Abstract
Rationale:
Regorafenib is the new standard third-line therapy in metastatic colorectal cancer (mCRC). However, the reported 1-year overall survival rate does not exceed 25%.
Patient concerns:
A 55-year-old man affected by mCRC, treated with regorafenib combined with stereotactic body radiotherapy (SBRT), showing a durable response.
Interventions:
After 6 months of regorafenib, a PET/CT scan revealed a focal uptake in a solid lung nodule which was treated with SBRT, whereas continuing regorafenib administration. Fourteen months later, the patient had further progression in a parasternal lymph node, but treatment with regorafenib was continued. The regorafenib-associated side effects, such us the hand-foot syndrome, were favorable managed by reducing the dose from 160 to 120 mg/day.
Outcomes:
Patient-reported outcome was characterized by a progression-free survival of approximately 3 years.
Lessons:
in presence of oligometastatic progression, a local SBRT while retaining the same systemic therapy may be a better multidisciplinary approach. Moreover, disease progression is no longer an absolute contraindication for continuing the regorafenib treatment.
Keywords: beyond progression, case report, colorectal cancer, oligometastatic, regorafenib, stereotactic body radiotherapy
1. Introduction
Regorafenib is a novel multikinase inhibitor indicated for previously treated metastatic colorectal cancer (mCRC) and currently it is considered a new standard of care in third-line treatment of mCRC. Treatment with regorafenib has demonstrated statistically significant improvements in terms of overall survival (OS), progression-free survival (PFS), and disease control in comparison to placebo in 2 randomized phase III trials.[1,2] The CORRECT study showed that the addition of regorafenib to best supportive care increases OS, compared with best supportive care only, in mCRC patients who had failed approved standard therapies.[1] In the CONCUR study, regorafenib resulted in significantly improved OS, PFS, and disease control rate (DCR) compared to placebo in Asian patients with refractory mCRC.[2] The efficacy and safety profiles of regorafenib for mCRC have also been demonstrated in a large, open-label, single-arm study[3] (CONSIGN) and in a real-life–based cohort study[4] (REBACCA).
The advent of regorafenib has improved the therapeutic approach to chemoresistant, late-stage mCRC; nevertheless, the great majority of patients still have a poor outcome, and the 1-year OS rate does not exceed 25%. Therefore, in these patients, an additional locoregional treatment could improve the control of metastatic disease, even if the optimal strategy for metastatic progressive disease is still debated.
Stereotactic body radiation therapy (SBRT) delivers large doses of radiation with great accuracy and it has been successfully used in both metastatic lung and breast tumors, obtaining a DCR of approximately 80%.[5,6] However, the clinical experience in the treatment of mCRC is still scarce.[7]
In this article, we describe the case of an mCRC patient who had a durable response to regorafenib in association to SBRT, and thus suggesting a possible new treatment opportunity for these patients.
2. Case report
In October 2011, a 55-year-old Caucasian man was diagnosed with mCRC. He underwent right hemicolectomy with omental and liver biopsy for a pT3pN0 pM1a (liver), undifferentiated (G3) mucinous adenocarcinoma of the ascending colon. Molecular analysis revealed a KRAS codon 12 mutation. Computed tomography (CT) scan at diagnosis showed multiple metastases in the right lobe of the liver (V, VI and VII segments, global diameter 7 cm) and multiple, small, with no malignant characteristics nodules in both lungs. FOLFOX-4 regimen was started as preoperative strategy for liver metastases resection. After 6 months of chemotherapy a partial response in the liver was achieved; therefore, the surgeon suggested a 2-stage right hepatectomy. However, a re-evaluation by the surgeon in February 2013 suggested how a right hepatectomy was not indicated for an inadequate contralateral hypertrophy. Therefore, a second-line chemotherapy with FOLFIRI plus aflibercept was started. After 12 cycles of chemotherapy, CT scan showed stable disease (SD), and aflibercept monotherapy was continued until severe proteinuria (5000 mg/24 h) and a blood pressure increase (150/100 mm Hg) occurred, in December 2013. Considering the long PFS, the patient was referred to a follow-up program. After 2 months, a 2 mm increase of the solid lung nodule in the superior lingular segment was reported, whereas liver metastases remained stable. Three months later, a new CT scan revealed a disease progression in lung (a lingular nodule change from 7 to 10 mm) and the onset of a peritoneal subdiaphragmatic nodule.
In August 2014, a third-line chemotherapy with regorafenib was started. After 3 cycles of regorafenib at a standard dosage (160 mg per day for 3 and 1 week rest), a grade 2 hand-foot-syndrome (HFS) was reported and the dose was reduced to 120 mg/day, leading to an improvement of symptoms. In February 2015, after 6 cycles of regorafenib, a CT scan showed a small size increase (Dmax 15 mm) of a lingular nodule. Our local multidisciplinary board of gastrointestinal experts suggested to perform an SBRT for lung lesion (54 Gy dose) and to maintain regorafenib at a dosage of 120 mg/day that was well tolerated. For metabolic evaluation of persistent disease after SBRT, a PET/CT 18-FDG was performed and no pathologic uptake areas were identified. The following CT and PET/CT scan revealed SD until April 2016, when a focal uptake (SUVmax 4.6) in a right parasternal lymph node was observed, without any other sites of progression. However, this oligoprogression in a single lymph node was not considered a suitable target and therefore the institutional board of gastrointestinal experts decided to treat the mediastinal lymph node with SBRT and to continue regorafenib treatment.
In July 2016, a PET/CT scan revealed a reduced metabolic uptake (SUV 1.1) in the lymphadenopathy. Treatment with regorafenib was maintained and no additional disease progression was observed. The patient is still undergoing treatment with regorafenib at a dosage of 120 mg/day, with a good tolerability and a PFS of 36 months (Fig. 1).
Figure 1.
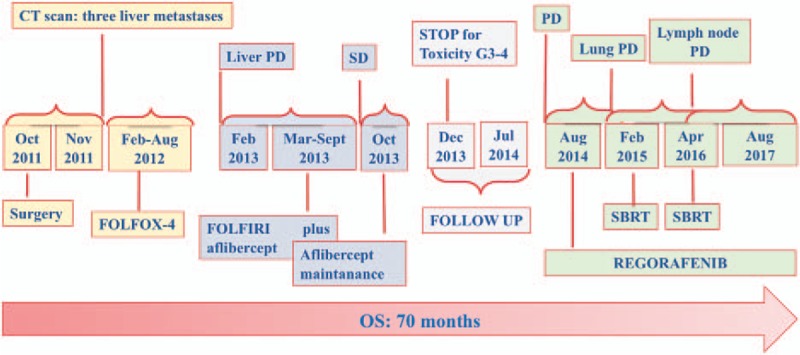
Long patient time-line and summary of all treatments carried out from diagnosis. OS = overall survival, PD = progression disease, SBRT = stereotactic body radiation therapy, SD = stable disease.
3. Discussion
The introduction of novel treatments, mainly consisting of chemotherapy agents and targeted therapy, has improved the OS of patients with mCRC.[8] Here, we report the case of a mCRC patient who had a durable response with regorafenib after the failure of a first-line treatment with FOLFOX and of a second-line therapy with FOLFIRI plus aflibercept. The regorafenib-associated side effects, in particular the HFS, were managed by reducing the dose from 160 to 120 mg/day.
In our view, this case reports the following interesting aspects: cases of such a long response to regorafenib, with a PFS of 36 months, have been rarely described in mCRC[9]; regorafenib was maintained despite the appearance of a lung nodule 6 months after the treatment initiation; SBRT was successfully used in association with regorafenib as a locoregional treatment. This novel therapeutic strategy was chosen because the patient had just a oligometastatic disease.
The term oligometastases, introduced by Hellmann and Weichselbaum[10] in 1995, describes an intermediate stage of cancer, between localized and metastatic disease, that is suitable for local treatment. Niibe and Chang[11] further defined the concept of oligorecurrence as a disease stage in which there are a limited number of metastases and in which the primary tumor has been controlled. In oligometastatic cases, we can use a multidisciplinary approach aiming to locally control the metastatic disease (i.e., surgery, radiotherapy, radiofrequency ablation) while controlling the systemic diseases with concomitant or subsequent oncological therapies (i.e., chemotherapy, target therapies, and immunotherapies).
In mCRC, surgery is the preferred approach for completely resectable oligometastases, especially for liver metastases.[12] Nevertheless, the patient's comorbidities/age, and the site and number of metastases could limit the surgical approach and lead to opt for others local strategies, such as SBRT and radiofrequency ablation. SBRT is widely used to treat metastases, because the radiation dose can be precisely delivered to the target lesion while sparing normal tissue, and therefore is usually well tolerated; moreover, the duration of the treatment is shorter in comparison with conventional radiation therapy.[13] SBRT has been mainly applied to treat nodal metastases, and it has been also used for solitary lung and liver metastases.[7] However, the clinical evidence supporting the use of SBRT for treating oligometastatic disease is still scarce.[7,13,14] Data from these small clinical studies suggest that liver, lung, isolated lymph nodes, spinal and adrenal metastasis, and postsurgical pelvic recurrence can be suitable for SBRT. In unresectable mCRC metastases, a local treatment with SBRT has shown to be able to improve patient's survival and quality of life with low morbidity.[15]
However, to the best of our knowledge, the combination of regorafenib and SBRT in mCRC has never been explored yet, and the present case is the first published case on this subject. A similar approach (i.e., SBRT delivered during regorafenib treatment to treat metastases) has been previously described only in 2 patients affected by metastatic gastrointestinal stromal tumor, achieving an objective response in the first case and a significant clinical benefit associated with a local tumor control in the second case.[16] Also, in our case, we obtained encouraging results, with an unexpected PFS of 36 months and a good tolerability of the combined treatment. This good response to regorafenib and SBRT in a late-stage mCRC suggests that radiotherapy can be used to treat focally progressing lesions in combination with systemic therapy, not only with a palliative intent but also to control the disease. The optimal, multidisciplinary management of the patient's disease and adverse events has probably also contributed to this positive outcome. However, further studies are needed to confirm the efficacy of SBRT in association to regorafenib in mCRC oligorecurrence patients.
The patient informed consent was given and the Ethical approval was not necessary, as it is only a case report.
4. Conclusions
This case report shows that regorafenib can improve disease control and significantly prolongs survival in mCRC, and that the concomitant use of SBRT is safe and effective. Moreover, it suggests that disease progression is no longer an absolute contraindication for continuing the treatment with regorafenib.
Footnotes
Abbreviations: DCR = disease control rate, HFS = hand-foot-syndrome, mCRC = metastatic colorectal cancer, OS = overall survival, PFS = progression-free survival, SBRT = stereotactic body radiotherapy, SD = stable disease.
The authors report no conflicts of interest.
References
- [1].Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. [DOI] [PubMed] [Google Scholar]
- [2].Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619–29. [DOI] [PubMed] [Google Scholar]
- [3].Van Cutsem E, Ciardiello F, Seitz JF, et al. 2139 CONSIGN: an open-label phase 3B study of regorafenib in patients with metastatic colorectal cancer (mCRC) who failed standard therapy. Eur J Cancer 2015;51:S378–9. [Google Scholar]
- [4].Adenis A, De la Fouchardiere C, Paule B, et al. Survival, safety, and prognostic factors for outcome with Regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBACCA) nested within a compassionate use program. BMC Cancer 2016;16:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013;14:e28–37. [DOI] [PubMed] [Google Scholar]
- [6].Ahmed KA, Torres-Roca JF. Stereotactic body radiotherapy in the management of oligometastatic disease. Cancer Control 2016;23:21–9. [DOI] [PubMed] [Google Scholar]
- [7].Seo YS, Kim M-S, Yoo H-J, et al. Stereotactic body radiotherapy for oligo-recurrence within the nodal area from colorectal cancer. World J Gastroenterol 2014;20:2005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- [9].Tang R, Kain T, Herman J, et al. Durable response using regorafenib in an elderly patient with metastatic colorectal cancer: case report. Cancer Manag Res 2015;7:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8–10. [DOI] [PubMed] [Google Scholar]
- [11].Niibe Y, Chang JY. Novel insights of oligometastases and oligo-recurrence and review of the literature. Pulm Med 2012;2012:261096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].House MG, Ito H, Gönen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg 2010;210:744–52. [DOI] [PubMed] [Google Scholar]
- [13].Fode MM, Høyer M. Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother Oncol 2015;114:155–60. [DOI] [PubMed] [Google Scholar]
- [14].Aoki M, Hatayama Y, Kawaguchi H, et al. Stereotactic body radiotherapy for lung metastases as oligo-recurrence: a single institutional study. J Radiat Res 2016;57:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takeda A, Sanuki N, Kunieda E. Role of stereotactic body radiotherapy for oligometastasis from colorectal cancer. World J Gastroenterol 2014;20:4220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gatto L, Nannini M, Saponara M, et al. Radiotherapy in the management of gist: state of the art and new potential scenarios. Clin Sarcoma Res 2017;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]


