessons learned. Am Surg 2011;77:517–26. [PubMed] [Google Scholar]
- [22].Nghiem HV, Jeffrey RB., Jr CT angiography of the visceral vasculature. Semin Ultrasound CT MR 1998;19:439–46. [DOI] [PubMed] [Google Scholar]
- [23].Kopka L, Rodenwaldt J, Vosshenrich R, et al. Hepatic blood supply: comparison of optimized dual phase contrast-enhanced three-dimensional MR angiography and digital subtraction angiography. Radiology 1999;211:51–8. [DOI] [PubMed] [Google Scholar]
- [24].Macdonald GA, Peduto AJ. Magnetic resonance imaging and diseases of the liver and biliary tract. Part 2. Magnetic resonance cholangiography and angiography and conclusions. J Gastroenterol Hepatol 2000;15:992–9. [DOI] [PubMed] [Google Scholar]
- [25].Lezzi R, Cotroneo AR, Giancristofaro D, et al. Multidetector-row CT angiographic imaging of the celiac trunk: anatomy and normal variants. Surg Radiol Anat 2008;30:303–10. [DOI] [PubMed] [Google Scholar]
- [26].Yang F, Di Y, Li J, et al. Accuracy of routine multidetector computed tomography to identify arterial variants in patients scheduled for pancreaticoduodenectomy. World J Gastroenterol 2015;21:969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]


