Abstract
Sarcoidosis is associated with cell-mediated immunodeficiency and treatment of symptomatic sarcoidosis usually includes systemic immunosuppressants. Data relative to incidence, prognosis factors, and outcome of infections are scarce.
Retrospective cohort study of 585 patients with biopsy proven sarcoidosis in a tertiary referral specialist clinic, with a nested case-control analysis. Twenty nine patients (4.9%) with severe infections were compared to 116 controls subjects with sarcoidosis, matched according to their gender, ethnicity, age at diagnosis, and treatment with corticosteroids.
After a median follow-up of 8 years [range; 1–46], 38 severe infections [mycobacterial infections (n = 14), fungal infections (n = 10), bacterial (n = 8), viral (n = 3) and parasitic (n = 1)] were observed in 30 patients. The incidence of severe infections was 0.71% persons-year (CI 95% 0.5–0.98) and 0.43% persons-year (CI 95% 0.27–0.66). Patients with severe infection were more frequently of male gender (60% vs 46%) and were more likely treated by ≥ 3 immunosuppressive agents (OR = 3.8, IC 95% [1.5–9.64], P = .005) and by cyclophosphamide (OR = 5.55, IC 95% [1.9–16.1], P = .002), and with neurological (OR = 3.36 CI 95% [1.37–8.25], P = .008), or cardiac (OR = 2.65 CI 95% [1.09–6.43], P = .031) involvement of the sarcoidosis, compared to the controls. Two patients died within the 6 months following infection, due to progressive multifocal leucoencephalopathy (n = 1), and of peritonitis (n = 1).
Severe infections are observed in 5.1% of our patients with sarcoidosis after a median follow-up of 8 years. Risk factors for severe infections included neurological or cardiac involvement of sarcoidosis, the use of immunosuppressive agents and mainly cyclophosphamide.
Keywords: immunodeficiency, immunosuppressants, infection, sarcoidosis, steroids
1. Introduction
Sarcoidosis is a multisystemic granulomatous disease of unknown etiology characterized by giant cell noncaseating granuloma.[1] It is an inflammatory disease whose pathophysiological mechanism is immunological but still misunderstood. It is an exaggerated immune response and granulomatous reaction to unidentified antigens (environmental or transmissing agents) in a context of genetic predisposition.[2,3] Significant lymphopenia involving CD4 (cluster of differenciation 4), CD8, and CD19 T-cells is common in sarcoidosis patients and correlated with disease activity. Data suggests that lymphopenia relates more to disease pathology than medical treatment.[4]
The treatment of symptomatic sarcoidosis usually includes glucocorticoids, and cytotoxic drugs like methotrexate, azathioprine, cyclosphosphamide, mycophenolate mofetil, and biologic agents like tumor necrosis factor alpha (TNF α) antagonist can be used in severe or refractory sarcoidosis. The use of immunosupressive drugs is associated with an increased risk for infection.[5]
In spite of CD4 + T-lymphocytopenia and treatment-induced immune suppression, the risk of severe or opportunistic infection is not usually considered to be higher in sarcoidosis, than in general population but this is still being debated.[6] Some studies have described cases of opportunistic infections even in untreated patients, in particular cryptococcosis and progressive multifocal leukoencephalopathy (PML).[7,8]
Few data are available with respect to the prevalence, risk factors, and outcome of severe infections in sarcoidosis. Some studies have specifically reported observations of opportunistic infections in patients with sarcoidosis. Baughman[9] reported 7 (0.9%) fungal infections out of 753 patients with sarcoidosis seen at their institution over a 18 months period. Rubinstein et al[10] followed up 197 patients for a mean duration of 7.2 years and reported no invasive opportunistic infection.
We conducted a single-center retrospective cohort study of 585 patients with biopsy proven sarcoidosis in order to assess the prevalence, main characteristics, and outcome of severe infections. We performed a nested case–control analysis in order to identify risk factors associated with severe infection in patients with sarcoidosis.
2. Materiel and methods
2.1. Patients
A single-center retrospective cohort analysis was conducted, including 585 consecutive patients with biopsy proven sarcoidosis followed at the hospital Pitié Salpêtrière in the internal medicine and clinical immunology department. One investigator (CCA) collected the data of the entire cohort. Patients presenting with a severe infection during their follow-up were identified and their data were collected (AD). To be included patients should have: (1) Clinical and paraclinical features consistent with the American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders (ATS/ERS/WASOG) criteria for diagnosis of sarcoidosis and with histological evidence of sarcoidosis;[11] (2) Serious infections were defined as those that led to hospitalization or death or required intravenous antibiotic treatment.[12] They included bacterial, mycobacterial, viral, and fungal infections.
Age at diagnosis of sarcoidosis, the organs involved by sarcoidosis, stage of sarcoidosis, treatment history and at the time of infection was recorded. The standard dosing regimen of cyclophosphamide in our center was 700 mg/m2 per month.
For severe nonmycobacterial infection, infection type, localization, treatment, and outcome were also reported. Laboratory parameters including blood lymphocytes count total and CD4, serum angiotensin-converting enzyme, gamma globulin level were noted at the time of diagnosis of sarcoidosis and at the time of severe infection.
The local ethics committee of La Pitié Salpétrière hospital, Paris VI University, approved this study.
2.2. Statistical analysis
Characteristics of patients according to the occurrence of infections are reported as median (interquartile range [IQR]) for quantitative variables and counts (percent) for categorical variables.
The incidence of severe infections among patients with sarcoidosis was estimated with its 95% confidence interval (95% CI) from the cohort using a Poisson model.
A first analysis compared the characteristics of patients between the groups (infected or not) among the whole cohort: quantitative variables were compared using the Wilcoxon rank sum tests while categorical variables are compared using the Fisher exact tests.
A second part consisted in a nested case–control analysis: each case patient (with a severe infection) was matched with 4 control subjects (without severe infection) within the cohort, according to their sex, age at diagnosis of sarcoidosis, ethnicity, and treatment with corticosteroids. Matching was performed by minimizing the Mahalanobis distance between cases and controls without replacement of controls. Factors associated with severe or opportunistic infection were identified in univariate analysis. Associations were estimated using Odds-Ratios (OR) and their 95%CIs in conditional logistic regression models to account for the matched design. All ORs estimated in this analysis should be interpreted as the estimated effect of variables, conditional on the matching factors (i.e., effect of variables while sex, ethnicity, age at diagnosis and corticosteroids use held constant).
Tests were two-sided and P-values lower than 5% were considered as indicating significant associations. Analyses were performed on R statistical platform, version 3.0.2.
3. Results
3.1. Characteristics of the cohort of sarcoidosis and according to the presence or not of severe infection
Main data are summarized in Tables 1–4 and Figure 1.
Table 1.
Main characteristics of the 16 patients with sarcoidosis and severe infection.
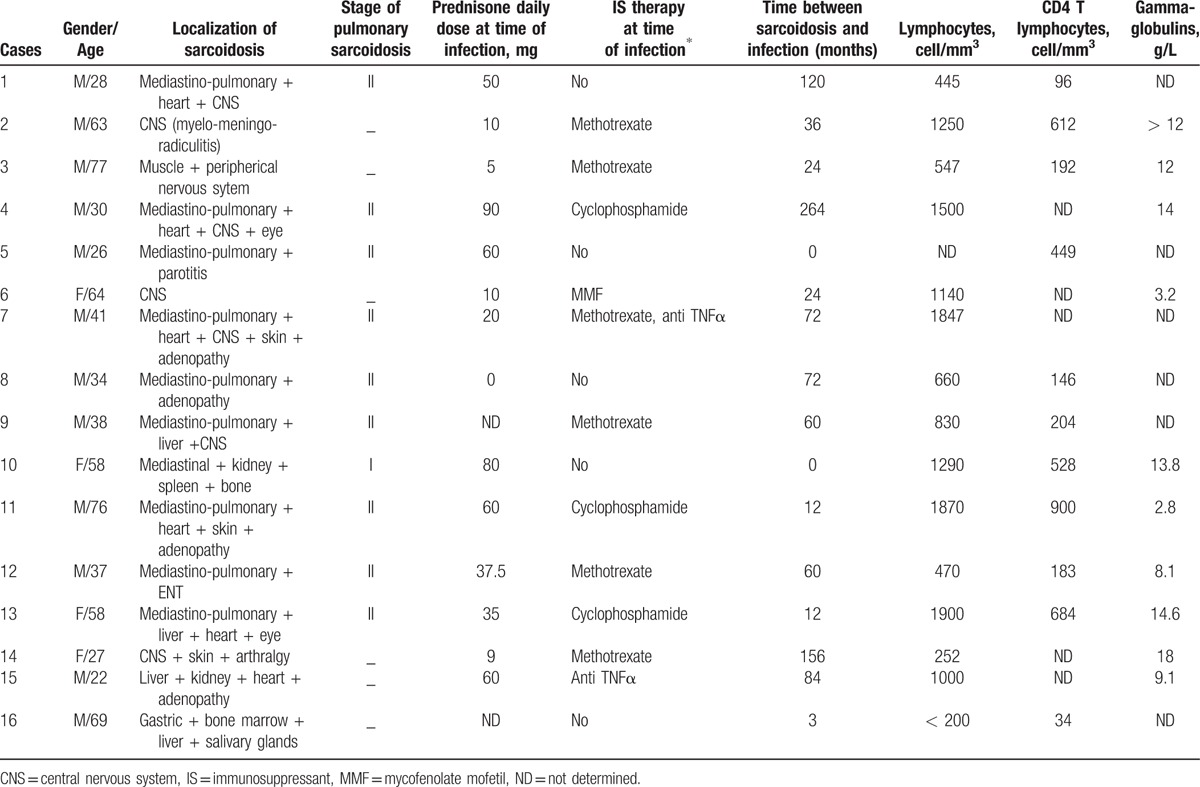
Table 4.
Case–control study of factors associated with severe infection∗.
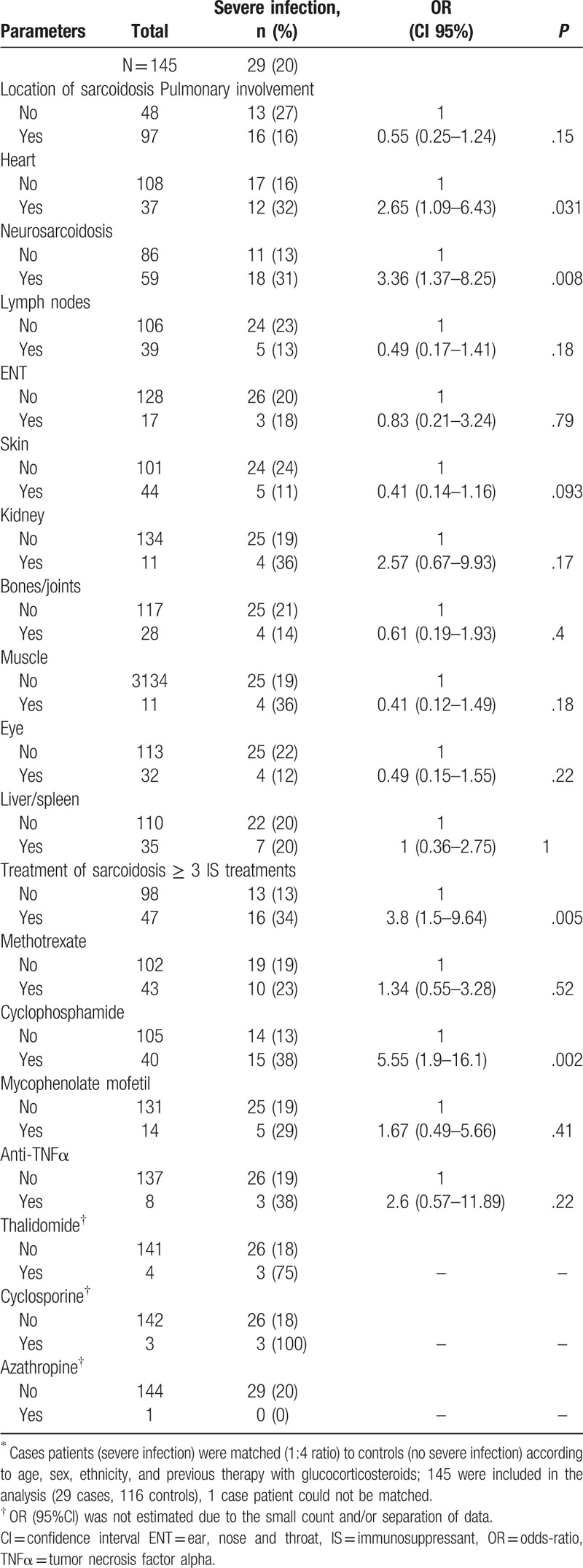
Figure 1.
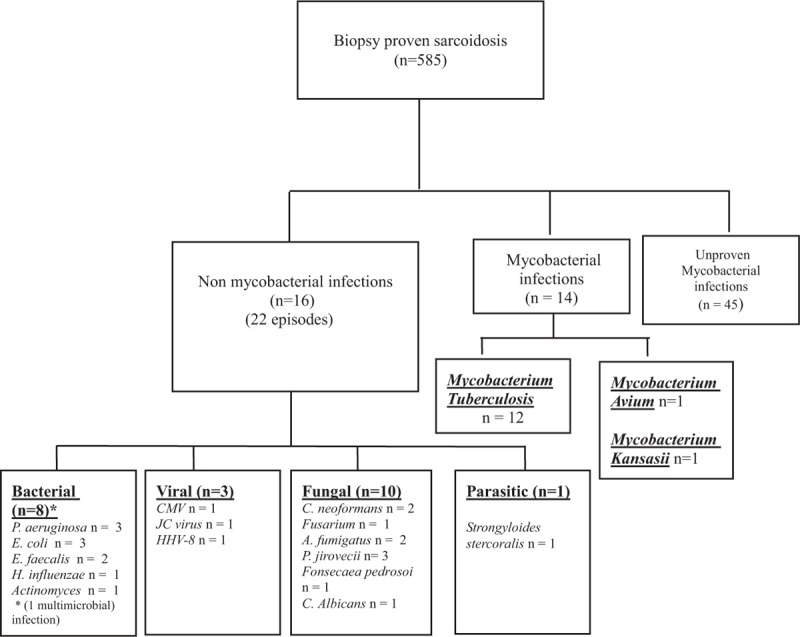
Flowchart of the cohort of patients with biopsy proven sarcoidosis.
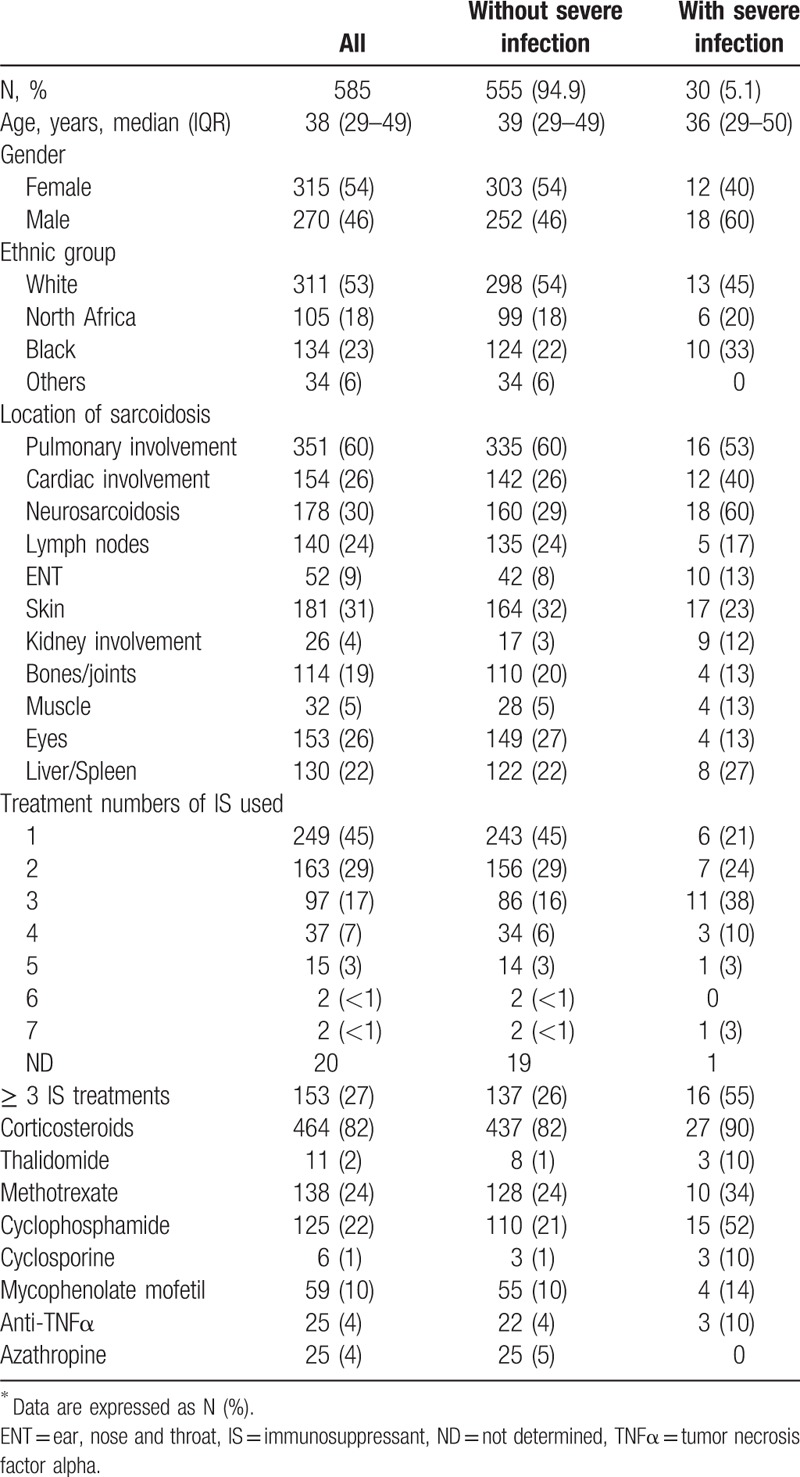
The cohort included 585 patients with biopsy proven sarcoidosis. A total of 22 episodes of severe nonmycobacterial infections were observed in 16 patients (median age: 38 years, IQR 29.5–63.2 and 75% of male) (Fig. 1 and Tables 1 and 2) and 14 episodes of mycobacterial infections were identified in 14 patients. Characteristics of the 585 patients included in this study are summarized in Table 3. There were 54% women (315 patients) and 46% men (270 patients), with median age of 38 years (29–49). The incidence of severe infections was 0.71% persons-year (CI 95% 0.50–0.98) and 0.43% persons-year (CI 95% 0.27–0.66) for nonmycobacterial severe infections. Patients originated from Europe (53%), Sub-Saharian Africa or Caribbean (23%), from Maghreb (18%) and other origin (6%). Main organ involvement of sarcoidosis included lung (60%), central nervous system (30%), cardiac (26%), skin (31%), eyes (26%), lymph nodes (24%), bones and joints (19%). About 27% of patients received at least 3 different immunosuppressive treatments including 82% of corticosteroids, 28% of hydroxychloroquine, 24% of methotrexate, and 22% of cyclophosphamide. Around 60% of patients with severe infection were male compared to 46% of patients without severe infection (P = .14). Patients with severe infection were more likely to be treated with ≥ 3 immunosuppressants (55% vs 26%, P = .0005) and were more frequently treated by thalidomide (10% vs 1%, P = .015), cyclophosphamide (52% vs 21%, P = .0003) and cyclosporine (10% vs < 1%, P = .002), respectively, as compared to those with no severe infection.
Table 2.
Outcome of the 16 patients with sarcoidosis and severe nonmycobacterial infection.
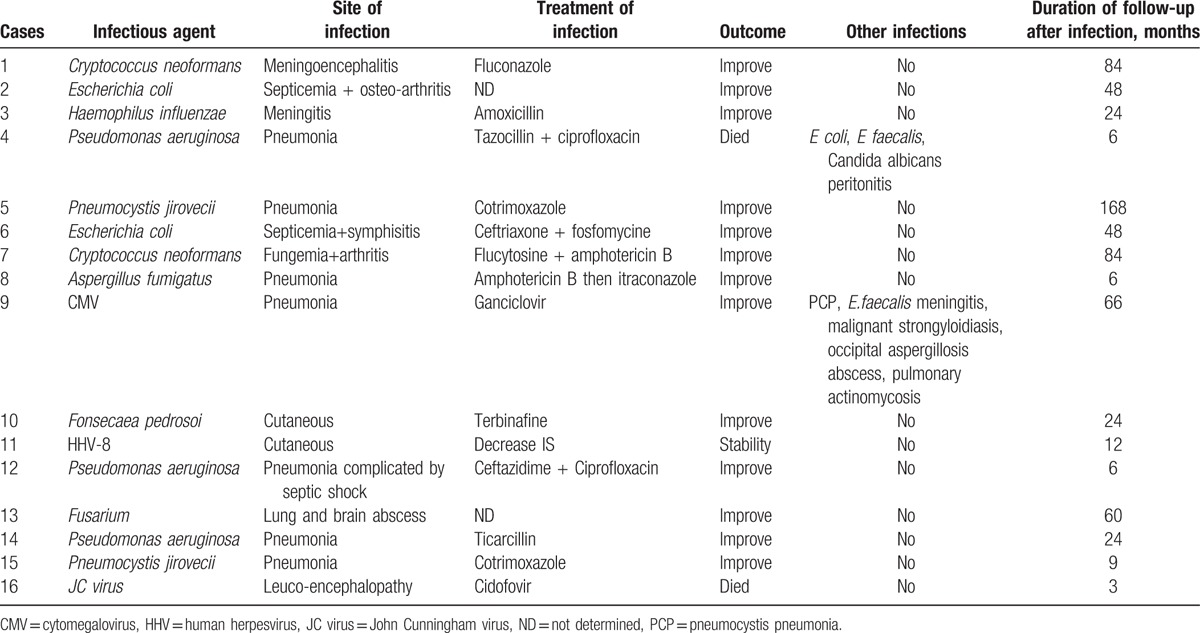
Table 3.
Characteristics of sarcoidosis patients according to the presence or not of severe infection∗.
3.2. Case–control study
Data are summarized in Table 4. Each sarcoidosis patient with a severe infection was matched with 4 control subjects who had a sarcoidosis but no actual or past severe infection (controls) according to their sex, ethnicity, age at diagnosis of sarcoidosis, and corticosteroids treatment. One case patient could not be matched leading to a sample size of n = 145 patients (29 cases matched to 116 controls) for this analysis. Conditionally on sex, age at diagnosis, ethnicity, and corticosteroids use, results were the following. Patients with severe infection presented more likely with neuro-sarcoidosis (OR = 3.36, CI 95% [1.37–8.25], P = .008) and cardiac sarcoidosis (OR = 2.65, CI 95% [1.09–6.43], P = .031). They were more likely to be treated by ≥ 3 immunosuppressants (OR = 3.8, IC 95% [1.5–9.64], P = .005) and by cyclophosphamide (OR = 5.55, CI 95% [1.9–16.1], P = .002) as compared to the controls.
3.3. Characteristics of the 16 patients with severe nonmycobacterial infections
The 22 severe infections included 14 patients with 1 infection, 1 patient with 2 infections and the remaining one with 6 infections (in 3 different time periods with a 1 year interval between periods 1 and 2 and a 4-year interval between periods 2 and 3). Nine (60%) patients were of Caucasian origin, 4 (26%) originated from Sub-Saharian Africa or West Indies and 2 (13%) originated from Maghreb. The average time between the diagnosis of sarcoidosis and the episode of infection was 5.2 years. No patient underwent pneumocystis prophylaxis by cotrimoxazole-thrimetoprim.
Fungal infections were the most frequent infections with 10 episodes. The most frequent pathogen (n = 3) was Pneumocystis jirovecci. Three patients developed a pneumocystis pneumonia with initial clinical presentation of febrile dyspnea, successfully treated by trimethoprim-sulfamethoxazole. There were 2 cryptococcosis including a Cryptococcus neoformans meningo-encephalitis (acute history of headache with fever associated with positive CSF [cerebrospinal fluid]) culture and CSF antigen for C neoformans) successfully treated by antifungal therapy (induction treatment by Amphotericin B–flucytosine for 2 weeks then fluconazole 600 mg/day for 3 months) and a C neoformans arthritis and septicemia (positive culture) treated by Amphotericin B–Flucytosine in induction phase followed by oral voriconazole with good outcome. Two Aspergillus related infections were noted, including one pulmonary aspergillosis treated by Amphotericin B then itraconazole and one patient presented a brain abscess with positive culture for Aspergillus fumigatus.
One infection was due to Fusarium (PCR [polymerase chain reaction] 16 S) with brain and lung abscess and one patient had a chromomycosis with skin localisation (right arm) with good outcome after treatment by terbinafine and lowering immunosuppressive therapy. The last one was a multimicrobial peritonitis due to fungal and bacterial pathogens (Candida albicans, Escherichia coli, and Enterococcus faecalis).
We reported 8 severe bacterial infections. The most common type was severe pneumonia due to Pseudomonas aeruginosa (n = 3). Six (67%) required intensive care unit, of whom one had a septic shock. The evolution was favorable in all cases with appropriate antibiotic therapy.
There were 2 bacterial meningitis, (one due to Haemophilus influenzae, and the other to E faecalis complicated by hydrocephalus requiring ventricular shunt), 2 osteoarticular infections (both due to E coli) and one multimicrobial peritonitis (E coli, E faecalis, C albicans). We found one episode of pulmonary actinomycosis, with positive bronchoalveolar lavage culture (nonacid fast, gram positive, identified as actinomycetes) treated by amoxicillin and with successful response.
There were 3 viral infections including one probable cytomegalovirus pneumonia (positive PCR in BAL [bronchoalveolar lavage] with new infiltrates on imaging and dyspnea)[13] treated by ganciclovir, one Kaposi's sarcoma who required significant decreased of immunosuppressive treatment and one progressive multifocal leukoencephalopathy (PML) with identification of JC (John Cunningham) virus by PCR in cerebrospinal fluid.
One patient, who originated from an endemic area (Central Africa), presented a disseminated strongylodiasis. No information was available about antiparasitic treatment before corticosteroids.
One patient was not receiving corticosteroids and/or immunosuppressive treatment at the time of infection. CD4 T cell lymphopenia was often present at the time of infection (median level of 204/mm3, IQR[146–612]). We observed that the median adjusted ePOST (extrapulmonary physician organ severity tool), an activity score of sarcoidosis[14] was higher at the time of diagnostic of sarcoidosis (8, range 5.75–9.25) compared with the time of severe infection (6, range 3.75–7.25). At the end of follow-up the median adjusted ePOST was lower (2.5, range 1–4).
Two patients (12.5%) died within the 6 months following infection.
3.4. Characteristics of the 14 patients with mycobacterial infections
A total of 14 episodes of mycobacterial infections were identified in 14 patients [12 Mycobacterium tuberculosis (n = 12), Mycobacterium avium (n = 1), and Mycobacterium kansasii (n = 1)].
4. Discussion
We examined the incidence, prognosis factors, and outcome of severe infections in a large cohort of patients with biopsy proven sarcoidosis.
The most striking conclusions drawn by this study are: (1) Severe infections, including mycobacterial ones, occurred in 5.1% of our patients; (2) Severe infections occurred predominantly in patients with neurological or cardiac sarcoidosis and in patients treated by immunosuppressants and mainly cyclophosphamide, conditionally on basic characteristics and known factors (sex, age at diagnosis, ethnicity, and corticosteroids use).[15,16]
Few data are available with respect to incidence of infections during sarcoidosis, but the risk is considered to be low. In the present study, the incidence of severe infections was of 0.71% persons-year and occurred in 5.1% of our cohort of patients with proven sarcoidosis. Mycobacterial infections occurred in 2.4% of our cohort.
Winterbauer and Kraemer[16] reported 5 (4.1%) opportunistic infections [3 cases of pulmonary aspergilloma, one case of pulmonary tuberculosis and one patient with disseminated herpes zoster infection] in a cohort of 122 patients over 7.2 years. Rubinstein et al[10] did not report any infection in 197 sarcoidosis patients followed 18 months in the 1980s. Baughman[9] reported 7 (0.9%) fungal infections in 753 patients over a 18 month period and with an incidence rate 0.6 per persons per year.
A systematic literature review conducted between 1966 and 2004 documented 65 cases reports of sarcoidosis complicated by opportunistic infection.[17] Cryptococcus was the most reported infection with 41 cases (59%) followed by mycobacterial infections (13%), nocardiosis (11%), histoplasmosis and pneumocystosis (9%), and aspergillosis (7%). Before the diagnosis of infection, patients were taking corticosteroids and immunosppressant in 50% and 3%, respectively. There were no data relative to CD4 T cell lymphocytopenia and the outcome was good as all patients improved under anti-infectious treatment. A retrospective study has reported 18 cases of cryptococcosis complicating sarcoidosis and reviewed 72 cases of the literature. They occurred in treatment naïve sarcoidosis patients in one-third of the cases. The mean CD4 lymphocyte count was 145/mm3 (range 55–1300). Risk factors for infection included extra-thoracic sarcoidosis and treatment with corticosteroids.[16] More recently, Lefaucheur et al[18] reported 10 cases of progressive multifocal encephalopathy associated with sarcoidosis and 20 observations from literature. The mean CD4 lymphocyte cell count was 235 ± 142 mm3), 10 patients were naive of treatment, and 17 (57%) patients died.
In the present study, we describe 39% mycobacterial infections (14/36), 28% fungal infections (10/36), 22% bacterial infection (8/36), 8% viral infection (8/36), and 3% parasitic infection (1/36). Fungal complications were the most frequent nonmycobacterial infections in our experience. This is consistent with previous report in the literature.[19] However, the type of pathogens found was different; Pneumocystis jirovecci was the main fungal complication in our cohort. This was likely due to our recruitment of severe extra-pulmonary sarcoidosis patients frequently treated by glucocorticosteroids. In contrast, Cryptococcus and Aspergillus pathogens have been largely described to be associated with sarcoidosis in the literature. In France, sarcoidosis represents 2.9% of HIV negative cryptococcosis.[6] A recent French series reported 18 cases of cryptococcosis and 72 additional cases from the literature.[7] Two-third of patients were treated by corticosteroids at the time of infection. Bacterial infections were evidenced in 22% of cases. They included mainly severe gram-negative infections (i.e., P aeruginosa, or E coli). Bacterial complications associated with sarcoidosis reported in the literature included mainly nocardiosis and M tuberculosis or atypical mycobacterial infections. In our series, mycobacterial infection was noted in 2.4% (14/585) of our patient with biopsy proven sarcoidosis. Viral infection was the fourth cause of infection and accounted for 1 of the 2 deaths due to PML. PML has been previously reported during sarcoidosis and also in treatment-naive patients. Sarcoidosis was found to account for 8–9% of the causes of PML.[20–22] Jamilloux et al[8] have reported that the mortality rate of PML was higher in sarcoidosis than in human immunodeficiency virus (HIV) infected patients treated by HAART (highly active antiretroviral therapy). We described one Kaposi disease that required a decrease in corticosteroids and immunosuppressant therapies to be controlled. This may represent a serious therapeutic issue especially in severe and difficult to treat patients with sarcoidosis.
Parasitic infections in sarcoidosis are anecdotal with only 2 reports of strongyloides infection in the literature. In our study, one case of disseminated strongyloidiasis was observed in a patient treated by corticosteroids and who was coming from an endemic area. It is important to consider and treat occult infection before initiating immunosuppressive treatments in these high risk patients.
Almost all severe nonmycobacterial infections occurred in patients treated with corticosteroids and/or immunosuppressive therapies, contrasting with previous reports who described opportunistic infections in treatment-naive patients with sarcoidosis.[7,8] This discrepancy might be due to the specific recruitment in our internal medicine department including high proportion of severe extra-pulmonary forms needing immunosuppressive therapies and steroids in most cases.
Conditionally on sex, age at diagnosis, ethnicity and corticosteroids use, we identified 2 types of risk factors associated with severe infection: treatment and localization of sarcoidosis. Treatment with ≥ 3 immunosuppressants or cyclophosphamide was significantly associated with severe infections (OR = 3.8 and 5.6, respectively). In addition, neurosarcoidosis, and cardiac sarcoidosis were associated with severe infection (OR = 3.4, and 2.7, respectively).
In rheumatoid arthritis, TNFα inhibitors have been associated with a 2- to 4-fold increased risk of serious bacterial infections and also with an increased risk of opportunistic infection.[12] In our study, the number of patients treated by TNFα inhibitors (4% of the cohort) was too low to draw any conclusion. Patients with severe sarcoidosis have been associated with increased risk for infections. Bernard et al have reported that cryptococcosis was more frequent in patients with neuro-sarcoidosis (28 vs. 8%), and cardiac involvement (17 vs. 0%).[7] In the present study, these 2 localizations were clearly associated with higher risk of severe infection.
The median level of CD4 lymphocytes was of 204/mm3 at the time of severe infection. Peripheral CD4 lymphocytopenia has been reported in up to 40% of sarcoidosis patients and significant peripheral lymphopenia is correlated with severe disease manifestations of sarcoidosis.[4,6] The 2.7% rate of severe nonmycobacterial infections reported here over 8 years of follow-up seems rather low considering this deficient immune status. However, lymphopenia is more severe during flare-ups of sarcoidosis but corrects with corticosteroids and the sequestration of lymphocytes in the granuloma is associated with the peripheral CD4 lymphocytopenia. It is still unclear if low CD4 count can be considered as a risk factor for opportunistic infections like pneumocystocis in non-HIV patients.[23] Indeed, peripheral CD4 lymphopenia is correlated to the severity of sarcoidosis.[4] However, the use of immunosuppressants is also associated with severity of disease and with a higher likelihood of infections.
Our study has some limitations. The number of cases of severe infections associated with sarcoidosis was small and precluded any complex multivariate model selection procedure to identify multiple risk factors. We therefore relied on a nested case–control design to identify factors associated with severe infection, while benefitting from the advantages of the cohort in terms of biases limitation.[24] This precluded the adjusted estimation of the association of the matching variables (sex, age at diagnosis, ethnicity, and corticosteroids use) with severe infections, which remains to be addressed in a larger cohort with a greater number of events, allowing complex multivariate modelling. The number of patients treated by TNFα inhibitors was too low to draw any definite conclusion about its possible association with the occurrence of infections in sarcoidosis.
In conclusion, severe nonmycobacterial infections are observed in 3% and mycobacterial infection in 2% of our patients with sarcoidosis. Severe nonmycobacterial were mostly fungal and bacterial infections who represent half of all infections episodes. Although outcomes are regularly good, 2 patients (7%, 2/30) died within the 6 months following infection. Infections are likely associated with severe CD4 lymphopenia. Risk factors for severe infections included the use of ≥ 3 immunosuppressants, mainly cyclophosphamide and the neurological and cardiac localizations of sarcoidosis.
Footnotes
Abbreviations: ATS = American Thoracic Society, BAL = bronchoalveolar lavage, CD4 = cluster of differentiation 4, CI = confidence interval, CSF = cerebrospinal fluid, ePOST = extrapulmonary physician organ severity tool, ERS = European Respiratory Society, HAART = highly active antiretroviral therapy, HIV = human immunodeficiency virus, IQR = interquartile range, JC virus = John Cunningham virus, OR = odds-ratios, PCR = polymerase chain reaction, PML = progressive multifocal leukoencephalopathy, TNF α = tumor necrosis factor alpha, WASOG = World Association of Sarcoidosis and Other Granulomatous disorder.
PC and DS are co-senior authors and DA and CC are the first co-authors
The authors have no conflicts of interest to disclose.
References
- [1].Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet 2014;383:1155–67. [DOI] [PubMed] [Google Scholar]
- [2].Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis. Am J Respir Crit Care Med 2004;170:1324–30. [DOI] [PubMed] [Google Scholar]
- [3].Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis, and therapeutics. JAMA 2011;305:391–9. [DOI] [PubMed] [Google Scholar]
- [4].Sweiss NJ, Salloum R, Gandhi S, et al. Significant CD4, CD8, and CD19 lymphopenia in peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PloS One 2010;5:e9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dixon WG, Watson K, Lunt M, et al. British Society for Rheumatology Biologics Register Control Centre Consortium. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2006;54:2368–76. [DOI] [PubMed] [Google Scholar]
- [6].Jamilloux Y, Valeyre D, Lortholary O, et al. The spectrum of opportunistic diseases complicating sarcoidosis. Autoimmun Rev 2015;14:64–74. [DOI] [PubMed] [Google Scholar]
- [7].Bernard C, Maucort-Boulch D, Varron L, et al. Cryptococcosis in sarcoidosis: cryptOsarc, a comparative study of 18 cases. QJM 2013;106:523–39. [DOI] [PubMed] [Google Scholar]
- [8].Jamilloux Y, Néel A, Lecouffe-Desprets M, et al. Progressive multifocal leukoencephalopathy in patients with sarcoidosis. Neurology 2014;82:1307–13. [DOI] [PubMed] [Google Scholar]
- [9].Baughman RP. Fungal infections as a complication of therapy for sarcoidosis. QJM 2005;98:451–6. [DOI] [PubMed] [Google Scholar]
- [10].Rubinstein I, Baum GL, Rosenthal T. Fungal infections complicating pulmonary sarcoidosis. J Infect Dis 1985;152:1360. [PubMed] [Google Scholar]
- [11].Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J 1999;14:735–7. [DOI] [PubMed] [Google Scholar]
- [12].Galloway JB, Hyrich KL, Mercer LK, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology 2011;50:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis Off Publ Infect Dis Soc Am 2017;64:87–91. [DOI] [PubMed] [Google Scholar]
- [14].Judson MA, Baughman RP, Costabel U, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J 2008;31:1189–96. [DOI] [PubMed] [Google Scholar]
- [15].Chew L-C, Maceda-Galang LM, Tan YK, et al. Pneumocystis jirovecii pneumonia in patients with autoimmune disease on high-dose glucocorticoid. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis 2015;21:72–5. [DOI] [PubMed] [Google Scholar]
- [16].Winterbauer RH, Kraemer KG. The infectious complications of sarcoidosis: a current perspective. Arch Intern Med 1976;136:1356–62. [PubMed] [Google Scholar]
- [17].Girard N, Cottin V, Hot A, et al. Opportunistic infections and sarcoidosis. Rev Mal Respir 2004;21(6 pt 1):1083–90. [DOI] [PubMed] [Google Scholar]
- [18].Lefaucheur R, Ahtoy P, Bouwyn JP, et al. Progressive multifocal leukoencephalopathy in patients with sarcoidosis. Neurology 2014;83:1301–2. [DOI] [PubMed] [Google Scholar]
- [19].Dhote R, Abad S, Valeyre D. Complications infectieuses de la sarcoïdose. Presse Médicale 2009;38:317–23. [DOI] [PubMed] [Google Scholar]
- [20].Aksamit AJ. Review of progressive multifocal leukoencephalopathy and natalizumab. Neurologist 2006;12:293–8. [DOI] [PubMed] [Google Scholar]
- [21].Rosenbloom MA, Uphoff DF. THe association of progressive multifocal leukoencephalopathy and sarcoidosis. Chest 1983;83:572–5. [DOI] [PubMed] [Google Scholar]
- [22].Richardson EP. Our evolving understanding of progressive multifocal leukoencephalopathy. Ann N Y Acad Sci 1974;230:358–64. [DOI] [PubMed] [Google Scholar]
- [23].Iriart X, Challan Belval T, Fillaux J, et al. Risk factors of Pneumocystis pneumonia in solid organ recipients in the era of the common use of posttransplantation prophylaxis. Am J Transplant 2015;15:190–9. [DOI] [PubMed] [Google Scholar]
- [24].Sedgwick P. Nested case-control studies: advantages and disadvantages. BMJ 2014;348:g1532–1532. [DOI] [PubMed] [Google Scholar]


