Abstract
Introduction:
Bazedoxifene may be promising to treat osteoporosis of postmenopausal women. We conducted a systematic review and meta-analysis to explore the efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis.
Methods:
PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases were systematically searched. Randomized controlled trials (RCTs) assessing the effect of bazedoxifene on osteoporosis of postmenopausal women were included. Two investigators independently searched articles, extracted data, and assessed the quality of included studies. The primary outcomes were vertebral fracture and spine BMD at 3 and 7 years.
Results:
Four RCTs are included in the meta-analysis. Overall, compared with placebo intervention in postmenopausal women with osteoporosis, bazedoxifene intervention can significantly reduce the risk of vertebral fracture [risk risks (RRs) = 0.69; 95% confidence interval (95% CI) = 0.52–0.93; P = .01], and increase spine BMD at 3 years (Std. mean difference = 1.71; 95% CI = 1.55–1.87; P < .005) and 7 years (Std. mean difference = 8.31; 95% CI = 8.07–8.55; P < .005). Bazedoxifene intervention results in no increase in adverse events (RR = 1.00; 95% CI = 0.99–1.00; P = .34), serious adverse events (RR = 1.04; 95% CI = 0.97–1.12; P = .31), myocardial infarction (RR = 0.88; 95% CI = 0.51–1.52; P = .64), stroke (RR = 0.97; 95% CI = 0.64–1.46; P = .87), venous thromboembolic event (RR = 1.56; 95% CI = 0.92–2.64; P = .10), and breast carcinoma (RR = 1.03; 95% CI = 0.59–1.79; P = .92).
Conclusions:
Compared with placebo intervention for the osteoporosis of postmenopausal women, bazedoxifene intervention is found to significantly reduce the incidence of vertebral fracture and increase spine BMD at 3 and 7 years, and results in no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic event, and breast carcinoma.
Keywords: bazedoxifene, fracture, meta-analysis, osteoporosis, postmenopausal women
1. Introduction
Osteoporosis is widespread in postmenopausal women because the decrease in estrogen production can accelerate bone loss.[1–3] High morbidity and mortality of osteoporosis-related fractures impose a heavy economic burden for patients and society.[4–7] Current prevention and treatment of postmenopausal osteoporosis mainly include bisphosphonates, hormone therapy, denosumab, strontium ranelate, and selective estrogen receptor modulators (SERMs).[8–10]
However, all currently available therapies cannot allow for long-term use. For instance, bisphosphonates may result in atypically low-impact subtrochanteric stress fractures with the increased duration of therapy.[11–13] Effective approaches are needed to prevent bone loss and reduce fracture risk with a favorable long-term safety/tolerability profile. Bazedoxifene is known as one novel SERM to treat osteoporosis in postmenopausal women with an increased risk of fracture. Previous studies have reported that bazedoxifene is able to effectively prevent bone loss and reduce the risk of new vertebral fractures in postmenopausal women, and has a favorable safety/tolerability profile with no adverse effects on the reproductive system.[14–16]
In contrast, 1 randomized controlled trial (RCT) shows that bazedoxifene fails to reduce the incidence of vertebral fractures and nonvertebral fractures in postmenopausal women with osteoporosis.[17] Considering these inconsistent effects, we therefore conduct a systematic review and meta-analysis of RCTs to investigate the influence of bazedoxifene on the osteoporosis of postmenopausal women.
2. Materials and methods
This systematic review and meta-analysis was conducted according to the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement[18] and the Cochrane Handbook for Systematic Reviews of Interventions.[19] Ethical approval was not necessary because all analyses were based on previous published studies.
2.1. Literature search and selection criteria
PubMed, EMbase, Web of science, EBSCO, and the Cochrane library were systematically searched from inception to December 2016, with the following keywords
Bazedoxifene, and osteoporosis, and postmenopausal. To include additional eligible studies, the reference lists of retrieved studies and relevant reviews were also hand-searched and the process above was performed repeatedly until no further article was identified. The inclusion criteria were as follows: study population were postmenopausal women with osteoporosis; intervention treatments were bazedoxifene (20 mg daily) versus placebo; and study design was RCT.
2.2. Data extraction and outcome measures
The following information was extracted for the included RCTs: first author, publication year, sample size, baseline characteristics of patients, bazedoxifene intervention, control, study design, vertebral fracture, spine bone mineral density (BMD) at 3 and 7 years, adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic event, and breast carcinoma. The author would be contacted to acquire the data when necessary.
The primary outcomes were vertebral fracture and spine BMD at 3 and 7 years. Secondary outcomes included adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic event, and breast carcinoma.
2.3. Quality assessment in individual studies
The Jadad Scale was used to evaluate the methodological quality of each RCT included in this meta-analysis.[20] This scale consisted of 3 evaluation elements: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points). One point would be allocated to each element if they have been mentioned in article, and another one point would be given if the methods of randomization and/or blinding have been detailed and appropriately described. If the methods of randomization and/or blinding were inappropriate, or dropouts and withdrawals had not been recorded, then 1 point was deducted. The score of Jadad Scale varied from 0 to 5 points. An article with Jadad score ≤2 was considered to be of low quality. If the Jadad score ≥3, the study was thought to be of high quality.[21]
2.4. Statistical analysis
Standard mean differences (Std. MDs) with 95% confidence intervals (95% CIs) for continuous outcomes (spine BMD at 3 and 7 years) and risk risks (RRs) with 95% CIs for dichotomous outcomes (vertebral fracture, adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic event, and breast carcinoma) were used to estimate the pooled effects. All meta-analyses were performed using random-effects models with DerSimonian and Laird weights. Heterogeneity was tested using the Cochran Q statistic (P < .1) and quantified with the I2 statistic, which described the variation of effect size that was attributable to heterogeneity across studies. An I2 value greater than 50% indicated significant heterogeneity. Sensitivity analysis was performed to detect the influence of a single study on the overall estimate via omitting 1 study in turn when necessary. Owing to the limited number (<10) of included studies, publication bias was not assessed. P < .05 in 2-tailed tests was considered statistically significant. All statistical analyses were performed with Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
3. Results
3.1. Literature search, study characteristics, and quality assessment
The flow chart for the selection process and detailed identification is presented in Fig. 1. Four hundred ninety-three publications were identified through the initial search of databases. Ultimately, 4 RCTs were included in the meta-analysis.[14–17]
Figure 1.
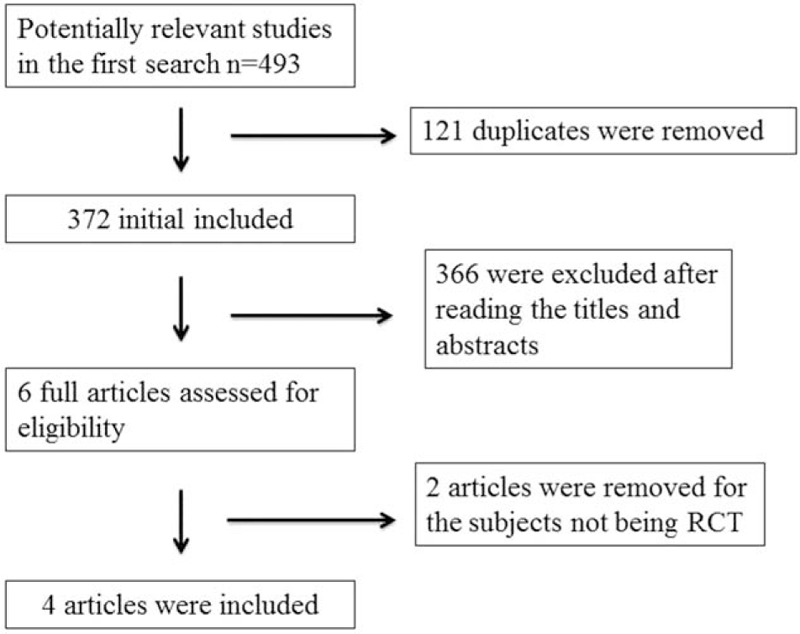
Flow diagram of study searching and selection process.
The baseline characteristics of 4 eligible RCTs in the meta-analysis are summarized in Table 1. The 4 studies were published between 2008 and 2015, and sample sizes ranged from 2105 to 3771. They showed similar baseline characteristics. Three included RCTs published the same sample of postmenopausal women, but at different follow-up time of 3, 5 , and 7 years.[15–17] We just took 1 index data in the most recent paper for analysis in order to avoid duplicate bias.
Table 1.
Characteristics of included studies.
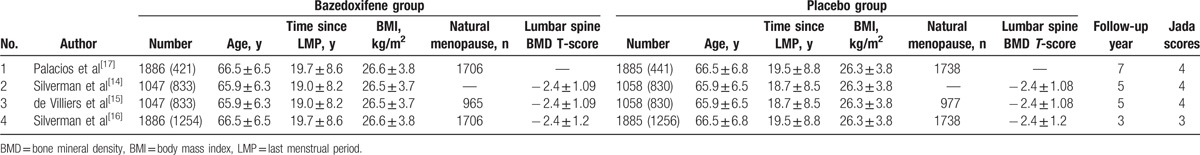
Among the 4 RCTs, 2 studies reported the vertebral fracture,[14,17] 1 study reported the spine BMD at 3 and 7 years,[17] and 2 studies reported the adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic event, and breast carcinoma.[14,17] Jadad scores of the 4 included studies varied from 3 to 4, and all 4 studies were considered to be high-quality ones according to quality assessment.
3.2. Primary outcome: vertebral fracture, spine BMD at 3 and 7 years
These 3 outcome data were analyzed with a random-effects model; the pooled estimate of the 2 included RCTs suggested that compared with placebo group for postmenopausal women, bazedoxifene intervention was associated with a significantly decreased incidence of vertebral fracture (RR = 0.69; 95% CI = 0.52–0.93; P = .01), with no heterogeneity among the studies (I2 = 0%, heterogeneity P = .71) (Fig. 2). Bazedoxifene intervention was found to significantly increase spine BMD at 3 years (Std. mean difference = 8.31; 95% CI = 8.07–8.55; P < .005; Fig. 3) and 7 years (Std. mean difference = 1.71; 95% CI = 1.55–1.87; P < .005; Fig. 4) than placebo intervention.
Figure 2.
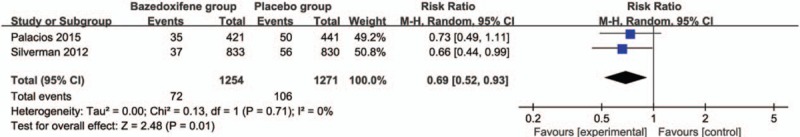
Forest plot for the meta-analysis of vertebral fracture.
Figure 3.
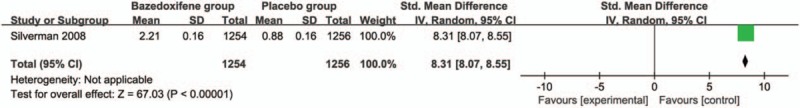
Forest plot for the meta-analysis of spine bone mineral density (BMD) at 3 years.
Figure 4.
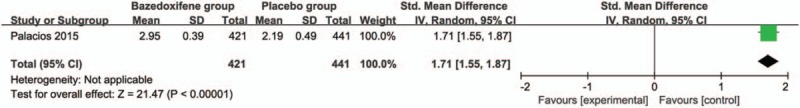
Forest plot for the meta-analysis of spine BMD at 7 years.
3.3. Sensitivity analysis
No heterogeneity was observed among the included studies for the incidence of vertebral fracture, and just 1 RCT was included for the analysis of spine BMD at 3 and 7 years. Thus, we did not perform sensitivity analysis by omitting 1 study in each turn to detect the source of heterogeneity.
3.4. Secondary outcomes
Compared with placebo group in postmenopausal women, bazedoxifene showed no increase in adverse events (RR = 1.00; 95% CI = 0.99–1.00; P = .34; Fig. 5), serious adverse events (RR = 1.04; 95% CI = 0.97–1.12; P = .31; Fig. 6), myocardial infarction (RR = 0.88; 95% CI = 0.51–1.52; P = .64; Fig. 7), stroke (RR = 0.97; 95% CI = 0.64–1.46; P = .87; Fig. 8), venous thromboembolic event (RR = 1.56; 95% CI = 0.92–2.64; P = .10; Fig. 9), and breast carcinoma (RR = 1.03; 95% CI = 0.59–1.79; P = .92; Fig. 10).
Figure 5.
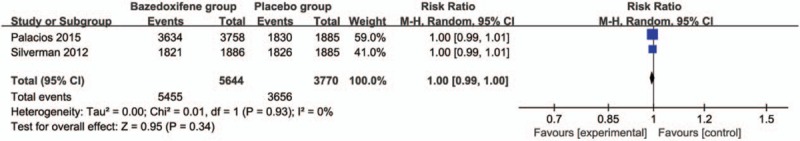
Forest plot for the meta-analysis of adverse events.
Figure 6.
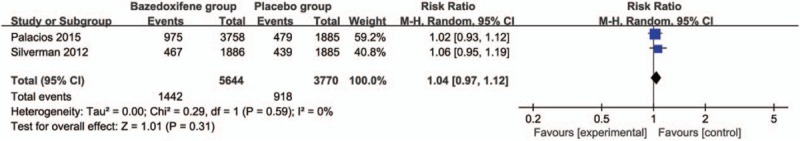
Forest plot for the meta-analysis of serious adverse events.
Figure 7.
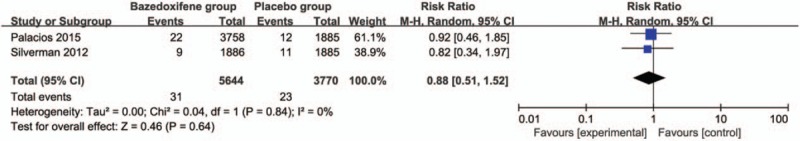
Forest plot for the meta-analysis of myocardial infarction.
Figure 8.
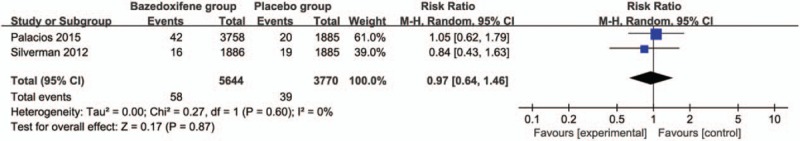
Forest plot for the meta-analysis of stroke.
Figure 9.
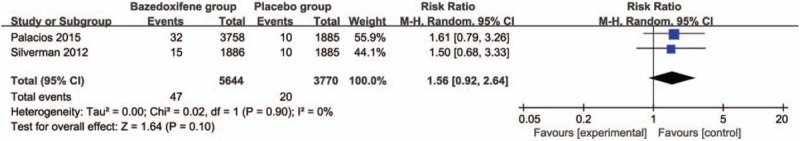
Forest plot for the meta-analysis of venous thromboembolic event.
Figure 10.
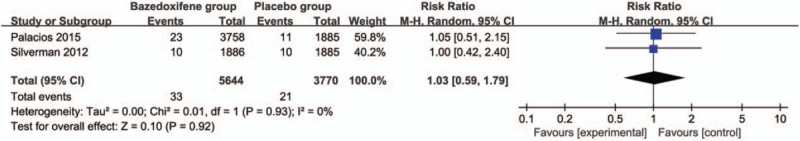
Forest plot for the meta-analysis of breast carcinoma.
4. Discussion
Our meta-analysis clearly suggests that compared with placebo intervention for postmenopausal women, bazedoxifene is associated with a significantly reduced incidence of vertebral fracture, and increased spine BMD at 3 and 7 years. To our knowledge, this is the first meta-analysis to analyze the effect of bazedoxifene on the osteoporosis of postmenopausal women.
This meta-analysis shows that bazedoxifene can significantly decrease the incidence of vertebral fracture after pooling the results of 2 RCTs at the follow-up of 5 and 7 years, which is consistent with the results of 1 clinical trial at the follow-up of 3 and 7 years.[16,17] However, 2 RCTs reported that bazedoxifene failed to reduce the occurrence of nonvertebral fracture.[14,17] Our results indicate that bazedoxifene has the ability to increase spine BMD. In contrast, bazedoxifene is not associated with the increase in hip BMD compared with baseline.[14] These reveal that bazedoxifene shows different influence on vertebral fracture and nonvertebral fracture, which may be caused by the reduction in bone turnover and potential improvement in bone material properties and/or microarchitecture, as shown by the significant reductions in bone turnover markers.[16,22–24]
In addition, this meta-analysis further confirms the long-term favorable safety/tolerability (ranging from 3 to 7 years) of bazedoxifene and there is no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic event, and breast carcinoma using bazedoxifene. However, bazedoxifene may result in high incidence of hot flushes and leg cramps across 7 years.[17]
Several limitations should be taken into account. First, our analysis is based on only 4 RCTs and more clinical trials with a large sample are needed to explore this issue. The follow-up time and basic characteristics of postmenopausal women in the included studies are different and it may have an influence on the pooling results. Next, bazedoxifene is found to have different effect on vertebral fracture and nonvertebral fracture. Finally, some unpublished and missing data may lead bias to the pooled effect.
5. Conclusion
Bazedoxifene shows an important ability to reduce the risk of vertebral fracture, and increase spine BMD at 3 and 7 years in postmenopausal women. Its long-term favorable safety/tolerability is confirmed. Bazedoxifene is recommended to be administrated for the osteoporosis of postmenopausal women with caution.
Footnotes
Abbreviations: BMD = bone mineral density, CIs = confidence intervals, RCTs = randomized controlled trials, RRs = risk risks, Std. MDs = standard mean differences.
This article does not contain any studies with human participants or animals performed by any of the authors.
The authors declare no conflict of interest.
References
- [1].Delaney MF. Strategies for the prevention and treatment of osteoporosis during early postmenopause. Am J Obstet Gynecol 2006;194:S12–23. [DOI] [PubMed] [Google Scholar]
- [2].Burger HG, Dudley EC, Robertson DM, et al. Hormonal changes in the menopause transition. Recent Progr Hormone Res 2002;57:257–75. [DOI] [PubMed] [Google Scholar]
- [3].Unni S, Yao Y, Milne N, et al. An evaluation of clinical risk factors for estimating fracture risk in postmenopausal osteoporosis using an electronic medical record database. Osteoporos Int 2015;26:581–7. [DOI] [PubMed] [Google Scholar]
- [4].Atik OS, Gunal I, Korkusuz F. Burden of osteoporosis. Clin Orthop Relat Res 2006;443:19–24. [DOI] [PubMed] [Google Scholar]
- [5].Daruwalla ZJ, Huq SS, Wong KL, et al. Hip fractures, preceding distal radius fractures and screening for osteoporosis: should we be screening earlier? A minimum 10-year retrospective cohort study at a single centre. Osteoporos Int 2016;27:361–6. [DOI] [PubMed] [Google Scholar]
- [6].Willson T, Nelson SD, Newbold J, et al. The clinical epidemiology of male osteoporosis: a review of the recent literature. Clin Epidemiol 2015;7:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Silverman SL, Kupperman ES, Bukata SV. Members of IOFFWG. Fracture healing: a consensus report from the International Osteoporosis Foundation Fracture Working Group. Osteoporos Int 2016;27:2197–206. [DOI] [PubMed] [Google Scholar]
- [8].Noto C, Pappagallo M. Current and emerging pharmacologic therapies for pain and challenges which still lay ahead. Methods Mol Biol 2010;617:539–54. [DOI] [PubMed] [Google Scholar]
- [9].Lewiecki EM. Current and emerging pharmacologic therapies for the management of postmenopausal osteoporosis. J Womens Health 2009;18:1615–26. [DOI] [PubMed] [Google Scholar]
- [10].Nakamura T, Nagahori M, Kanai T, et al. Current pharmacologic therapies and emerging alternatives in the treatment of ulcerative colitis. Digestion 2008;77(Suppl 1):36–41. [DOI] [PubMed] [Google Scholar]
- [11].Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014;29:1–23. [DOI] [PubMed] [Google Scholar]
- [12].Park KT, Lee KB. Sequential subtrochanteric femoral fracture after atypical diaphyseal fracture in a long-term bisphosphonate user: a case report. Acta Chir Orthop Traumatol Cech 2015;82:157–60. [PubMed] [Google Scholar]
- [13].Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2010;25:2267–94. [DOI] [PubMed] [Google Scholar]
- [14].Silverman SL, Chines AA, Kendler DL, et al. Sustained efficacy and safety of bazedoxifene in preventing fractures in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled study. Osteoporos Int 2012;23:351–63. [DOI] [PubMed] [Google Scholar]
- [15].de Villiers TJ, Chines AA, Palacios S, et al. Safety and tolerability of bazedoxifene in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled phase 3 trial. Osteoporos Int 2011;22:567–76. [DOI] [PubMed] [Google Scholar]
- [16].Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 2008;23:1923–34. [DOI] [PubMed] [Google Scholar]
- [17].Palacios S, Silverman SL, de Villiers TJ, et al. A 7-year randomized, placebo-controlled trial assessing the long-term efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: effects on bone density and fracture. Menopause 2015;22:806–13. [DOI] [PubMed] [Google Scholar]
- [18].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. Available at: www.cochrane-handbook.org. [Google Scholar]
- [20].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [21].Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982–9. [DOI] [PubMed] [Google Scholar]
- [22].Bauer DC, Black DM, Garnero P, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res 2004;19:1250–8. [DOI] [PubMed] [Google Scholar]
- [23].Seeman E, Delmas PD. Bone quality: the material and structural basis of bone strength and fragility. N Engl J Med 2006;354:2250–61. [DOI] [PubMed] [Google Scholar]
- [24].Eastell R, Hannon RA, Garnero P, et al. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate: review of statistical analysis. J Bone Miner Res 2007;22:1656–60. [DOI] [PubMed] [Google Scholar]


