Abstract
The aim of this study was to investigate the roles of serum interleukin-6 (IL-6), IL-8, IL-10, squamous cell cancer antigen (SCC-Ag), and cytokeratin 21-1 fragment (CYFRA 21-1) in the metastasis and prognosis of breast cancer.
A total of 534 breast cancer patients admitted to our department between January 2011 and December 2014 were enrolled in this study. Besides, 452 matched healthy individuals received physical examination at the same period served as the normal control. Serum IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α) were determined using an immunoradiometric assay. SCC-Ag level was evaluated using chemiluminescent microparticle immunoassay. CYFRA 21-1 was determined using the chemiluminescence assay.
Compared with the control group, a significant increase was noticed in the serum IL-6, IL-8, and IL-10 in breast cancer patients, especially those with severe conditions (P < .01). Serum IL-6, IL-8, and IL-10 showed a significant increase in the patients with severe breast cancer compared with those with mild conditions (P < .05). For the patients with response after radiotherapy, the serum IL-6, IL-8, and IL-10 were significantly decreased compared with the baseline levels (P < .05). The median survival duration for the patients of SCC-Ag negative patients was 25 months, while that for the SCC-Ag positive group was 16 months. Significant difference was noticed in the survival of SCC-Ag negative group compared with that of SCC-Ag positive group (P < .05).
Serum IL-6, IL-8, IL-10, SCC-Ag, and CYFRA 21-1 were considered as potential markers in the metastasis and prognosis of breast cancer.
Keywords: breast cancer, cytokine, metastasis, serum
1. Introduction
Breast cancer is the most common cancer for women in China, with about 21,000 new cases diagnosed and 8000 deaths recorded annually.[1,2] Nowadays, radical resection has been preferred for the treatment of breast cancer. Unfortunately, the outcome of patients is not satisfactory with a 5-year survival rate of less than 30.0% due to recurrence.
Currently, the diagnosis of breast cancer is basically through the imaging techniques such as computed tomography and magnetic resonance imaging.[3] Nowadays, extensive studies have been carried out to investigate the feasibility of serum tumor markers in the diagnosis, prognosis, or clinical management of malignant diseases. Several studies have been performed to investigate the correlation between serum cytokines the pathogenesis of various cancer types.[4,5] For example, interleukin-6 (IL-6), IL-8, and IL-10 were strongly associated with an increased risk of breast cancer.[6] On the contrary, 7 common polymorphisms in genes of inflammatory cytokines including IL-6, IL-8, and IL-10 and tumor necrosis factor-α (TNF-α) were reported to show no roles in the prostate cancer.[7] Besides, studies have been conducted to investigate the feasibility of squamous cell cancer antigen (SCC-Ag), and cytokeratin 21-1 fragment (CYFRA 21-1) in cancer patients, especially those with esophageal cancer.[8] However, rare studies have been carried out to investigate the feasibility of these factors in the pathogenesis and prognosis of breast cancer.
In this study, 534 patients with breast cancer admitted to our department from January 2011 to December 2014 were included. We aim to investigate the roles of serum I IL-6, IL-8 and IL-10, SCC-Ag, and CYFRA 21-1 in the pathogenesis and prognosis of breast cancer.
2. Methods
2.1. Patients
A total of 534 patients with breast cancer admitted to our department between January 2011 and December 2014 were enrolled in this study. The diagnosis of breast cancer was based on imaging technique, cytological pathological analysis as previously described. The criteria of exclusion were as follows: those who received radical resection; concurrent with other types of tumor; died from treatment-related complications; and those with a Karnofsky Performance Status (KPS) score of less than 60 showed no tolerance to radiotherapy or the combination of radiotherapy and chemotherapy. Patients were divided into 2 groups according to the TNM staging: group I, those with a TNM stage of I or II; group 2, those of TNM stage of III or IV. Besides, 452 matched healthy individuals received physical examination served as the normal control. Written informed consent was obtained from each patient or the guardians. The study is in line with the Declaration of Helsinki, and the protocols were approved by the Ethic Committee of Weihai Maternal and Child Hospital.
2.2. Laboratory analysis
Venous blood was collected from each patient under fasting conditions. Serum tumor markers were determined before and 1 month after treatment. Serum IL-6, IL-8, IL-10, and TNF-α were determined using an immunoradiometric assay as previously described.[9] SCC-Ag level was evaluated using chemiluminescent microparticle immunoassay with the commercial kit purchased from Abbott Laboratories (CA). Cyfra 21-1 was determined using the chemiluminescence assay with the kit purchased from Roche (MA). All the procedures were carried out at least in triplicate according to the manufacturer's instructions.
2.3. Treatment regimen
Three hundred fifty-six patients (66.7%) received radiotherapy, and the other 168 patients (33.3%) received radiotherapy combined with chemotherapy. For the patients who received radiotherapy, the lesions at the deep of the thoracic and abdominal cavity were exposed to 3-dimensional conformal radiation therapy or intensity-modulated radiation therapy using 6-MV X-ray beams. The cervical and supraclavicular lesions were treated by the mixed irradiation by 6-MV X-ray beams and 9-Mev electron rays with a dosage of 1.8 to 2.0 Gy once (total dosage: 50–74 Gy). For the chemotherapy, patients received 1 to 6 cycles of platinum-based chemotherapy.
2.4. Follow-up and survival analysis
The patients were followed up once every 3 to 6 months within 3 years after the treatment. Three years after treatment, the patients were followed up once every year until January 2016 or death. The survival was defined as the duration from presentation of local recurrence to death. Besides, the survival rates were compared in the patients with negative results in the tumor marker screening and those with positivity. The normal ranges for SCC-Ag and CYFRA 21-1 were defined as less than 1.5 and 4 ng/mL, respectively.
2.5. Statistical analysis
All data were presented as mean ± standard deviation. SPSS19.0 software (IBM, NY) was used for the statistical analysis. The survival rates were measured using Kaplan–Meier method, and were tested using Logrank analysis. Student t test was used for the comparison between groups. Chi-square test was used for the comparison among groups. Multiple logic regression analysis was utilized for the analysis of prognostic factors. P value of less than .05 was considered to be statistically significant.
3. Results
3.1. Patient characteristics
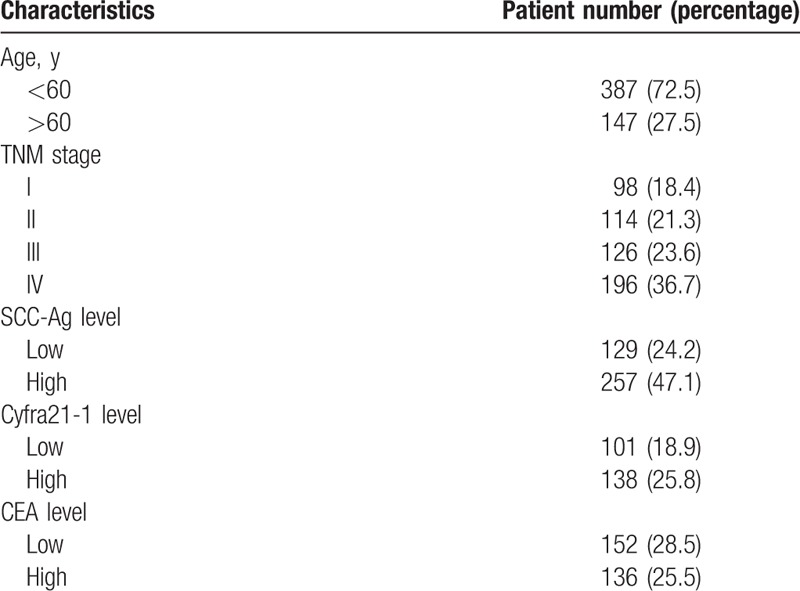
Five hundred thirty-four patients with breast cancer were included in this study. The patients were aged 39 to 75 years (median: 56 years). For the pathological staging, the number of patients of stage I, II, III, and IV was 98, 114, 126, and 196, respectively. In addition, 138 patients (25.8%) showed recurrence in single site, while 224 patients (41.9%) showed recurrence in multiple sites. Compared with the normal control, no statistical difference was noticed in the age and body weight in the patients with breast cancer (P > .05, Table 1).
Table 1.
Patient characteristics.
3.2. Comparison of serum IL-6, IL-8, and IL-10
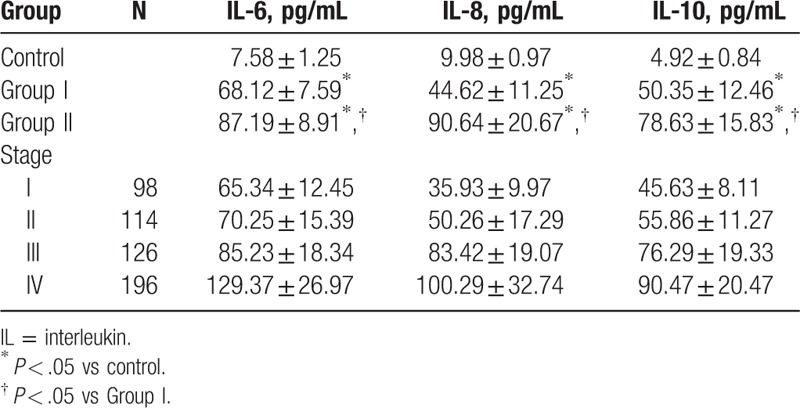
Compared with the normal individuals, a significant increase was noticed in the serum IL-6, IL-8, and IL-10 in patients with breast cancer of group I and II, respectively (P < .05). Compared with the serum markers in group I, a significant increase was noticed in the IL-6, IL-8, and IL-10 in patients with severe conditions (P < .05, Table 2).
Table 2.
Comparison of serum IL-6, IL-8, and IL-10 in patients and normal individuals.
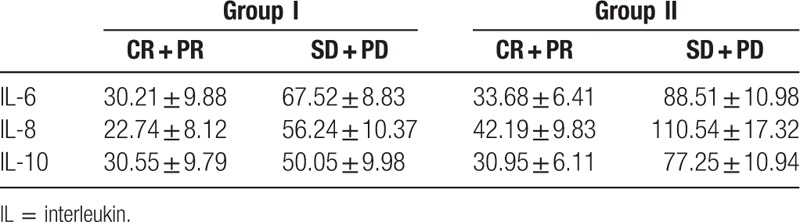
In this study, we also determined the serum IL-6, IL-8, and IL-10 after radiotherapy and/or chemotherapy. Among the patients with partial response and complete response, a significant decrease was noticed in these patients compared with the baseline levels (P < .05). For the patients with stable disease or progressive disease, a remarkable increase was noticed in the serum IL-6 and IL-8 compared with the baseline levels, while no statistical difference was identified in the IL-10 in the breast cancer patients compared with the baseline levels (Table 3).
Table 3.
Comparison of serum IL-6, IL-8, and IL-10 in patients before and after treatment.
3.3. Comparison of SCC-Ag and CYFR21-1
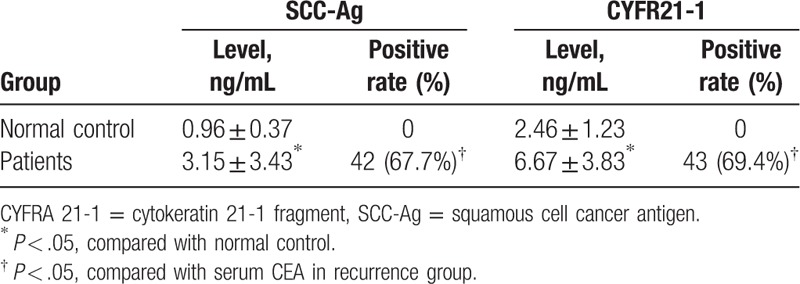
Significant elevation was noticed in the SCC-Ag and CYFR21-1 in patients compared with those of the normal individuals (P < .05, Table 4). Besides, the positive rates of SCC-Ag and CYFR21-1 in the patients were 67.7% and 69.4%, which were higher than that of the CEA (16.1%), respectively (P < .05, Table 2). Compared with the SCC-Ag, no statistical difference was observed in the positive rate of CYFR21-1 in the patients.
Table 4.
Expression of serum SCC-Ag, CYFR21-1, and CEA in esophageal carcinoma patients with postoperative recurrence.
3.4. Survival analysis
The median survival duration was 12 months after radiotherapy [95% confidence interval (95% CI): 6.98–10.93] or a combination of chemotherapy and radiotherapy (95% CI: 13.07–17.59). The survival rates at 1 year, 2, 3, and 5 years were 44.7%, 18.6%, 13.3%, and 7.8%, respectively.
The median survival duration for the patients of SCC-Ag negative patients was 25 months, while that for the SCC-Ag positive group was 16 months. Significant difference was noticed in the survival of SCC-Ag negative group compared with that of SCC-Ag positive group (P < .05). The median survival duration for the patients of CYFR21-1 negative group was 14 months, which was superior to the serum CYFR21-1 positive group with a median survival duration of 9 months (P < .05).
3.5. Risk factors for overall survival in breast cancer
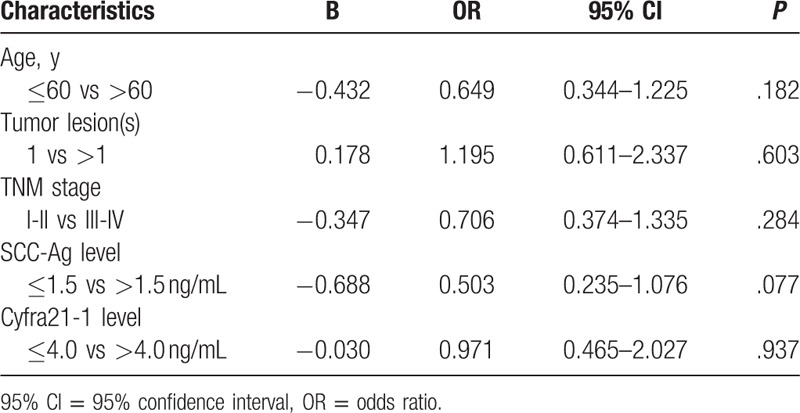
In this study, multivariate Cox regression analysis was performed to identify the risk factors for overall survival in patients, including age, gender, pathological stage, tumor lesions, IL-6, IL-8, IL-10, SCC-Ag, CYFR21-1, and serum cytokines. Our data indicated that age, gender, tumor lesions, TNM stage, SCC-Ag, and CYFRA 21-1 were not the risk factors for the survival of breast cancer patients (Table 5).
Table 5.
Multivariate Cox regression analysis for overall survival in patients with breast cancer.
4. Discussion
Cytokines have been reported to play crucial roles in the immune response to cancer cells. In this study, we aim to investigate the roles of serum IL-6, IL-8, IL-10 and TNF-α, SCC-Ag, and CYFRA 21-1 in the pathogenesis and prognosis of breast cancer. Besides, we also determined their alternations before and after treatment.
IL-6, a pleiotropic cytokine that was initially identified as a T cell derived lymphokine, has been reported to play crucial roles in the immune system and acute-phase responses.[10] To our best knowledge, it is closely involved in several biological processes, including regulating cell growth, apoptosis, and proliferation.[11] For example, it acts as an autocrine growth factor in the pathogenesis of malignancy.[12] Besides, it could regulate the proliferation and secretion of matrix metalloproteinases in ovarian carcinoma SKOV-3 cell line.[13] Moreover, it has been reported to activate several intracellular signaling pathways. For instance, the binding of IL-6 to its receptor could activate the Janus family of kinases bound to the cytoplasmic domain of gp130, which could promote its nuclear transfer and transcriptional function.[14] In this study, elevation of IL-6 was noticed in breast cancer patients, especially in patients of advanced stages. Our results indicated that patients with metastasis showed higher serum IL-6 than those without. Patients with response to treatment showed remarkable decrease of serum IL-6 compared with the baseline levels.
IL-8, a cytokine initially discovered by Yoshimura, involves in inflammation and immunity, and shows tumorigenic and pro-angiogenic properties.[15] Increased expression of IL-8 and its receptors has been reported in various cancer cells. In a previous study based on primary breast cancer tissues, a high expression of IL-8 was correlated with the angiogenesis process that was essential for tumor growth and progression.[16] Besides, IL-8 was correlated with the increased proliferation of cancer cells.[17] The role of IL-8 in cancer progression was reported to be associated with lymph node positive status, higher TNM staging, as well as the hormone receptors.[18] In this study, IL-8 expression was significantly elevated compared with that of the normal control. Patients with response to treatment showed decreased IL-8 compared with the baseline levels. On this basis, we propose that IL-8 may serve as an important prognostic marker for breast cancer, and may contribute to the identification of genes and proteins differentially expressed in the breast cancer patients.
IL-10, with potent immunosuppressive and anti-inflammatory properties,[19] facilitates to the progression of different human tumors. It has been considered as a cytokine of Th2 response, and is able to suppress the antigen-presenting cells (APCs). In cancer cells, the expression of IL-10 was elevated, which can downregulate the cytokines of cancer and finally attenuate the phagocytosis of the macrophages. Furthermore, IL-10 could attenuate the apoptosis of small cell lung cancer.[20] Our results showed that the concentration of serum IL-10 was significantly higher in the breast cancer patients with severe conditions than the normal control and patients with moderate conditions. Besides, the expression of IL-10 showed reduction in patients with responses after treatment.
Serum cancer biomarkers levels were reported to be related to the postoperative pathological stage. Simultaneously, the levels of serum SCC-Ag and CYFR21-1 in late-stage patients were higher than those of the early-stage patients.[21] To our best knowledge, a higher recurrence has been frequently reported in patients of late stage, especially those received no chemotherapy and/or radiotherapy. To date, there are still disputes on the correlation between serum cancer markers and the pathological stages, as well as the patients’ survival.[22] For example, there was a tendency for higher serum CYFRA 21-1 concentrations in advanced T-stage rather than N or M stages in cancer patients.[23] In addition, the positive rate of CYFR21-1 increased with the progression of esophageal cancer with a ratio of 22.2% of pTNM stage 0-IIA and 77.8% pTNM stage IIB/III; however, SCC-Ag and CEA rates were not correlated to the pTNM stages. On this basis, the authors concluded that CYFR21-1 elevation contributed to the diagnosis of recurrence in the absence of clinical data and imaging monitoring. Furthermore, Mao et al[24] revealed the serum SCC-Ag and CYFR21-1 were closely related to the TNM staging in the esophageal carcinoma patients (1). According to our data, serum SCC-Ag and CYFR21-1 were correlated to the TNM staging in breast cancer patients. To be exact, the serum SCC-Ag and CYFR21-1 in the stage III patients were obviously higher than those in patients of stage I. Similarly, the serum CYFR21-1 in stage III patients was also higher than that in stage II patients. Our results indicate the monitoring of serum SCC-Ag and CYFR21-1 is of prime importance in the advanced esophageal cancer patients after radical resection, as the elevation of these markers contributes to the diagnosis of recurrence.
In this study, we explored the roles of serum I IL-6, IL-8 and IL-10, SCC-Ag, and CYFRA 21-1 in the pathogenesis and prognosis of breast cancer. Our data demonstrated that serum IL-6, IL-8, IL-10, SCC-Ag, and CYFRA 21-1 may serve as potential markers for predicting metastasis and prognosis of breast cancer. Our study may contribute to development of cancer markers for the evaluation of breast cancer metastasis and prognosis.
Footnotes
Abbreviations: APCs = antigen-presenting cells, CYFRA 21-1 = cytokeratin 21-1 fragment, IL = interleukin, KPS = Karnofsky Performance Status, SCC-Ag = squamous cell cancer antigen, TNF-α = tumor necrosis factor-α.
All the authors declare that we have no conflict of interests.
References
- [1].Stewart B, Wild C. World cancer report 2014. Lyon, France: International Agency for Research on Cancer; 2014. [Google Scholar]
- [2].Exadaktylos AK, Buggy DJ, Moriarty DC, et al. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? J Am Soc Anesthesiol 2006;105:660–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fuster D, Duch J, Paredes P, et al. Preoperative staging of large primary breast cancer with [18F] fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin Oncol 2008;26:4746–51. [DOI] [PubMed] [Google Scholar]
- [4].Landi S, Moreno V, Gioia-Patricola L, et al. Association of common polymorphisms in inflammatory genes interleukin (IL) 6, IL8, tumor necrosis factor (, NFKB1, and peroxisome proliferator-activated receptor ( with colorectal cancer. Cancer Res 2003;63:3560–6. [PubMed] [Google Scholar]
- [5].Hartman ZC, Poage GM, Den Hollander P, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res 2013;73:3470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kozłowski L, Zakrzewska I, Tokajuk P, et al. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst 2003;48:82–4. [PubMed] [Google Scholar]
- [7].Michaud DS, Daugherty SE, Berndt SI, et al. Genetic polymorphisms of interleukin-1B (IL-1B), IL-6, IL-8, and IL-10 and risk of prostate cancer. Cancer Res 2006;66:4525–30. [DOI] [PubMed] [Google Scholar]
- [8].Kulpa J, Wójcik E, Reinfuss M, et al. Carcinoembryonic antigen, squamous cell carcinoma antigen, CYFRA 21-1, and neuron-specific enolase in squamous cell lung cancer patients. Clin Chem 2002;48:1931–7. [PubMed] [Google Scholar]
- [9].Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer 1999;85:2526–31. [DOI] [PubMed] [Google Scholar]
- [10].Cui G, Florholmen J. Polarization of cytokine profile from Th1 into Th2 along colorectal adenoma-carcinoma sequence: implications for the biotherapeutic target? Inflamm Allergy Drug Targets 2008;7:94–7. [DOI] [PubMed] [Google Scholar]
- [11].Takeda K, Kaisho T, Yoshida N, et al. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol 1998;161:4652–60. [PubMed] [Google Scholar]
- [12].Ruef C, Budde K, Lacy J, et al. Interleukin 6 is an autocrine growth factor for mesangial cells. Kidney Int 1990;38:249–57. [DOI] [PubMed] [Google Scholar]
- [13].Rabinovich A, Medina L, Piura B, et al. Regulation of ovarian carcinoma SKOV-3 cell proliferation and secretion of MMPs by autocrine IL-6. Anticancer Res 2007;27:267–72. [PubMed] [Google Scholar]
- [14].Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000;19:2548–56. [DOI] [PubMed] [Google Scholar]
- [15].Kunkel SL, Standiford T, Kasahara K, et al. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Exp Lung Res 1991;17:17–23. [DOI] [PubMed] [Google Scholar]
- [16].Penson RT, Kronish K, Duan Z, et al. Cytokines IL-1(, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNF( in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer 2000;10:33–41. [DOI] [PubMed] [Google Scholar]
- [17].Waugh DJJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res 2008;14:6735–41. [DOI] [PubMed] [Google Scholar]
- [18].Ren Y, Law S, Huang X, et al. Macrophage migration inhibitory factor stimulates angiogenic factor expression and correlates with differentiation and lymph node status in patients with esophageal squamous cell carcinoma. Ann Surg 2005;242:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].de Vries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med 1995;27:537–41. [DOI] [PubMed] [Google Scholar]
- [20].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hua S, Xia Z, Chang LIU. Relationships between tumor markers level and the clinical pathological characteristics and survival time in 514 patients with lung cancer. Jie Fang Jun Yi Xue Za Zhi 2013;5:16–7. [Google Scholar]
- [22].Karayiannakis AJ, Syrigos KN, Polychronidis A, et al. Circulating VEGF levels in the serum of gastric cancer patients: correlation with pathological variables, patient survival, and tumor surgery. Ann Surg 2002;236:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Suyama T, Nakajima K, Kanbe S, et al. Prognostic significance of preoperative serum CYFRA 21-1 in patients with upper urinary tract urothelial carcinoma. Int J Urol 2011;18:43–7. [DOI] [PubMed] [Google Scholar]
- [24].Mao YS, Zhang DC, Zhao XH, et al. Significance of CEA, SCC and Cyfra21-1 serum test in esophageal cancer. Chin J Oncol 2003;25:457. [PubMed] [Google Scholar]


