Abstract
Background:
Depending on the type of injury, the pain mechanisms are multifactorial. Preoperative pregabalin administrations as an adjunct to a multimodal postoperative pain management strategy have been tested in various surgical settings. The purpose of current study was to evaluate the effects of preoperative pregabalin administration on postoperative pain intensity and rescue analgesic requirement following video-assisted thoracoscopic surgery (VATS).
Methods:
Sixty adult patients undergoing VATS were randomly assigned either to receive pregabalin 150 mg (Pregabalin group) or placebo (Control group) 1 hour before anesthesia. Primary efficacy variable was pain intensity. Secondary efficacy variables were the requirement of rescue analgesics, total volume of intravenous patient-controlled analgesia (IV-PCA), and adverse effects induced by pregabalin or IV-PCA.
Results:
Pain intensity scores at post-anesthesia care unit (PACU), 6 and 24 hours were lower significantly in the Pregabalin group compared with the Control group (mean [SD]; 5.6 [2.0] vs 6.8 [1.8]; mean difference: 1.2, 95% CI of difference: 0.2166–2.1835, P = .018, mean [SD]; 3.8 [1.9] vs 5.6 [1.4]; mean difference: 1.8, 95% CI of difference: 1.0074–2.7260, P = .001 and mean [SD]; 2.6 [1.6] vs 3.5 [1.5]; mean difference: 0.9, 95% CI of difference: 0.0946–1.7054, P = .029, respectively]. Also, the frequency of additional rescue drug administered at PACU (median [interquartile range]; 2 [2–3] vs 1 [1–2], P = .027) was significantly less in the Pregabalin group. The incidences of adverse effects related to pregabalin or IV-PCA were not different between the groups.
Conclusion:
A single administration of pregabalin 150 mg before VATS decreased postoperative pain scores and incidence of additional rescue analgesics in the immediate postoperative period without increased risk of adverse effects.
Keywords: opioid-sparing effect, postoperative pain, pregabalin
1. Introduction
Although many efforts have been made to achieve pain control after surgical procedures, approximately 80% of surgical patients still complain of postoperative pain.[1] To date, several modalities have been tested to reduce postoperative pain including multimodal analgesia,[2] preemptive analgesia,[3,4] and minimal invasive surgery such as video-assisted thoracoscopic surgery (VATS).[5] It is well known that VATS requires only a small incision, without rib retraction. This minimizes the damage to the thoracic wall, thereby reducing the postoperative pain, pulmonary dysfunction, morbidity, and mortality.[6–8] Nevertheless, VATS still calls for intravenous patient-controlled analgesia (IV-PCA) or epidural analgesia to control the postoperative pain.[9,10]
These methods are commonly associated with adverse effects, including procedure-related complications, respiratory depression, pruritus, constipation, as well as nausea and vomiting.
Gabapentinoids have been found to adjuvants to multimodal postoperative pain management strategies in various surgical settings. Several clinical trials have demonstrated the effects of preemptive gabapentin on postoperative pain.[11,12] In particular, pregabalin, a synthesized derivative of gabapentin, has attracted attention as it exhibits high predictability due to its linear pharmacokinetics,[13] and has more rapid absorption and higher bioavailability (90%),[14] than gabapentin.
Unlike the pain caused by other procedures, the postoperative pain caused by thoracic surgery has a multifactorial pain mechanism that involves intercostal incision, iatrogenic rib fracture, pleural irritation, and wound pain from the chest tube insertion, and referred shoulder pain.[15] Several clinical trials have demonstrated a significant opioid sparing effect and pain intensity reduction after thoracotomy. Considering the need for pain treatment regimens adapted to specific surgical procedures, there is a lack of evidence for the efficacy of pregabalin on acute perioperative pain and for its opioid-sparing effect following VATS. We hypothesized that preoperative oral pregabalin would lower the severity of postoperative pain and the need to use opioids for postoperative pain control. Therefore, this study set out to evaluate whether preoperative oral pregabalin would reduce the severity of postoperative pain and the rescue analgesic requirements after VATS.
2. Methods
After approval of the institutional review board (http://cris.nih.go.kr, registration number: KCT0000577), written informed consent was obtained from all participating patients. From December 2012 to April 2014, 60 adult patients (aged 20–65 years), ASA class 1 or 2, scheduled to undergo elective wedge resection or lobectomy under VATS were enrolled in this randomized, placebo-controlled, double-blind trial. Patients were excluded in this study if they had severe cardiovascular or respiratory diseases, impaired hepatic and/or renal function, history of chronic use of analgesics and drug abuse, history of dizziness or frequent headache or were morbidly obese patients. The flow chart showing the patients’ enrollment for randomization is shown in Fig. 1.
Figure 1.

Flow chart of patient participation.
Using computerized randomization table, the patients were assigned to the placebo group (n = 30) or to the pregabalin group (n = 30). Sixty patients were prospectively included and were randomly assigned to either the placebo group (n = 30) or the pregabalin group (n = 30) 1 day prior to surgery according to a computerized randomization table created by the hospital investigational pharmacy, who was not involved in the current study. The placebo group received placebo drug (vitamin B complex formula, General Nutrition Corp., Pittsburgh, PA) orally 1 hour before the anesthetic induction. The pregabalin groups received oral pregabalin 150 mg at the same time points. All the medications of study drug were performed by a single researcher, who was not involved in other process of this study. No other sedative premedication was given to all patients.
On arrival at the operating theatre, standard monitoring for all patients including electrocardiogram, pulse oximeter, and noninvasive blood pressure were applied. Anesthesia was induced with propofol at an initial target-effect site concentration of 4 μg mL−1 and remifentanil at a target-effect site concentration of 3 ng mL−1 (Orchestra, Fresenius Vial, France). Endotracheal intubation was facilitated with intravenous administration of rocuronium bromide 0.8 mg kg−1. General anesthesia was maintained with a continuous infusion of propofol, which was titrated to keep bispectral index scale (BIS) values of 40 to 60 and target-controlled infusion (TCI) of remifentanil was titrated to stabilize hemodynamic response during surgery. At the end of the surgery, continuous infusion of propofol and remifentanil was stopped and residual neuromuscular paralysis was antagonized with pyridostigmine 0.2 mg kg−1 and glycopyrrolate 0.04 mg kg−1. After completion of the surgical procedure, IV-PCA (AutoMed-3200, Acemedical, Korea) was initiated. The IV-PCA regimen consisted of fentanyl 20 μg kg−1 in 0.9% saline (total volume; 100 mL) was programmed to deliver 1 mL each time the patient pressed the activation button, with a 15 minutes lockout interval, no fentanyl bolus before initiation. After successful extubation and recovery, the patients were transferred to the post-anesthesia care unit (PACU). The duration of surgery and anesthesia as well as type of surgery were recorded.
Primary outcome was postoperative pain intensity. Secondary outcomes were rescue analgesic requirement, total volume of administered IV-PCA, incidence and severity of postoperative nausea and vomiting (PONV), and side effect associated with pregabalin including headache, sedation, and visual disturbance. Assessment of pain intensity during respiration was done by numerical rating scales (NRS; 0 = no pain and 10 = worst imaginable pain) on arrival to the PACU and at postoperative 6, 24, and 48 hours. If the patient requested additional analgesic or the patient's NRS score was ≥5, tramadol 0.7 mg kg−1 was administered intravenously and repeated if required. All additional doses of rescue analgesic were also recorded at every visit. The severity of nausea was assessed by NRS (0 = no nausea and 10 = worst imaginable nausea) and the use of antiemetic drugs were recorded at all time points. The adverse effects of pregabalin including sedation, headache, dizziness, and visual disturbance were also evaluated during the study period. Sedation was assessed by a numeric score of 1–5 (1, completely awake; 2, awake but drowsy; 3, asleep but responsive to verbal commands; 4, asleep but responsive to tactile stimulus; 5, asleep and not responsive to any stimulus). Patients with scores of 4 and 5 were regarded as significantly sedated and the number of these patients was recorded. Sedated patients were closely observed and managed properly as necessary. All the variables were checked by independent anesthesiologist blinded to group allocation.
Based on an institutional preliminary study, the anticipated NRS was 4 (standard deviation [SD] = 2), and we considered a 50% reduction of NRS to be clinically significant. We determined that 27 patients would be required in each group to demonstrate this difference with α error of 5% and power of 95% using the independent t test. Allowing for about 10% drop-out rate during the study period, 30 patients were enrolled in each group.
All data were expressed as number (proportion), mean ± SD, or median (interquartile range). Chi-square test or Fisher exact test for categorical variables and the independent t test or Mann–Whitney U test for continuous variables were used as appropriate. Values measured repeatedly were compared by repeated measures ANOVA using the Bonferroni correction for post hoc analysis. The package SPSS version 15.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. A P value <.05 was considered as statistically significant.
3. Results
VATS was performed successfully in all patients and none of the patients developed any perioperative complications associated with anesthesia or surgery. Thus, all data from 60 adult patients could be collected and analyzed.
Patients’ characteristics and operation data including the duration and type surgery were not different statistically between the groups (Table 1).
Table 1.
Patients’ characteristics.

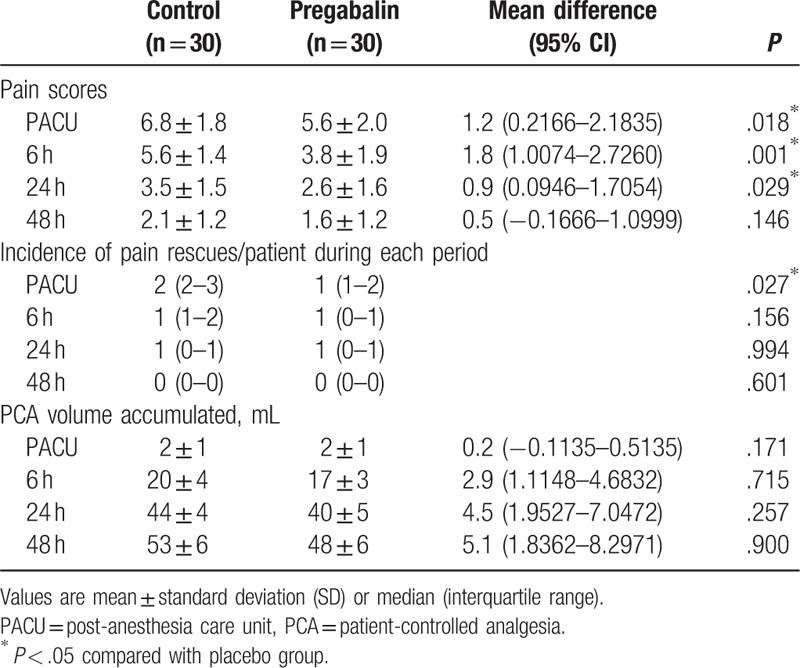
The preoperative pain scores of the all enrolled patients were <2 and were similar statistically between the groups (data not shown). Pain intensity scores expressed on the NRS at PACU and until 6 and 24 hours after surgery were significantly lower in the Pregabalin group (mean [SD]; 6.8 [1.8] vs 5.6 [2.0]; mean difference: 1.2, 95% CI of difference: 0.2166–2.1835, P = .018, mean [SD]; 5.6 [1.4] vs 3.8 [1.9]; mean difference: 1.8, 95% CI of difference: 1.0074–2.7260, P = .001 and mean [SD]; 3.5 [1.5] vs 2.6 [1.6]; mean difference: 0.9, 95% CI of difference: 0.0946–1.7054, P = .029, respectively). At 48 hours after surgery, pain intensity score only showed a trend towards being lower in the pregabalin group without any statistical significance, while none of the patients in the pregabalin group complained of pain intensity scores >3 and required rescue analgesics. In contrast, 2 patients in the control group complained of pain intensity score ≥5 requiring rescue analgesics. The incidence of additional pain rescue drug (tramadol) administered at PACU (median [interquartile range]; 2 [2–3] vs 1 [1–2], P = .027) was significantly less in the Pregabalin group but not different between the groups at 6, 24, and 48 hours after surgery. The total amount of cumulative IV-PCA volume infused was similar between the groups during each study period (Table 2).
Table 2.
Pain scores and analgesic consumption.
The occurrence of complications of pregabalin including sedation, headache, dizziness, and blurred vision were not different and the incidence and severity of PONV were also similar between the groups (Table 3).
Table 3.
Side effects of pregabalin and the incidence of postoperative nausea.

4. Discussion
This randomized controlled study was designed to evaluate the efficacy of a single preoperative administration of pregabalin 150 mg before VATS as an adjunct to IV-PCA. A significant beneficial effect was observed on the pain intensity and the requirement for pain rescue medication in the immediate postoperative period, beyond the action duration of the pregabalin. Although there were no differences in the total amount of fentanyl used in the IV-PCA and in the incidences of postoperative PONV, the aforementioned beneficial effects of the pregabalin were not accompanied by any increase in side effects.
Elements of nociceptive and neuropathic pain may greatly contribute to patient discomfort. In thoracic surgery especially, the multilayer intercostal incisions, thoracotomy tube insertion, and pleural irritation are intensely painful.[16] During inspiration, the tube can mechanically irritate the parietal and more sensitive visceral pleura, causing intense discomfort. There is no single pharmaceutical agent or route of administration to address every individual pain. Therefore, treatment regimens should be multimodal and tailored to the patient and the procedure.[15] VATS operations decrease the extent of the incision, as identical tissue layers are traversed. In general, patients recovering from VATS operations have fewer respiratory complications and lower pain scores postoperatively.[17]
Thoracic epidural analgesia is still regard as a gold standard for postoperative pain management after thoracotomy.[18] However, the performance of epidural catheterization for VATS remains controversial due to the complication risks related to the catheterization procedure and the low NRS score after VATS. For this reason, IV-PCA has become the mainstay of pain management in VATS.
Although opioid-based IV-PCA has been widely used for its analgesic efficacy and its perceived ease of use for a selected duration, several studies have demonstrated its association with several adverse effects, including respiratory depression, sedation, and nausea and vomiting. As a way to reduce these adverse effects from opioids, a multimodal approach to the management of acute postoperative pain is recommended.[19]
Meanwhile, the pain after surgery is normally perceived as nociceptive pain, since surgical injuries trigger nociceptive signals that are transmitted by the polymodal A-fiber and the C-fiber nociceptors to the dorsal horn neurons of the spinal cord, which reduces the nociceptive threshold and increases the sensitivity to nociceptive stimuli. However, surgical trauma has been known to induce hyperalgesia and tactile allodynia, which can contribute to persistent postoperative pain after surgery.[20,21] In this respect, it is well-known that the preventive inhibition of the pain pathway before the establishment of the injury-induced hypersensitivity by means of preemptive analgesia can reduce the postoperative pain. For that purpose, the analgesic efficacies of various anodynes administered preoperatively as adjuvants, including NSAIDs, ketamine, and local anesthetics, have been evaluated.[3,4,22,23]
Among adjuvant medications, pregabalin has been drawing attention as an adjuvant to a multimodal postoperative pain control strategy. Like other gabapentinoids, pregabalin reduces the hyperexcitability of the dorsal horn neurons induced by tissue damage, rather than reducing the afferent input from the site of the tissue injury.[20] It also inhibits the release of excitatory neurotransmitters, including glutamate, noradrenalin, and substance P.[24] Moreover, it prevents an induction phase and can produce long-lasting antihyperalgesic and antiallodynic actions over the half-life of the drug. These theoretical backgrounds have prompted clinical studies to validate the analgesic efficacy of preoperative pregabalin for postoperative pain following many kinds of surgeries.[25–27] Yet, certain important aspects, such as the type of surgery, need to be considered when evaluating the efficacy of a pain control regimen. There are no comprehensive data about the analgesic efficacy of a single preemptive pregabalin administration in patients undergoing VATS.
In this study, the preoperative administration of pregabalin 150 mg was beneficial in reducing the pain intensity without the introduction of untoward side effects in the immediate postoperative period after VATS. This is supported by the results of a recent meta-analysis which showed that the administration frequency of pregabalin is not a significant predictor of the 24 hours pain scores at rest.[28] Previous studies which evaluated the effect of pregabalin on acute postoperative pain yielded many contradictory results. These different results may have come from the different doses, the timing of the doses, or the type of surgery.[26,27]
Along with the analgesic effects, it is difficult to assess the degree of central sensitization and hyperalgesia clinically. We did not assess these aspects in our study. Nevertheless, considering that the time to maximal plasma concentration and the elimination half-life of pregabalin are approximately 1 and 6.3 hours, respectively,[14] and that the beneficial influence of pregabalin was evident beyond its action duration, we can only speculate that the above mentioned action mechanisms may have been involved. Nonetheless, the current study provides primary evidence of the analgesic efficacy of a single preemptive pregabalin 150 mg administration in patients undergoing VATS in lowering the pain intensity scores and reducing the additional rescue analgesic requirement in immediate postoperative period.
The limitations of this study are as follows. Several meta-analyses of the impact of pregabalin on acute and persistent postoperative pain concluded that perioperative pregabalin administration did not reduce the pain intensity for the first 24 hours after surgery, although significant differences were found in individual studies in comparison with placebos.[28–31] The opioid consumption during the first 24 hours after surgery was significantly reduced by pregabalin.[30] In contradiction with other results, in our trial, a single administration of pregabalin 150 mg before VATS was effective in reducing the postoperative pain score and the administration of additional pain rescue drugs with no opioid-sparing effect. We suppose that the reason for the diminishing opioid-sparing effect though the pain score reduction, was due to the administration of tramadol as a rescue medication instead of NASIDs or paracetamol. Tramadol works by binding to the μ-opioid receptor and inhibits the reuptake of serotonin and norepinephrine. It has been used to treat moderate to moderately severe pain with less resulting respiratory depression than opioids. Considering the pain intensity (NRS ≥5), we chose the first line rescue drug as tramadol rather than NSAIDs. This was found to be sufficient for control of the postoperative pain, regarding to incidence of pain rescue postoperatively and leads reduction of opioid consumption on PCA. As mentioned above, in our study, we administrated single dose of pregabalin only 150 mg preoperatively, that was effective in reducing the pain intensity and need for additional analgesic drugs.
Moreover, there was no anxiety evaluation in this study. Regarding the influence of anxiety on pain perception, and the anxiolytic and sedative properties of pregabalin,[32] further evaluation of the correlation between perioperative anxiety and postoperative pain is needed.
In conclusion, a single administration of pregabalin 150 mg before VATS was effective in reducing the postoperative pain score and the incidence of additional pain rescue drugs in the immediate postoperative period, without an increased risk of untoward side effects. Pregabalin may be a useful adjuvant to a multimodal postoperative pain management strategy in selected patients.
Acknowledgments
The authors thank Jae-Kwang Shim, MD, PhD (Yonsei University College of Medicine, Seoul, South Korea) for his valuable comments on the manuscript.
Footnotes
Abbreviations: IV-PCA = intravenous patient-controlled analgesia, VATS = video-assisted thoracoscopic surgery.
Author Contributions: JCK designed this study, analyzed data, and prepared the manuscript. SB acquired data and analyzed data. SK helped conducted the study and acquired data. S-YL helped conducted the study and acquired data.
JHL helped conducted the study and acquired data. SA designed this study and prepared the manuscript.
All authors reviewed the manuscript.
Funding: This study was not funded by any companies or organizations.
Clinical Trial Registration Site: http://cris.nih.go.kr
Registration Number: KCT0000577.
Conflict of Interest: No conflicts of interest declared.
References
- [1].Apfelbaum JL, Chen C, Mehta SS, et al. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 2003;97:534–40. table of contents. [DOI] [PubMed] [Google Scholar]
- [2].Grass JA, Sakima NT, Valley M, et al. Assessment of ketorolac as an adjuvant to fentanyl patient-controlled epidural analgesia after radical retropubic prostatectomy. Anesthesiology 1993;78:642–8. discussion 21A. [PubMed] [Google Scholar]
- [3].Aida S, Yamakura T, Baba H, et al. Preemptive analgesia by intravenous low-dose ketamine and epidural morphine in gastrectomy: a randomized double-blind study. Anesthesiology 2000;92:1624–30. [DOI] [PubMed] [Google Scholar]
- [4].Reuben SS, Bhopatkar S, Maciolek H, et al. The preemptive analgesic effect of rofecoxib after ambulatory arthroscopic knee surgery. Anesth Analg 2002;94:55–9. table of contents. [DOI] [PubMed] [Google Scholar]
- [5].Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 1993;56:1285–9. [DOI] [PubMed] [Google Scholar]
- [6].Stammberger U, Steinacher C, Hillinger S, et al. Early and long-term complaints following video-assisted thoracoscopic surgery: evaluation in 173 patients. Eur J Cardiothorac Surg 2000;18:7–11. [DOI] [PubMed] [Google Scholar]
- [7].Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362–5. [DOI] [PubMed] [Google Scholar]
- [8].Li WW, Lee RL, Lee TW, et al. The impact of thoracic surgical access on early shoulder function: video-assisted thoracic surgery versus posterolateral thoracotomy. Eur J Cardiothorac Surg 2003;23:390–6. [DOI] [PubMed] [Google Scholar]
- [9].Yie JC, Yang JT, Wu CY, et al. Patient-controlled analgesia (PCA) following video-assisted thoracoscopic lobectomy: comparison of epidural PCA and intravenous PCA. Acta Anaesthesiol Taiwan 2012;50:92–5. [DOI] [PubMed] [Google Scholar]
- [10].Lee JH, Yang WD, Han SY, et al. Effect of epidural magnesium on the incidence of chronic postoperative pain after video-assisted thoracic surgery. J Cardiothorac Vasc Anesth 2012;26:1055–9. [DOI] [PubMed] [Google Scholar]
- [11].Dirks J, Fredensborg BB, Christensen D, et al. A randomized study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy. Anesthesiology 2002;97:560–4. [DOI] [PubMed] [Google Scholar]
- [12].Agarwal A, Gautam S, Gupta D, et al. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth 2008;101:700–4. [DOI] [PubMed] [Google Scholar]
- [13].Frampton JE, Foster RH. Pregabalin: in the treatment of postherpetic neuralgia. Drugs 2005;65:111–8. discussion 119–120. [DOI] [PubMed] [Google Scholar]
- [14].Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia 2004;45(Suppl):13–8. [DOI] [PubMed] [Google Scholar]
- [15].Elmore B, Nguyen V, Blank R, et al. Pain management following thoracic surgery. Thorac Surg Clin 2015;25:393–409. [DOI] [PubMed] [Google Scholar]
- [16].Ochroch EA, Gottschalk A. Impact of acute pain and its management for thoracic surgical patients. Thorac Surg Clin 2005;15:105–21. [DOI] [PubMed] [Google Scholar]
- [17].Yamashita Y, Mukaida H, Harada H, et al. Post-thoracotomy pain and long-term survival associated with video-assisted thoracic surgery lobectomy methods for clinical T1N0 lung cancer: a patient-oriented, prospective cohort study. Eur J Cardiothorac Surg 2013;44:e71–6. [DOI] [PubMed] [Google Scholar]
- [18].Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107:1026–40. [DOI] [PubMed] [Google Scholar]
- [19].Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg 1993;77:1048–56. [DOI] [PubMed] [Google Scholar]
- [20].Field MJ, Holloman EF, McCleary S, et al. Evaluation of gabapentin and S-(+)-3-isobutylgaba in a rat model of postoperative pain. J Pharmacol Exp Ther 1997;282:1242–6. [PubMed] [Google Scholar]
- [21].Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 1993;77:362–79. [DOI] [PubMed] [Google Scholar]
- [22].Wilson RJ, Leith S, Jackson IJ, et al. Pre-emptive analgesia from intravenous administration of opioids. No effect with alfentanil. Anaesthesia 1994;49:591–3. [PubMed] [Google Scholar]
- [23].Obata H, Saito S, Fujita N, et al. Epidural block with mepivacaine before surgery reduces long-term post-thoracotomy pain. Can J Anaesth 1999;46:1127–32. [DOI] [PubMed] [Google Scholar]
- [24].Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca (2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology 2002;42:229–36. [DOI] [PubMed] [Google Scholar]
- [25].Jokela R, Ahonen J, Tallgren M, et al. Premedication with pregabalin 75 or 150 mg with ibuprofen to control pain after day-case gynaecological laparoscopic surgery. Br J Anaesth 2008;100:834–40. [DOI] [PubMed] [Google Scholar]
- [26].Yadeau JT, Paroli L, Kahn RL, et al. Addition of pregabalin to multimodal analgesic therapy following ankle surgery: a randomized double-blind, placebo-controlled trial. Reg Anesth Pain Med 2012;37:302–7. [DOI] [PubMed] [Google Scholar]
- [27].Chaparro LE, Clarke H, Valdes PA, et al. Adding pregabalin to a multimodal analgesic regimen does not reduce pain scores following cosmetic surgery: a randomized trial. J Anesth 2012;26:829–35. [DOI] [PubMed] [Google Scholar]
- [28].Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 2015;114:10–31. [DOI] [PubMed] [Google Scholar]
- [29].Engelman E, Cateloy F. Efficacy and safety of perioperative pregabalin for post-operative pain: a meta-analysis of randomized-controlled trials. Acta Anaesthesiol Scand 2011;55:927–43. [DOI] [PubMed] [Google Scholar]
- [30].Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth 2011;106:454–62. [DOI] [PubMed] [Google Scholar]
- [31].Lam DM, Choi SW, Wong SS, et al. Efficacy of pregabalin in acute postoperative pain under different surgical categories: a meta-analysis. Medicine (Baltimore) 2015;94:e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kumar KP, Kulkarni DK, Gurajala I, et al. Pregabalin versus tramadol for postoperative pain management in patients undergoing lumbar laminectomy: a randomized, double-blinded, placebo-controlled study. J Pain Res 2013;6:471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


