Abstract
Objective:
Pretreatment of myoinositol is a very new method that was evaluated in multiple small studies to manage poor ovarian response in assisted reproduction. This study was to determine the efficacy of myoinositol supplement in infertile women undergoing ovulation induction for intracytoplasmic sperm injection (ICSI) or in vitro fertilization embryo transfer (IVF-ET).
Methods:
A meta-analysis and systematic review of published articles evaluating the efficacy of myo-inositol in patients undergoing ovulation induction for ICSI or IVF-ET was performed.
Results:
Seven trials with 935 women were included. Myoinositol supplement was associated with significantly improved clinical pregnancy rate [95% confidence interval (CI), 1.04–1.96; P = .03] and abortion rate (95% CI, 0.08–0.50; P = .0006). Meanwhile, Grade 1 embryos proportion (95% CI, 1.10–2.74; P = .02), germinal vescicle and degenerated oocytes retrieved (95% CI, 0.11–0.86; P = .02), and total amount of ovulation drugs (95% CI, –591.69 to –210.39; P = .001) were also improved in favor of myo-inositol. There were no significant difference in total oocytes retrieved, MII stage oocytes retrieved, stimulation days, and E2 peak level.
Conclusions:
Myoinositol supplement increase clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. It may improve the quality of embryos, and reduce the unsuitable oocytes and required amount of stimulation drugs.
Keywords: intracytoplasmic sperm injection, inositol, in vitro fertilization embryo transfer, ovulation induction, pregnancy rate
1. Introduction
Inositol, as vitamin factor, was considered part of B group, but not a true vitamin. It's found that inositol could stimulate the endogenous production of lecithin and perform a check on the metabolism of fats and sugar and in cellular activities of the nervous system.[1] Inositol have 2 relevant isomeric forms including myoinositol and D-chiro-inositol (DCI).[2] Myoinositol can be synthesized by the body in the follicular microenvironment, however, DCI is converted from epimerization of myoinositol by an epimerase precursors, other than synthesized by precursors.[3] Recently, an international consensus conference, further confirmed that, during IVF program, when inositol supplement, the oocyte and embryo quality in assisted reproduction might be better.[4,5] Inositol, as one of the second messenger in signal transduction pathways, regulates the secretion of some exocrine glands such as pancreas and other organs,[6] including the ovaries, thus it may also play positive roles in assisted reproduction.
It's clear that polycystic ovary syndrome (PCOS) is associated with hyperinsulinemia,[7] and this instigated extensive investigation on the relationship across metabolic complications, reproductive morbidities, and insulin sensitivity and resistance. It's found that some of the actions of insulin are mediated by putative inositol-containing phosphoglycan mediators, also known as putative insulin mediators or second messengers. So, inositol was classified as an insulin sensitizing agent other than metformin and troglitazone,[8,9] and was also recommended in PCOS treatment.[10] Besides, our previous study found that myo-inositol supplements had promising benefits to prevent gestational diabetes mellitus (GDM) of significantly reduced incidence.[11] There are also evidences that DCI administration played direct roles in increasing insulin sensitivity,[12] and in addition combined with myoinositol they were capable of improving ovarian function that it reduced quantities of the follicle-stimulating hormone (FSH) required in ovarian stimulation and in oocyte recoveries to the best pick-up both in number and in quality.[13] Indeed, it was also found that, high level of myoinositol in ovarian is crucial for improved FSH signaling, oocyte maturation and embryo development.[14]
Taking together, it can be speculated that the relevance of these studies is mainly related to a possibility that inositol supplement have active roles of a therapeutic approach to manage poor ovarian response women in assisted reproduction. Previously, a number of prospective trials suggested the quality of oocytes and embryos were improved by inositol[1,15–20]; however, the sample size of these studies is relatively small, and a consensus was not achieved yet. Meanwhile, its efficacy in clinical pregnancy rate was also not determined, thus there is a necessity of collating the accruing evidence on myoinositol supplement to be beneficial for women in assisted reproduction. The aim of this meta-analysis is to evaluate the current efficacy of myoinositol in ovulation induction for intracytoplasmic sperm injection (ICSI) or in vitro fertilization embryo transfer (IVF-ET).
2. Methods
2.1. Study selection
PubMed (1966.01–2017.06), the Cochrane Library (2017 Issue 6), EMBASE (1974.01–2017.06), and ISI (2017 Issue 6) were searched. Free text terms used for the search were (“intracytoplasmic sperm injection” OR “ICSI” OR “in vitro fertilization embryo transfer” OR “IVF-ET” OR “assisted reproduction”) AND “myoinositol,” and both medical subject headings and free text terms were adopted. Additional searches were also conducted mainly through reviewing related articles, references in relevant reviews and citations of potential studies. The publication language was not limited.
2.2. Inclusion and exclusion criteria
Search results were critically reviewed by 2 reviewers independently for eligibility in the meta-analysis. Only articles reported clinical controlled trials with prospective design, investigating myoinositol supplement efficacy for infertile women undergoing ovulation induction for ICSI or IVF-ET were considered. Age of patients was limited to <42 years. Myoinositol was additionally supplemented in the treatment group (myoinositol group) before ovulation induction compared with control group, in which only folic acid was administered. Primary outcome measures included clinical pregnancy rate and clinical abortion rate, and secondary outcome measures included grade 1 embryos, total amount of agent used to reach follicular maturation, the number of days of stimulation, E2 peak level, the number of total oocytes retrieved, germinal vescicle, and degenerated (GV-DEG) oocytes retrieved and meta-phase II (M II) stage oocytes retrieved.
2.3. Data extraction and quality evaluation
Data of basic characteristics and outcome measures were both independently extracted and cross-checked by 2 reviewers. The methodological quality of the selected trials was assessed using the Cochrane Handbook methods. For randomized controlled trials (RCTs), items of risks in randomization, allocation concealment, blinding of participant and outcome measurement, incomplete outcome data, selective reporting, and other bias were judged by Cochrane tool of risk of bias.[21] For prospective cohort trials, item of risks in patient selection, baseline comparability, and outcomes selection and measurement with total 9 stars were judged by Newcastle-Ottawa Scale (NOS).[22]
2.4. Statistical analysis
Available data of outcome measures was combined and analyzed using ReMan 5.2 software (Cochrane Collaboration, Demark). The statistic heterogeneity across the trials was calculated by χ2 statistic, and inconsistency was judged by the value of I2 statistic. When I2 >50%, random-effects model was used, otherwise fix-effects model was used. For continuous outcomes mean difference (MD) was chosen and for dichotomous outcomes odds ratios (OR) was chosen for the combined estimate effects, followed by the respective 95% confidence intervals (95% CI). Because of high clinical homogeneity and limited number of selected trials, subgroup analyses were performed to separate the different kinds of ovulation drugs and patients diagnosed with PCOS or not, whereas inverted funnel plot was not performed. Sensitivity analysis was performed though omitting the non-RCT trial, of low quality trials and high weight trial, or changing meta-analysis combined effect model.
The meta-analysis was reported according to the PRISMA statement. All the included studies declared that the study was approved by local ethics committee, and our meta-analysis itself did not involve any ethics issues.
3. Results
3.1. Literature search
The preliminary search identified 469 potentially relevant citations. Articles such as not relevant to the proposed interventions, reviews, not controlled studies, and duplicates were excluded. Then 16 articles were full-text assessed, and a total of 7 trials were retrieved from the electronic databases. Figure 1 shows the flow chart of studies from initial results of publication search to final inclusion or exclusion.
Figure 1.
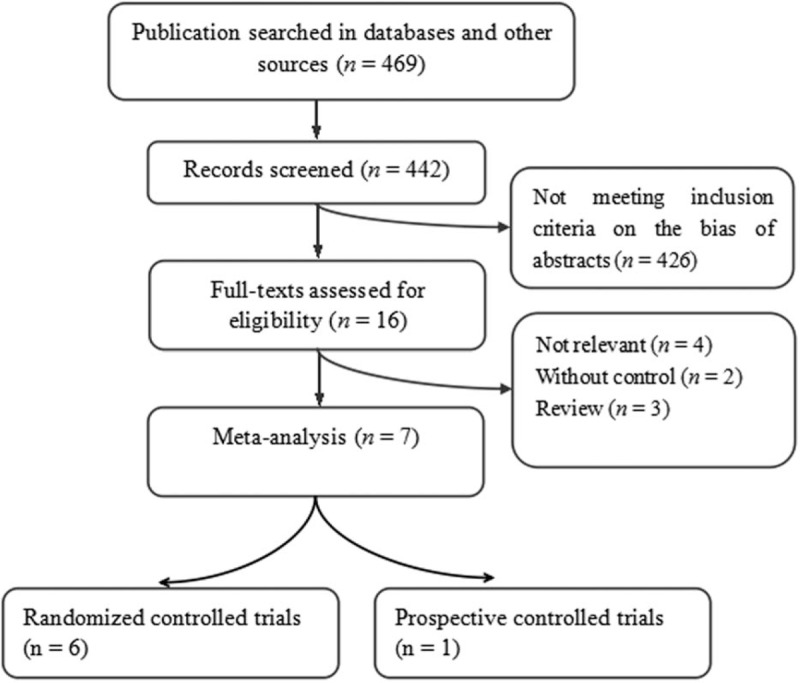
Flow chart of trials selection.
3.2. Basic characteristics and methodological quality
There were 6 prospectively RCTs and 1 cohort study[18] including 935 patients in the combined study, and among them 453 women were in the myoinositol group and 482 women in the control group. Table 1[1,15–17,19,20] presents the basic characteristic of the included studies. PCOS patients were included in 4 of the trials, and only 2 trials completely exclude PCOS patients in their study.[17,18] All the included trials adopted myoinositol plus folic acid in treatment group, and folic acid alone in control group. The daily dose of folic acid was 400 ug and dose of myoinositol was 4 g, which was administered once a day in 2 trials[18,19] when twice a day in 4 trial. The myoinositol was administrated for 3 months before and during controlled ovulation induction in a total of 5 trials,[1,15,16,17,20] and for 3 month before enrollment in 1 trial,[18] and from the first day of the cycle until 14 days after embryo transfer in 1 trial.[19] Case number ranged from 30 to 196 participants, and reported average age ranged from 31.5 to 36.2 years. Reported average duration of infertility ranged from 21.6 to 46.1 months, and body mass index ranged from 22.7 to 26.7 kg/m2. Among the included trials, only 1 trial excluded patients diagnosed as PCOS whereas the others included such patients.
Table 1.
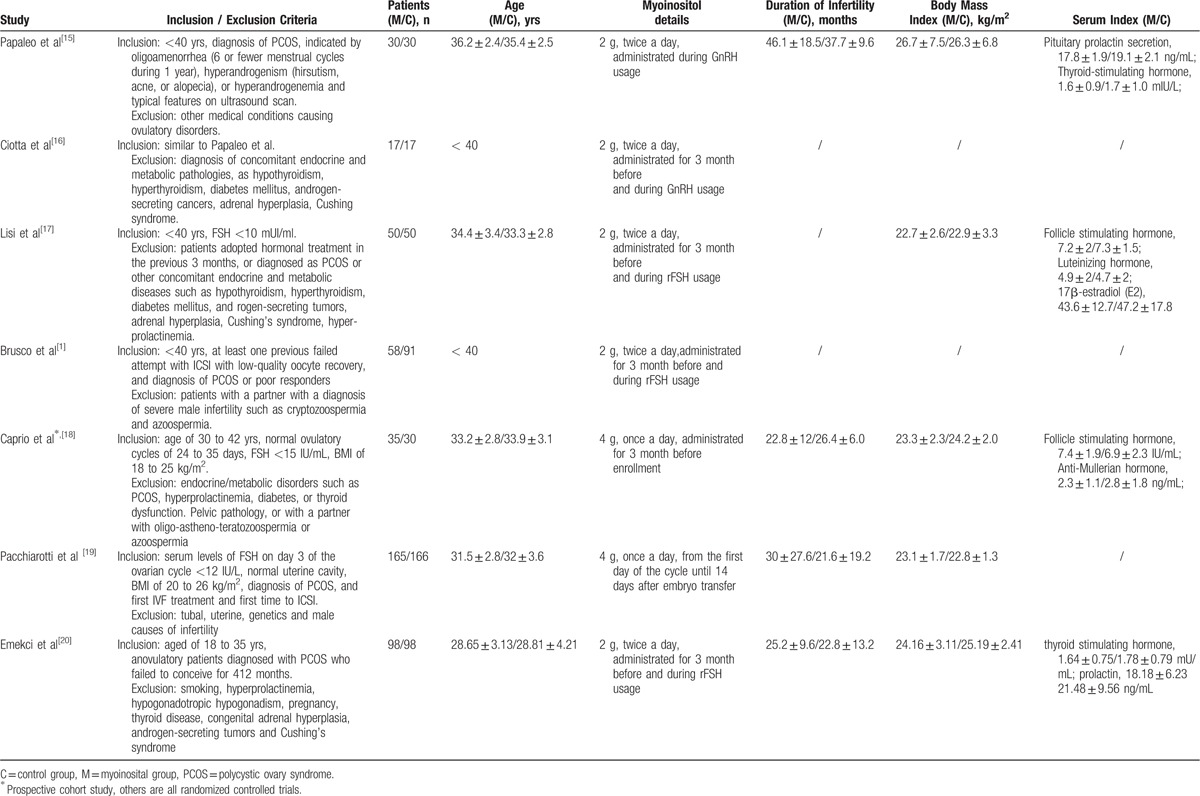
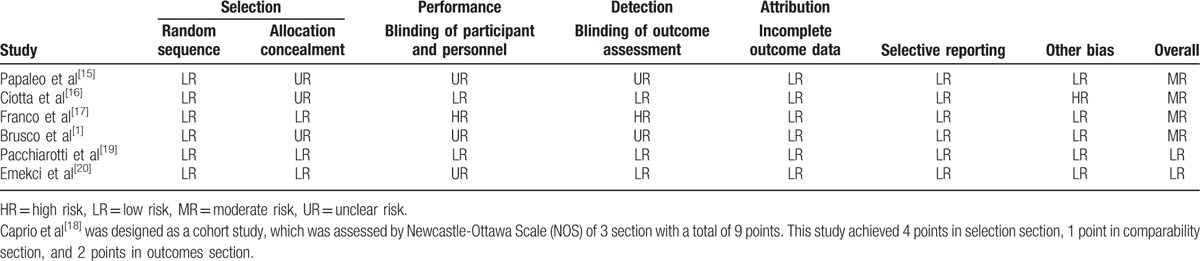
Table 2[1,15–17,19,20] shows the methodological quality of the included RCTs and cohort study, and overall quality of the combined study was good.
Table 2.
3.3. Clinical pregnancy rate
Clinical pregnancy rate was reported in 6 trials involving 913 patients, as shown in Figure 2. The meta-analysis results showed that the clinical pregnancy rate was significantly higher in the myoinositol group than in the control group (33.33%/27.62%, OR, 1.45; 95% CI, 1.08–1.95; P = .01).
Figure 2.
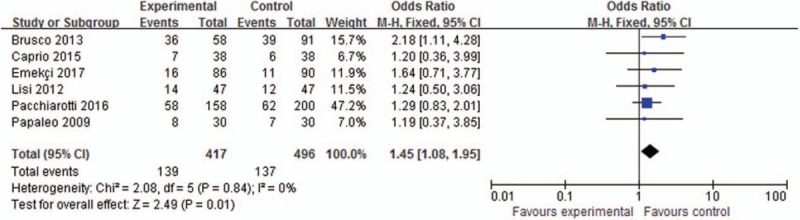
Meta-analysis of clinical pregnancy rate.
3.4. Abortion rate
Abortion rate was reported in 3 trials involving 311 patients, as shown in Figure 3. The meta-analysis results showed that the abortion rate was also significant lower in the myoinositol group than in the control group (5.92%/17.61%, OR, 0.26; 95% CI, 0.12–0.59; P = .001).
Figure 3.
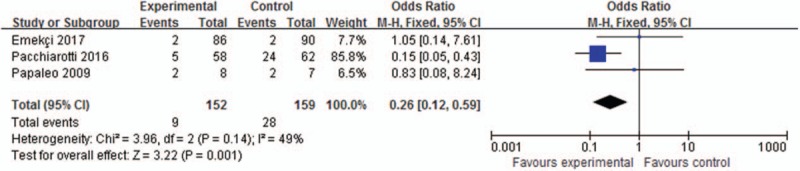
Meta-analysis of clinical abortion rate.
3.5. Grade 1 embryos proportion
Grade 1 embryos proportion was reported in 3 trials involving a total of 1294 embryos (Fig. 4). Meta-analyses in random-effects model demonstrated that the proportion of grade 1 embryos was significantly higher in the myoinositol than in the control group (45.17%/41.93%, OR, 1.73; 95% CI, 1.10–2.74; P = .02).
Figure 4.
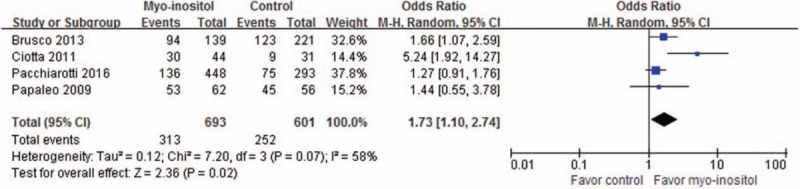
Meta-analysis of grade 1 embryos proportion.
3.6. Total oocytes retrieved
The number of total oocytes retrieved was reported in 4 trials involving 686 patients. The meta-analysis results in random-effects model revealed no significant difference in the number of total oocytes retrieved between the two groups (MD, −0.11; 95% CI, −0.79 to 0.58; P = .75).
3.7. GV-DEG oocytes retrieved
The proportion of GV-DEG oocytes retrieved was reported in 3 trials including 1186 oocytes, as shown in Figure 5. The meta-analysis results in random-effects model showed that the proportion of GV-DEG oocytes retrieved was significantly lower in the myoinositol group than in the control group (10.23%/22.81%, OR, 0.31; 95% CI, 0.11–0.86; P = .02).
Figure 5.
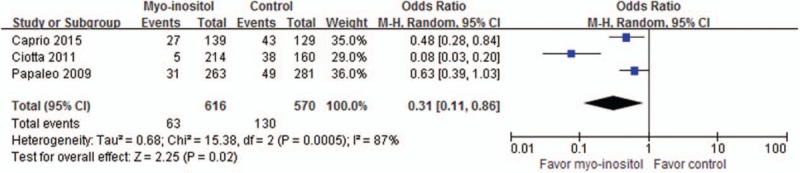
Meta-analysis of GV-DEG oocytes retrieved.
3.8. MII stage oocytes retrieved
The proportion of MII stage oocytes retrieved was reported in 6 trials including 4880 oocytes from 771 women. The meta-analysis results in random-effects model did not find significant difference between the groups (58.65%/57.10%, OR, 1.06; 95% CI, 0.97–1.15; P = .21) (Fig. 6).
Figure 6.
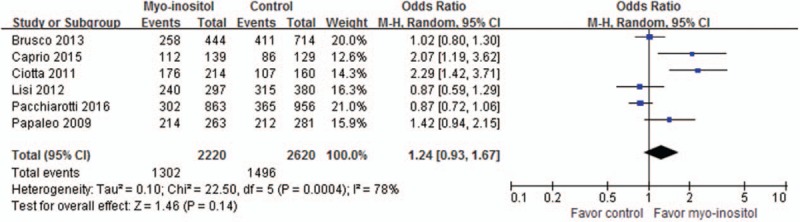
Meta-analysis of MII stage oocytes retrieved proportion.
3.9. Total amount of ovulation drugs
Total amount of ovulation drugs was reported in 5 trials involving 773 patients, and the meta-analysis results in random-effect model showed that the total amount was significant lower in the myoinositol group than in the control group (MD, −327.40; 95% CI, −567.38 to −87.41; P = .007), as shown in Figure 7. Meta-analyses results showed that the myoinositol group required significantly less total units of rec-FSH (4 trials, MD, −229.42; 95% CI, −387.65 to −71.18; P = .004) and gonadotropin (1 trial, MD, −544; 95% CI, −647.19 to −440.81; P < .00001) than control.
Figure 7.
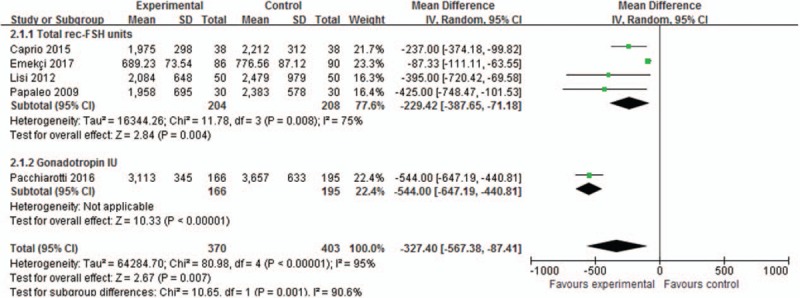
Meta-analysis of ovulation drug dosage.
3.10. Stimulation days
Stimulation days were reported in 3 trials involving 521 patients. The meta-analysis results in random-effects model demonstrated that there was no significant difference in the stimulation days between the 2 groups (MD, −0.55; 95%CI, −1.17 to −0.07; P = .08).
3.11. E2 peak level
E2 peak level was reported in 3 trials involving 521 patients. The meta-analysis results in random-effects model demonstrated that there was no significant difference in the E2 peak level between the 2 groups (MD, −222.48; 95% CI, −579.22 to −134.25; P = .22).
3.12. Subgroup analysis of non-PCOS patients
Meta-analyses of most of the outcomes did not exclude patients diagnosed of PCOS. Subgroup analysis of 2 trials completely excluded PCOS patients was performed. The results showed that the trend was altered in the aspect of clinical pregnancy rate (n, 85/85; OR, 0.04; 95% CI, −0.09 to 0.16; P = .58) for non-PCOS infertile women, compared with infertile women including PCOS patients (n, 332/411; OR, 0.08; 95% CI, 0.02 to 0.15; P = .01). No different located in aspects of total oocytes retrieved, MII stage oocytes retrieved, and total amount of ovulation drugs.
3.13. Sensitivity analysis
After both changing combined model, and omitting non-RCT low quality trials[18] and high weight trial,[19] the trends of clinical pregnancy rate, grade 1 embryos proportion, total oocytes retrieved, MII stage oocytes retrieved, GV-DEG oocytes retrieved, and ovulation drug dosage were not changed. The trends of abortion rate, stimulation days and E2 peak level were altered, indicating unstable results in the outcome measures.
4. Discussion
It is clear that oocyte and embryo quality depression are widely considered to be the main challenge of IVF in assisted reproduction. Many studies have already investigated and proposed potential predictive factors for negative and positive IVF-ET outcomes.[23,24] Confirmed by a previous meta-analysis, female age, duration of subfertility, and basal FSH level are also predictors of pregnancy after IVF-ET.[25] Attempt of MI administration in IVF-ET has lasted for nearly 2 decades. Firstly an elevated level of inositol was first found in serum samples of females achieved successful IVF-ET pregnancies, and proposed a positive role of inositol in the early in vitro phase of IVF-ET and embryonic development.[26] Further study revealed that local concentration of myoinositol in human follicular fluid partly reflecting the oocytes quality.[14] Clear mechanism was absent, and primary research indicated that MI may promote meiotic progression of germinal vesicle oocytes in mouse,[27] and may improve insulin resistance[12] through the enhancement of intracellular calcium oscillation.
This meta-analysis showed very promising benefits that, as a result of pretreatment with inositol the clinical pregnancy rate obtained was increased by 6.13%, and abortion rate obtained was reduced by 27.08%. Myoinositol used to be classified as an insulin sensitizing agent and commonly used in PCOS for hyperandrogenemia and GDM. Inositol also had some roles in reestablishing menstrual cyclicity and ovulation, and increasing the chance of a spontaneous pregnancy by rescuing the ovarian response to endogenous gonadotropins. Meanwhile, its use in pregnancy female is confirmed to be safe that only mild gastrointestinal side effects in limited dose of 12 g/day. Thus, myoinositol has emerged as a newly “pregnancy-promoting” agent in assisted reproduction, especially for PCOS patients.
There was no difference in the number of total oocytes retrieved. A positive trend was supposed in patients pretreated with myoinositol in mature oocytes proportion of MII stage oocytes retrieved; however, it failed to reach a statistical difference. The combination results of 3 trials reporting unmature oocytes of GV-DEG oocytes retrieved proportion presented a significant reduction. What's more, myoinositol therapy had significant higher proportion in good quality embryos of score I embryos or grade 1 embryos. Omitting 2 trials, this result only included PCOS patients, confirming that patients diagnosed as PCOS could get benefit by myo-inositol in embryos development.[28,29] As second passenger in signal transduction pathways, inositol are involved in the release of cortical granules, in the inhibition of polyspermy, in the completion of meiosis and in the activation of the cell cycle and insulin sensitivity roles that may subsequently result in embryonic development.[15,17]
Besides, the total amount of gonadotropins/FSH used to reach follicular maturation was significantly reduced in myoinositol group. Two trials[15,19] including PCOS patients indicated a reduction in stimulation days and E2 peak when patients are treated with inositol and folic acid instead of folic acid alone; however, 1 trial[17] including non-PCOS patients showed no difference. The efficacy of inositol is related to the role by 2 different molecules at ovarian level. Myoinositol is the second messenger of FSH, and literature data supposed that myoinositol signaling may to some extent adjust the level of anti-Mullerian hormone production induced by FSH in granulosa cells.[30] Myoinositol may improved the quality of follicles, and large follicles was always had higher level of progesterone and E2 concentrations than the small ones. Likely, follicles containing mature oocyte also always have a higher level of melatonin concentrations than unmature ones.[31] So, elevated concentrations of melatonin might firstly induce progesterone production, and then involved in the induction of luteinizing hormone sensibility of the developing follicle to oogenesis. Stimulated luteinizing hormone receptors expression resulted in luteinization and ovulation.[19] Perhaps, there is a different mechanism for ovulation during PCOS patients and none PCOS patients.
There are some weaknesses with present evidence. In 3 including RCTs,[1,15,17] myoinositol supplement was open labeled, and absence of blinding of the female or outcome measures, in such RCTs and 1 non-CRT[18] had negative influence. In addition, Caprio et al[18] was designed as a prospective cohort study, which may have a risk of selection bias although sensitivity was performed. This meta-analysis was also limited by differences in the participants as variation located in the inclusion criteria and the components of the intervention such as comparable metformin and folic acid intake in both groups. Furthermore, women across the trials and groups may not have absolutely same age, infertility duration and ovarian function; this may affect the beneficial effect of myoinositol unavoidably as confirmed predictor factors. After all, 1 trial[1] adopt very low daily dose of DCI supplement besides of myo-inositol. And both ICSI or IVF-ET was adopted widely in current practice as no significant difference, however, it seemed that the drugs used for ovarian stimulation and devices for procedure also have influence on outcomes.[32,33]
Another limitation concerns the generalizability of this meta-analysis. All subjects were Caucasian women from Italy and no other ethnic groups were represented, so publication bias may also existed although comprehensive search performed, and it remains unclear whether the findings are applicable to the other countries’ pregnant female. The beneficial effects of myo-inositol supplement in infertility women undergoing ovulation induction for ICSI or IVF-ET is encouraging, whereas the optimal dose, frequency and type of inositol isomer were unclear, and the effects of different genera and varied dose need to be identified.
5. Conclusions
On the basis of current evidence, myoinositol supplement has promising benefits to females undergoing ovulation induction for ICSI or IVF-ET in assisted reproduction. Myoinositol is really cost effective, and it is an attractive treatment options warranted further evaluation by large multicentre randomized controlled trials, involving different racial of individuals.
Footnotes
Abbreviations: CI = confidence interval, DCI = D-chiro-inositol, FSH = follicle-stimulating hormone, GDM = gestational diabetes mellitus, ICSI = intracytoplasmic sperm injection, IPG = inositol-containing phosphoglycan, IVF-ET = in vitro fertilization embryo transfer, MD = mean difference, NOS = Newcastle-Ottawa Scale, OR = odds ratios, PCOS = polycystic ovary syndrome, RCTs = randomized controlled trials.
XZ and DL are co-first authors and contributed equally to this work.
This work was supported by Fujian Provincial Natural Science Foundation (number 2016J0105).
The authors declared no conflicts of interest.
References
- [1].Brusco GF, Mariani M. Inositol: effects on oocyte quality in patients undergoing ICSI. An open study. Eur Rev Med Pharmacol Sci 2013;17:3095–102. [PubMed] [Google Scholar]
- [2].Zacchè MM, Caputo L, Filippis S, et al. Efficacy of myo-inositol in the treatment of cutaneous disorders in young women with polycystic ovary syndrome. Gynecol Endocrinol 2009;25:508–13. [DOI] [PubMed] [Google Scholar]
- [3].Unfer V, Carlomagno G, Rizzo P, et al. Myo-inositol rather than D-chiro-inositol is able to improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Eur Rev Med Pharmacol Sci 2011;15:452–7. [PubMed] [Google Scholar]
- [4].Bevilacqua A, Carlomagno G, Gerli S, et al. Results from the International Consensus Conference on myo-inositol and D-chiro-inositol in obstetrics and gynecology-assisted reproduction technology. Gynecol Endocrinol 2015;31:441–6. [DOI] [PubMed] [Google Scholar]
- [5].Facchinetti F, Bizzarri M, Benvenga S, et al. Results from the International Consensus Conference on Myo-inositol and d-chiro-inositol in obstetrics and gynecology: the link between metabolic syndrome and PCOS. Eur J Obstet Gynecol Reprod Biol 2015;195:72–6. [DOI] [PubMed] [Google Scholar]
- [6].Granata R, Settanni F, Biancone L, et al. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic (-cells and human islets: involvement of 3’, 5’-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-kinase/Akt signaling. Endocrinology 2007;148:512–29. [DOI] [PubMed] [Google Scholar]
- [7].Burghen GA, Givens JR, Kitabchi AE. Correlation of Hyperandrogenism with Hyperinsulinism in Polycystic Ovarian Disease. J Clin Endocrinol Metab 1980;50:113–6. [DOI] [PubMed] [Google Scholar]
- [8].Azziz R, Ehrmann D, Legro RS, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial 1. J Clin Endocrinol Metab 2001;86:1626–32. [DOI] [PubMed] [Google Scholar]
- [9].Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: a randomized controlled study. Hum ReprodV 17 2002;1729–37. [DOI] [PubMed] [Google Scholar]
- [10].Tang T, Lord JM, Norman RJ, et al. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev V 5 2012;CD003053.doi: 10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- [11].Zheng X, Liu Z, Zhang Y, et al. Relationship between myo-inositol supplementary and gestational diabetes mellitus: a meta-analysis. Medicine 2015;94:e1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Larner J. D-chiro-inositol-its functional role in insulin action and its deficit in insulin resistance. J Diabetes Res 2002;3:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colazingari S, Treglia M, Najjar R, et al. The combined therapy myo-inositol plus D-chiro-inositol, rather than D-chiro-inositol, is able to improve IVF outcomes: results from a randomized controlled trial. Arch Gynecol Obstet 2013;288:1405–11. [DOI] [PubMed] [Google Scholar]
- [14].Chiu TTY, Rogers MS, Law ELK, et al. Follicular fluid and serum concentrations of myo-inositol in patients undergoing IVF: relationship with oocyte quality. Hum Reprod 2002;17:1591–6. [DOI] [PubMed] [Google Scholar]
- [15].Papaleo E, Unfer V, Baillargeon JP, et al. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil Steril 2009;91:1750–4. [DOI] [PubMed] [Google Scholar]
- [16].Ciotta L, Stracquadanio M, Pagano I, et al. Effects of myo-inositol supplementation on oocyte's quality in PCOS patients: a double blind trial. Eur Rev Med Pharmacol Sci 2011;15:509–14. [PubMed] [Google Scholar]
- [17].Lisi F, Carfagna P, Oliva MM, et al. Pretreatment with myo-inositol in non polycystic ovary syndrome patients undergoing multiple follicular stimulation for IVF: a pilot study. Reprod Biol Endocrinol 2012;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Caprio F, D’Eufemia MD, Trotta C, et al. Myo-inositol therapy for poor-responders during IVF: a prospective controlled observational trial. J Ovarian Res 2015;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pacchiarotti A, Carlomagno G, Antonini G, et al. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol Endocrinol 2016;32:69–73. [DOI] [PubMed] [Google Scholar]
- [20].Emekçi Özay Ö, Özay AC, Çağlıyan E, et al. Myo-inositol administration positively effects ovulation induction and intrauterine insemination in patients with polycystic ovary syndrome: a prospective, controlled, randomized trial. Gynecol Endocrinol 2017;1–5. [DOI] [PubMed] [Google Scholar]
- [21].Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [23].Pacchiarotti A. Antioxidative capacity of melatonin in follicular fluid of aged IVF patients: beneficial effects on oocytes and embryo. J Gynecol Neonatal Biol 2015;1:1–5. [Google Scholar]
- [24].Andersen AN, Witjes H, Gordon K, et al. Predictive factors of ovarian response and clinical outcome after IVF/ICSI following a rFSH/GnRH antagonist protocol with or without oral contraceptive pre-treatment. Hum Reprod 2011;26:3413–23. [DOI] [PubMed] [Google Scholar]
- [25].Van Loendersloot LL, Van Wely M, Limpens J, et al. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update 2010;16:577–89. [DOI] [PubMed] [Google Scholar]
- [26].Chiu TTY, Tam PPL. A correlation of the outcome of clinical in vitro fertilization with the inositol content and embryotrophic properties of human serum. J Assist Reprod Genet 1992;9:524–30. [DOI] [PubMed] [Google Scholar]
- [27].Chiu TTY, Rogers MS, Briton-Jones C, et al. Effects of myo-inositol on the in-vitro maturation and subsequent development of mouse oocytes. Hum Reprod 2003;18:408–16. [DOI] [PubMed] [Google Scholar]
- [28].Unfer V, Carlomagno G, Dante G, et al. Effects of myo-inositol in women with PCOS: a systematic review of randomized controlled trials. Gynecol Endocrinol 2012;28:509–15. [DOI] [PubMed] [Google Scholar]
- [29].Papaleo E, Unfer V, Baillargeon JP, et al. Contribution of myo-inositol to reproduction. Eur J Obstet Gynecol Reprod Biol 2009;147:120–3. [DOI] [PubMed] [Google Scholar]
- [30].Taieb J, Grynberg M, Pierre A, et al. FSH and its second messenger cAMP stimulate the transcription of human anti-Müllerian hormone in cultured granulosa cells. Mol Endocrinol 2011;25:645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tamura H, Nakamura Y, Korkmaz A, et al. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril 2009;92:328–43. [DOI] [PubMed] [Google Scholar]
- [32].Buckett WM. A review and meta-analysis of prospective trials comparing different catheters used for embryo transfer. Fertil Steril 2006;85:728–34. [DOI] [PubMed] [Google Scholar]
- [33].Al-Inany HG, Abou-Setta AM, Aboulghar MA, et al. Highly purified hMG achieves better pregnancy rates in IVF cycles but not ICSI cycles compared with recombinant FSH: a meta-analysis. Gynecol Endocrinol 2009;25:372–8. [DOI] [PubMed] [Google Scholar]


