Abstract
We evaluated a system-wide impact of a health intervention to improve treatment of osteoporosis after a fragility fracture. The intervention consisted of assigning a screening coordinator to selected fracture clinics to identify, educate, and follow up with fragility fracture patients and inform their physicians of the need to evaluate bone health. Thirty-seven hospitals in the province of Ontario (Canada) were assigned a screening coordinator. Twenty-three similar hospitals were control sites. All hospitals had orthopedic services and handled moderate-to-higher volumes of fracture patients. Administrative health data were used to evaluate the impact of the intervention.
Fragility fracture patients (≥50 years; hip, humerus, forearm, spine, or pelvis fracture) were identified from administrative health records. Cases were fractures treated at 1 of the 37 hospitals assigned a coordinator. Controls were the same types of fractures at the control sites. Data were assembled for 20 quarters before and 10 quarters after the implementation (from January 2002 to March 2010). To test for a shift in trends, we employed an interrupted time series analysis—a study design used to evaluate the longitudinal effects of interventions, through regression modelling. The primary outcome measure was bone mineral density (BMD) testing. Osteoporosis medication initiation and persistence rates were secondary outcomes in a subset of patients ≥66 years of age.
A total of 147,071 patients were used in the analysis. BMD testing rates increased from 17.0% pre-intervention to 20.9% post-intervention at intervention sites (P < .01) compared with no change at control sites (14.9% and 14.9%, P = .33). Medication initiation improved significantly at intervention sites (21.6–23.97%; P = .02) but not at control sites (17.5–18.5%; P = .27). Persistence with bisphosphonates decreased at all sites, from 59.9% to 56.4% at intervention sites (P = .02) and more so from 62.3% to 54.2% at control sites (P < .01) using 50% proportion of days covered (PDC 50).
Significant improvements in BMD testing and treatment initiation were observed after the initiation of a coordinator-based screening program to improve osteoporosis management following fragility fracture.
Keywords: bone mineral density testing, compliance, coordinator, fragility fracture, osteoporosis, screening program, treatment initiation
1. Introduction
Fragility fractures are the most concerning presentation of osteoporosis (OP) and are a signal for increased refracture risk[1–4] and increased mortality risk[5] particularly after a hip fracture.[6] Systematic reviews confirm the efficacy of bone-sparing medication (medication commonly used to treat OP) in reducing the risk of future fractures[7,8] and have been integrated into clinical practice guidelines and position papers in Canada[9,10] and abroad[11–14] that recommend fracture-risk assessment and, when indicated, bone-sparing medication for the post-fragility fracture population. Despite this agreement, <20% of fragility fracture patients undergo testing or receive bone-sparing medication after their fragility fracture.[15,16] Post-fracture programs have emerged to address this gap, with those using dedicated coordinators to facilitate guideline uptake having the best impact.[17–20] Results from randomized trials support this model for testing and treatment after hip[21] and wrist fractures[22,23] with Morrish demonstrating a dose-response relationship between the intensity of the coordinator's role and testing and OP outcomes.[24] Program evaluations have suggested reduced refracture rates with these program[25,26] and even reduced mortality, attributing this to the initiation of proven bone-sparing medication.[27] Even with the cost of a dedicated coordinator, the programs are cost-effective because of the costly hip fractures prevented[28–30]: the mean attributable direct health care costs for a hip fracture range from $36,929 (women) to $39,479 (men) in the first year.[31]
Despite testing the benefit of a coordinator in clinical trials and implementation in various forms,[14,32] we were unable to find studies that rigorously evaluated the relative effectiveness of a coordinator-based screening or liaison program versus usual care (no coordinator in place) in a wide-scale implementation of the model while attempting to control for risk of bias, or secular trends. In Ontario (population 13.8 million), a coordinator-based screening program was initiated in 2007 as part of a broader Ontario Osteoporosis Strategy.[33] Twenty coordinators were placed in 37 hospitals that had higher volumes of these fragility fractures, and offered orthopedically managed outpatient fracture clinics. Another 23 similar hospitals existed at the time but were not assigned a coordinator due to budget limits (control hospitals in our analysis). All hospitals had orthopedic services and handled moderate-to-higher volumes of fracture patients. Hospitals chosen for a coordinator were selected based on the ability to integrate a coordinator into their post-fracture care, and proximity to another hospital site to allow sharing of a coordinator within a reasonable distance. Each hospital was approached by the Ministry of Health's delegate regarding participating in the program and hospital-specific agreements were negotiated for the coordinator and their role. The coordinator's role is to identify fragility fracture patients (defined as over 50 years of age with a low-trauma fracture) usually by case finding in the fracture clinic, and to facilitate investigation and interventions through the patient's primary care provider (PCP). At the control hospitals, no specific additions were made to the fracture management program and any care was dependent on the initiative of the usual health care team and their practices.
The purpose of our study was to evaluate the impact of the implementation of the Fracture Clinic Screening Program (FCSP) of the Ontario Osteoporosis Strategy on bone mineral density (BMD) testing, medication initiation, and medication persistence in the year after a fragility fracture.
2. Methods
2.1. Fracture Clinic Screening Program
The FCSP was started in January 2007 to case find (identify) fragility fracture patients (age 50 or over, and low energy fractures, that is, fractures due to slips, trips, or falls from standing height or less), to offer education to the patient and their PCP, to offer advice, and recommendations on testing needed for fracture-risk assessment, the role of Vitamin D and calcium, and to provide follow-up to those not on treatment at baseline to ensure they follow through with their PCP on suggestions made in clinic. In this model, much of the OP-related action needs to be taken by the PCP and the patients themselves (ie, investigation, intervention, and compliance).[9,10] Coordinators are hired and trained centrally through Osteoporosis Canada to provide consistent, up-to-date information. Outcomes for this program have been reported and suggest up to 50% of patients will go on to have BMD testing, and 21% initiate prescription pharmacotherapy,[34] reflecting advances over previous estimates of <10% to 20% having any discussion of bone health with their health care providers.
All sites had approval from their respective research ethics board for research use of the data in consenting patients. This specific analysis was also approved by the research ethics board of the senior investigator's (DEB) home institution, St. Michael's Hospital (REB# 08–304C).
2.2. Study design
This study made use of administrative health data for all insured Ontarians which are maintained at the Institute for Clinical Evaluative Sciences (ICES) in Toronto. ICES is a prescribed entity funded through the Ministry of Health and Long Term Care (single insurer in the province) and holding linked sociodemographic and health information for insured health events. It supports linked data across billing data for physicians and emergency room visits, diagnostic testing billings, hospital separations and, in those over the age of 65, prescription drug billings covered by the Ontario Drug Benefits Program. All persons eligible for universal health care in Ontario have health care expenses and activity tracked in these databases. The various data are linked across sources and longitudinally and are maintained at ICES. Data housed at ICES are widely used in health services research and are considered to be of high quality.[35,36]
Fragility fracture cases from January 2002 (5 years, 20 quarters) before the program to March 2010 (3 years after the start of the program) were identified using the Public Health Agency of Canada's (PHAC) case definition for a fragility fracture. This definition sought diagnostics codes (ICD9-CM and ICD10) for humerus, forearm, hip, spine, and pelvis fractures on billing data or hospital discharge records and restricts cases to those over age of 50[36] with no similar fracture codes billed to that person's file in the 6 months before that identification (ie, thus defining a “new” fracture). If billing codes related to a similar fracture were found in the past 6 months, we assumed the current episode of care was for the previous fracture.[36] The PHAC case definition relies on the fracture type and age as being likely fragility fractures and does not specify low trauma etiology. This might attenuate intervention effects for programs like ours by including low and high trauma fractures; however, its universal application across the population makes it the only means to compare before and after the program's initiation in intervention hospitals or control.
Cases meeting PHAC fracture types were identified and restricted to those seen by orthopedic surgeons in the first 30 days post fracture, and seen at a moderate or high fragility fracture volume hospital (determined by the Ministry of Health and Long Term Care as having >350 fragility fracture cases per year). The cases were restricted to those over age of 50 and then separated into control and intervention hospitals based on whether that hospital had been assigned 1 of the 20 coordinators by the FCSP (intervention). The start time for the intervention varied over the ramp up of the program, so the individual start date was assigned time zero for each site and quarters defined as 3-month intervals before and after that date. Control hospitals had no such start date, and were assigned a start date by randomly selecting (without replacement) from the start dates of intervention hospitals. Quarters were then assigned again before and after that date. Each individual was then followed for outcome indicators for 1 year after the quarter in which their fracture visit occurred.
2.3. Administrative outcomes
2.3.1. Primary outcome: bone mineral density testing within 1 year of fracture
BMD testing was considered the primary outcome, as it is the most universal marker (ie, includes all eligible age groups, whereas medication use is only for those over 65) of post-fracture OP activity in our cohort.[35] Billing for BMD testing in those eligible for BMD was calculated to create the primary outcome rate. Those who had a BMD test in the year before their fracture were not eligible for retesting, as their previous results would be considered adequate for fracture-risk assessment.
2.3.2. Secondary outcome: treatment initiation within 1 year of screening in those over 65 years of age
Treatment initiation was defined as the filling of a prescription for a bone-sparing agent in the year after a fragility fracture (ie, the first clinic visit after fracture).[35] Those who had filled a prescription in the year before the fracture were excluded from the analysis on treatment initiation, as they were considered previously treated and would not reflect program impact. Medication billing was available for those 66 years and older, as all Ontarians 65 years and older are eligible for free prescription medications, including bone-sparing medication, through the Ontario Drug Benefits Program. In this analysis, we could therefore only include persons 66 years and older at time of fracture, to permit examination of the year before fracture to exclude those taking bone-sparing medication before the index fracture. Medications considered in this definition were: alendronate, risedronate, calcitonin, etidronate, teriparatide, and zoledronic acid.
2.3.3. Secondary outcome: persistence with taking medication
Persistence with medication was measured by calculating the proportion of days covered (PDC) by the drug prescriptions that were filled[37,38] as an indicator of medication taking behavior or adherence. A PDC threshold of at least 80% (ie, the person filled enough prescriptions to comply with 80% of the usual dose)[37,38] was required, as this has been shown to be the acceptable threshold for fracture prevention benefit.[39] Compliance was calculated as the proportion of patients who initiated care in the year following their fracture who met the PDC threshold. We also report a lower threshold, PDC-50 (50% rather than 80%).
2.4. Analysis
2.4.1. Description of sample
The sample capture and characteristics were described using descriptive statistics.
2.4.2. Description of outcome rates
The sample description and the rates of the target outcomes described for all PHAC fracture cases seen in the control and intervention hospitals before and after program implementation were calculated and summarized. The numerator and denominator were defined only by those eligible for that outcome in that 1-year observation window. Thus, follow-up of an individual was censored at the 1-year post-screening visit to prevent late events (ie, BMD testing beyond 1 year) from favorably influencing rates.
2.4.3. Interrupted time series
To test for a shift in trends, we employed an interrupted time series analysis—a study design used to evaluate the longitudinal effects of interventions, through regression modelling. Since the interrupted time series is well suited for evaluation of effectiveness of population-level health interventions that have been implemented at a clearly defined point in time,[40] we employed this study design to evaluate whether the program led to increased BMD testing, treatment initiation and persistence in the intervention group when compared with the control hospitals. Interventional autoregressive, integrated moving average (ARIMA) models were used to examine the impact of program initiation on rates of outcomes.[41,42] Models were represented by their structural parameters and the best ARIMA model was selected based on the Schwarz-Bayesian criterion.[43–45] Initial model specification was guided by visual inspection of correlograms, and ramp intervention functions were used to allow for more gradual intervention uptake. To test the appropriateness of model assumptions, we examined the autocorrelation, partial autocorrelation, and inverse autocorrelation functions. We performed the augmented Dickey-Fuller test to test for stationarity and examined the Ljung-Box χ2 statistic at various lags to assess autocorrelation.
The anticipated shape of the impact of the intervention was either an immediate or delayed, yet sustained, higher rate of outcome after the intervention compared with before. The control hospitals were expected to experience no such change in trend but to continue as per pre-intervention trends. Analysis was carried out in SAS 9.3 (Cary, NC: SAS Institute).
3. Results
A cohort flowchart can be found in Figure 1. A final set of 147,071 cases were identified as having met the PHAC operational case definition for fragility fracture, age, and site criteria and had a visit date for the fracture before April 10, 2010 and were used in the analysis. At the intervention hospitals 109,173 fractures met the PHAC definition: 69,856 before the intervention and 39,317 after (some of which would have been in our program but not all). The control hospitals had 24,676 fractures before the program began and 13,222 fractures in the post-intervention period.
Figure 1.
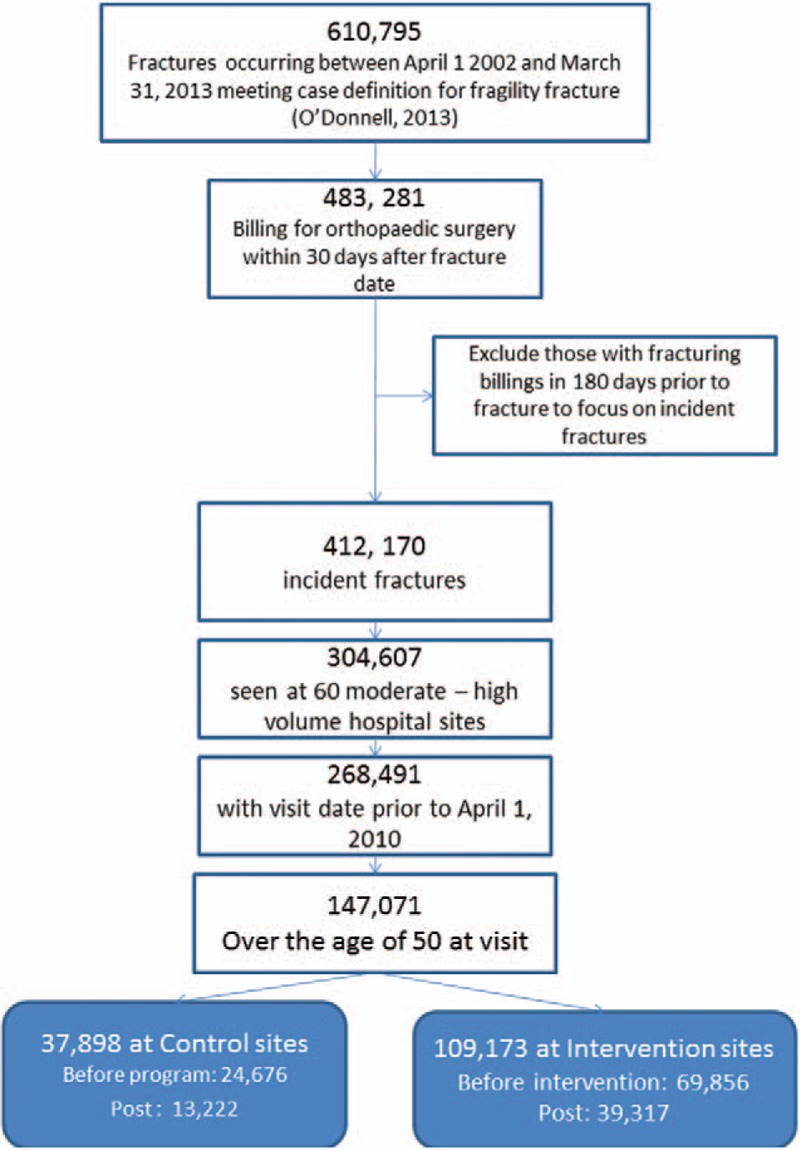
Flowchart depicting creation of the dataset from administrative health data used in this time series analysis.
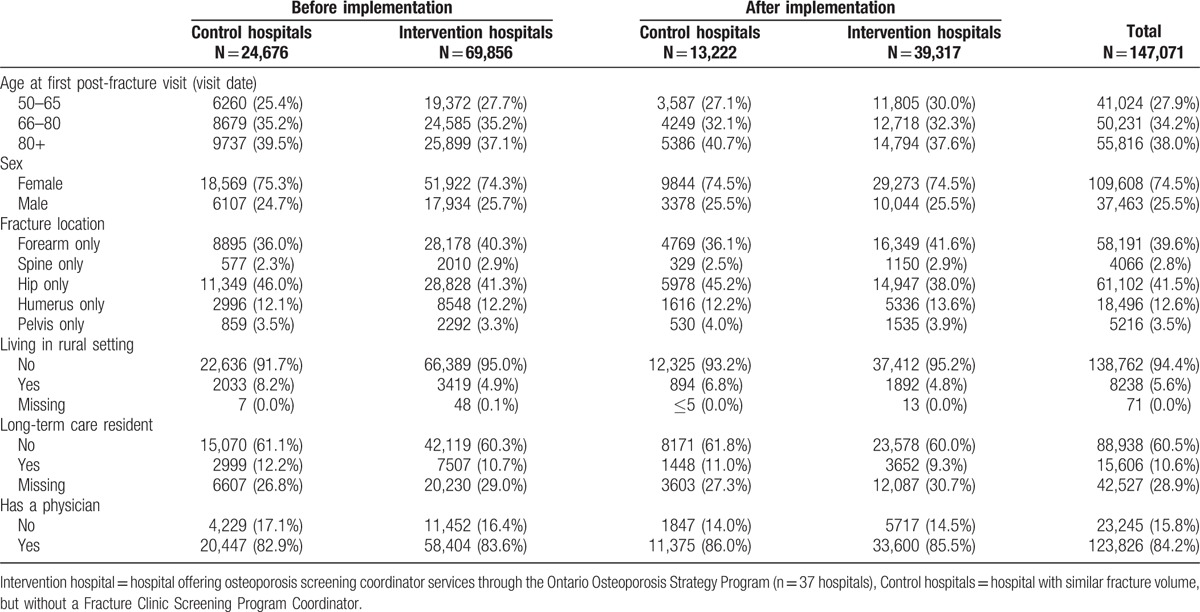
The demographic information is summarized in Table 1. Comparison of the pre-intervention group at the intervention hospitals and control hospitals demonstrated considerable similarities in terms of age, gender, and breakdown of fracture type.
Table 1.
Description of samples used in the interrupted time series analyses (control and intervention hospitals before and after implementation).
3.1. Time series analysis
Table 2 summarizes the rates and their statistical significance for OP outcomes in the cases identified for the interrupted time series analysis before and after program initiation at hospitals with and without coordinators (intervention and control, respectively).
Table 2.
Observed outcome rates for pre- and post-intervention samples at intervention and control hospitals.

3.2. Primary outcome
Average BMD testing rates increased significantly among intervention hospitals (from 17.0% before program implementation to 20.9% following implementation; P < .01, Table 2). The BMD testing rates at the control hospitals were stable at 14.9% (P = .33). This is depicted graphically in Figure 2.
Figure 2.
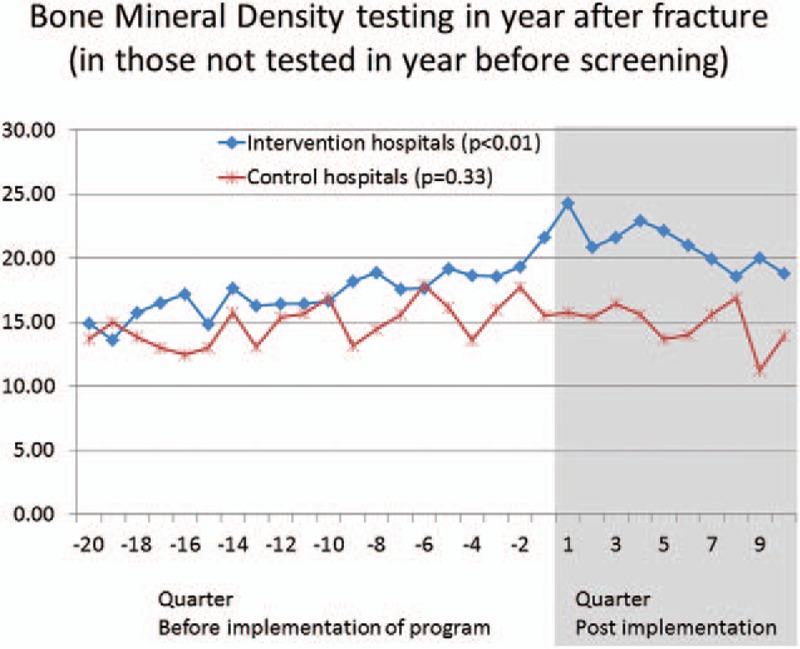
Graphical depiction of trends for outcomes, divided into pre- and post-implementation phases (vertical line). Main lines (continuing before and after implementation) depict overall trends for intervention sites (blue) and control sites (red boxes).
3.3. Secondary outcomes
Treatment initiation (pharmacotherapy dispensing in those over 65) showed statistically significant increases in intervention hospital (P < .02) moving from 21.6% pre-implementation to 24.0% post-implementation, but there was no change in the control hospitals (17.5% and 18.5%, respectively, P = .27). Medication persistence estimated by the PDC of either 80% or 50% declined in all groups from pre- to post-intervention period. Persistence in medication taking among intervention hospital cases dropped from 59.9% pre-intervention to 56.4% post-intervention (P = .02 at PDC50). At the PDC 80, the rates decreased from 45.8% to 40.01% (P < .001). In control hospitals, a larger decrease in persistence (PDC 50) was found: 62.3% to 54.2%, P < .01 or 47.6% to 38.5% (P < .01) using a PDC-80 threshold.
4. Discussion
Interrupted time series analysis demonstrated a statistically significant increase in BMD testing rates in persons 50 years and older who had sustained a low-trauma fracture after the implementation of a province-wide FCSP compared with before implementation. Further, similar changes were not found in hospitals that were not participating in the program over the same time frame. Higher rates of treatment initiation were also observed following the program implementation in intervention over control hospitals. Persistence with medication taking in the year following initiation declined in both groups in the post-implementation period, and this should be the focus of further inquiry as to whether it is an artifact of media attention leading to discontinuation[14] or changes in the dispensing practices biasing toward lower adherence rates.[46] Treatment initiation rates were higher in the intervention hospitals (24.0%) than the control hospitals (18.5%) after the program started. However, these levels continue to be below what we have seen achieved in clinical trials, and what we might expect as the target in this higher risk group. One might expect a lower rate in an evaluation of a widespread implementation such as our program compared with a randomized trial, but ideal levels should always be the aim.
The program described in this paper would be considered a type C (education, targeted communication with providers) in the review by Ganda and this was associated with an estimated 30% absolute increase in testing rates—we found a lower rate when looking at the average for the interrupted time series analysis, but a comparable rate when, like Ganda,[18] we described only the confirmed low-trauma fracture participants in this data (43% testing).
5. Strengths
Interrupted time series analyses are considered the most robust quasi-experimental design for inferring causality between intervention and outcome in the absence of a randomized controlled trial.[40,47,48] Our ability to also examine a complementary comparison group strengthens our conclusion that the outcomes we observed were not due to a competing shift in provincial practices, or secular advances in guideline implementation,[49,50] or other facets of the Ontario Osteoporosis Strategy itself—for example, public and health care professional education and efforts in rehabilitation and long-term care.[14,33]
A key feature in the quality of an interrupted time series analysis is the independent, unbiased ascertainment of outcomes,[48] and in our situation independence from the embedded coordinator him/herself. We used administrative data to allow greater objectivity across all subjects and less influence on outcome in those who were seen by the coordinator for follow-up information. The primary outcome was BMD testing billings, because they were available for all patients in the cohort (ie, not limited to those over age 66 years as was the case for medication initiation and persistence outcomes) and were recorded in the administrative health billings consistently over the study duration. An amendment to the government's funding of BMD testing came into place in 2007 in an effort to curb overuse in low-risk patients. It was not intended to impact the testing of these higher-risk patients, and we believe that if it did, it would have done so equally in the intervention and control hospitals. BMD testing was therefore considered the most universal indicator of OP-related activity after a fragility fracture.
Ramsay et al[48] suggest a set of 8 criteria for a high quality, interrupted time series analysis. Our study meets these criteria, with the exception that the observed impact of our intervention was more immediate and sustained than anticipated in our a priori hypothesis.
6. Limitations
There were 2 limitations to this study that should be acknowledged. First, the use of the PHAC case definition, though well accepted and recommended,[36] focuses on accurate coding for a probable or likely fragility fractures (type of fracture type and age), but it cannot ascertain the low-trauma nature of the fracture. All of our groups could therefore be a mix of high and low trauma. This would suggest that the focus of our message is on the trend, the shift that we observed and it significance, recognizing the actual point estimates could be attenuated by the inclusion of people who may have had high-trauma fractures (and would not be seen in our program) or indeed people whom the coordinators missed due to their being part time at each site. For the interrupted time series analysis the case definition had to be the same across all groups and we chose the PHAC as recommended for chronic disease surveillance in Canada.[36] The significance of our findings despite this attenuation builds confidence in our results.
A second limitation is the potential that there were uncontrolled biases in this analysis. Interrupted time series assumes populations of independent individuals, but there could have been clustering within hospitals in both our intervention and our control sites. Because we had so many sites, and we had control sites showing no advances in testing and treatment rates, we felt confident that the impact of any within hospital trends was not the cause of our findings.
Future research will continue to monitor the impact of the Ontario FCSP as changes are made. Recently a shift was made to allow the coordinators to order and book BMD testing allowing fracture-risk assessments to be communicated back to the PCP bringing more information to that visit. Future research can test for further impact of that program change. This shift will also allow risk stratified analyses based on the access to BMD test results and fracture-risk assessments that will be linked to the administrative data at ICES and provide more specific need-based analyses.
7. Conclusions
This study evaluated the impact of the implementation of a program designed to reduce a gap between clinical practice guidelines and front-line OP care following a fragility fracture. The program was implemented across 37 different hospitals in the province, reflecting some of the busiest fracture clinics in that publically funded health system. We demonstrated a positive, statistically significant impact of a coordinator, who acts as case finder, educator, and facilitator of bone-health screening and treatment in the fracture clinic setting, on BMD testing and OP treatment initiation. This study adds support to the growing body of knowledge on the effectiveness of fracture liaison service coordinator models[21–24,28] and is the first to use an interrupted time series analysis to evaluate the impact.
Acknowledgments
The authors gratefully acknowledge the support of Osteoporosis Canada, which implements the Ontario Osteoporosis Strategy Fracture Clinic Screening program on behalf of the MOHLTC. The authors also thank the Fracture Clinic Screening Program Evaluation Team for their contributions to the program and to overall evaluation of the program.
Footnotes
Abbreviations: ARIMA = autoregressive integrated moving average (an interrupted time series analysis), BMD = bone mineral density, FCSP = Fracture Clinic Screening Program, ICES = Institute for Clinical Evaluative Sciences, OP = osteoporosis, PCP = primary care provider, PDC = proportion of days covered, PHAC = Public Health Agency of Canada.
The data used in this study were linked using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences.
This study was supported by funding from the Ontario Ministry of Health and Long Term Care (MOHLTC) through the Ontario Osteoporosis Strategy. The views expressed are those of the researchers and do not necessarily reflect those of the MOHLTC.
MM received Honoraria and served as a consultant/advisory board of AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Hoffman-La Roche, Novartis, Novo Nordisk and Pfizer. ERB has received an unrestricted research grant and speaker fees from Amgen Canada Inc, and is an Editorial Board member for the Journal of Rheumatology.
The rest of the authors declare no conflict of interest.
Contributor Information
Collaborators: the Ontario Osteoporosis Strategy Fracture Clinic Screening Program Evaluation Team
References
- [1].Colón-Emeric C, Kuchibhatla M, Pieper C, et al. The contribution of hip fracture to risk of subsequent fractures: data from two longitudinal studies. Osteoporos Int 2003;14:879–83. [DOI] [PubMed] [Google Scholar]
- [2].Haentjens P, Autier P, Collins J, et al. Colles fracture, spine fracture, and subsequent risk of hip fracture in men and women. A meta-analysis. J Bone Joint Surg Am 2003;85-A:1936–43. [DOI] [PubMed] [Google Scholar]
- [3].Johnell O, Kanis JA, Odén A, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int 2004;15:175–9. [DOI] [PubMed] [Google Scholar]
- [4].Klotzbuecher CM, Ross PD, Landsman PB, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000;15:721–39. [DOI] [PubMed] [Google Scholar]
- [5].Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009;301:513–21. [DOI] [PubMed] [Google Scholar]
- [6].Papaioannou A, Kennedy CC, Ioannidis G, et al. The impact of incident fractures on health-related quality of life: 5 years of data from the Canadian Multicentre Osteoporosis Study. Osteoporos Int 2009;20:703–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cranney A, Waldegger L, Zytaruk N, et al. Risedronate for the prevention and treatment of postmenopausal osteoporosis. Cochrane Database Syst Rev 2003;4:CD004523. [DOI] [PubMed] [Google Scholar]
- [8].Cranney A, Tugwell P, Adachi J, et al. Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev 2002;23:517–23. [DOI] [PubMed] [Google Scholar]
- [9].Papaioannou A, Morin S, Cheung AM, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010;182:1864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brown JP, Josse RG. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ 2002;167:S1–34. [PMC free article] [PubMed] [Google Scholar]
- [11].Javid KS, Thien A, Hill R. Implementation of and compliance with NICE guidelines in the secondary prevention of osteoporotic fractures in postmenopausal women. Ann R Coll Surg Engl 2008;90:213–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lewiecki EM, Watts NB. New guidelines for the prevention and treatment of osteoporosis. South Med J 2009;102:175–9. [DOI] [PubMed] [Google Scholar]
- [13].Akesson K, Marsh D, Mitchell PJ, et al. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int 2013;24:2135–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Marsh D, Akesson K, Beaton DE, et al. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int 2011;22:2051–65. [DOI] [PubMed] [Google Scholar]
- [15].Elliot-Gibson V, Bogoch ER, Jamal SA, et al. Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int 2004;15:767–78. [DOI] [PubMed] [Google Scholar]
- [16].Giangregorio L, Papaioannou A, Cranney A, et al. Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum 2006;35:293–305. [DOI] [PubMed] [Google Scholar]
- [17].Bell K, Strand H, Inder WJ. Effect of a dedicated osteoporosis health professional on screening and treatment in outpatients presenting with acute low trauma non-hip fracture: a systematic review. Arch Osteoporos 2014;9:167. [DOI] [PubMed] [Google Scholar]
- [18].Ganda K, Puech M, Chen JS, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 2013;24:393–406. [DOI] [PubMed] [Google Scholar]
- [19].Sale JE, Beaton D, Posen J, et al. Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int 2011;22:2067–82. [DOI] [PubMed] [Google Scholar]
- [20].Gidwani S, Davidson N, Trigkilidas D, et al. The detection of patients with ‘fragility fractures’ in fracture clinic—an audit of practice with reference to recent British Orthopaedic Association guidelines. Ann R Coll Surg Engl 2007;89:147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Majumdar SR, Beaupre LA, Harley CH, et al. Use of a case manager to improve osteoporosis treatment after hip fracture: results of a randomized controlled trial. Arch Intern Med 2007;167:2110–5. [DOI] [PubMed] [Google Scholar]
- [22].Majumdar SR, Johnson JA, McAlister FA, et al. Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: a randomized controlled trial. CMAJ 2008;178:569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jaglal SB, Donescu OS, Bansod V, et al. Impact of a centralized osteoporosis coordinator on post-fracture osteoporosis management: a cluster randomized trial. Osteoporos Int 2012;23:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morrish DW, Beaupre LA, Bell NR, et al. Facilitated bone mineral density testing versus hospital-based case management to improve osteoporosis treatment for hip fracture patients: additional results from a randomized trial. Arthritis Rheum 2009;61:209–15. [DOI] [PubMed] [Google Scholar]
- [25].Nakayama A, Major G, Holliday E, et al. Evidence of effectiveness of a fracture liaison service to reduce the re-fracture rate. Osteoporos Int 2016;27:873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lih A, Nandapalan H, Kim M, et al. Targeted intervention reduces refracture rates in patients with incident non-vertebral osteoporotic fractures: a 4-year prospective controlled study. Osteoporos Int 2011;22:849–58. [DOI] [PubMed] [Google Scholar]
- [27].Center JR, Bliuc D, Nguyen ND, et al. Osteoporosis medication and reduced mortality risk in elderly women and men. J Clin Endocrinol Metab 2011;96:1006–14. [DOI] [PubMed] [Google Scholar]
- [28].Majumdar SR, Lier DA, Beaupre LA, et al. Osteoporosis case manager for patients with hip fractures: results of a cost-effectiveness analysis conducted alongside a randomized trial. Arch Intern Med 2009;169:25–31. [DOI] [PubMed] [Google Scholar]
- [29].McLellan AR, Wolowacz SE, Zimovetz EA, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int 2011;22:2083–98. [DOI] [PubMed] [Google Scholar]
- [30].Sander B, Elliot-Gibson V, Beaton DE, et al. A coordinator program in post-fracture osteoporosis management improves outcomes and saves costs. J Bone Joint Surg Am 2008;90:1197–205. [DOI] [PubMed] [Google Scholar]
- [31].Nikitovic M, Wodchis WP, Krahn MD, et al. Direct health-care costs attributed to hip fractures among seniors: a matched cohort study. Osteoporos Int 2013;24:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Eisman JA, Bogoch ER, Dell R, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res 2012;27:2039–46. [DOI] [PubMed] [Google Scholar]
- [33].Jaglal SB, Hawker G, Cameron C, et al. The Ontario Osteoporosis Strategy: implementation of a population-based osteoporosis action plan in Canada. Osteoporos Int 2010;21:903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Beaton DE, Dyer S, Jiang D, et al. Factors influencing the pharmacological management of osteoporosis after fragility fracture: results from the Ontario Osteoporosis Strategy's fracture clinic screening program. Osteoporos Int 2014;25:289–96. [DOI] [PubMed] [Google Scholar]
- [35].Cadarette SM, Jaglal SB, Raman-Wilms L, et al. Osteoporosis quality indicators using healthcare utilization data. Osteoporos Int 2011;22:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].O’Donnell S. Canadian Chronic Disease Surveillance System (CCDSS) Osteoporosis Working Group. Use of administrative data for national surveillance of osteoporosis and related fractures in Canada: results from a feasibility study. Arch Osteoporos 2013;8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cadarette SM, Burden AM. Measuring and improving adherence to osteoporosis pharmacotherapy. Curr Opin Rheumatol 2010;22:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Melo M, Qiu F, Sykora K, et al. Persistence with bisphosphonate therapy in older people. J Am Geriatr Soc 2006;54:1015–6. [DOI] [PubMed] [Google Scholar]
- [39].Siris ES, Selby PL, Saag KG, et al. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med 2009;122:S3–13. [DOI] [PubMed] [Google Scholar]
- [40].Lopez-Bernal J, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017;46:348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hartmann DP, Gottman JM, Jones RR, et al. Interrupted time-series analysis and its application to behavioral data. J Appl Behav Anal 1980;13:543–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Box GEP, Jenkins GM. Time Series Analysis: Forecasting and Control. San Francisco, CA: Holden-Day; 1976. [Google Scholar]
- [43].Andersson K, Petzold MG, Sonesson C, et al. Do policy changes in the pharmaceutical reimbursement schedule affect drug expenditures? Interrupted time series analysis of cost, volume and cost per volume trends in Sweden 1986–2002. Health Policy 2006;79:231–43. [DOI] [PubMed] [Google Scholar]
- [44].Gustafsson NK, Ramstedt MR. Changes in alcohol-related harm in Sweden after increasing alcohol import quotas and a Danish tax decrease--an interrupted time-series analysis for 2000–2007. Int J Epidemiol 2011;40:432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hopewell S, Ravaud P, Baron G, et al. Effect of editors’ implementation of CONSORT guidelines on the reporting of abstracts in high impact medical journals: interrupted time series analysis. BMJ 2012;344:e4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Burden AM, Huang A, Tadrous M, et al. Variation in the days supply field for osteoporosis medications in Ontario. Arch Osteoporos 2013;8:128. [DOI] [PubMed] [Google Scholar]
- [47].Calsyn RJ, Fergus EO, York JL. Interrupted time series analysis: a research technique for evaluating social programs for the elderly. J Gerontol 1977;32:89–96. [DOI] [PubMed] [Google Scholar]
- [48].Ramsay CR, Matowe L, Grilli R, et al. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care 2003;19:613–23. [DOI] [PubMed] [Google Scholar]
- [49].Johansson PM, de Leon AP, Sadigh S, et al. Statistical modelling needed to find the effects from a community-based elderly safety promotion program. Eur J Public Health 2009;19:100–5. [DOI] [PubMed] [Google Scholar]
- [50].Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27:299–309. [DOI] [PubMed] [Google Scholar]


