Supplemental Digital Content is available in the text
Keywords: anesthetics, critical care, inhalation, meta-analysis, sedation
Abstract
Background:
Volatile sedation in the intensive care unit (ICU) may reduce the number of adverse events and improve patient outcomes compared with intravenous (IV) sedation. We performed a systematic review and meta-analysis comparing the effects of volatile and IV sedation in adult ICU patients.
Methods:
We searched the PubMed, Embase, Cochrane Central Register, and Web of Science databases for all randomized trials comparing volatile sedation using an anesthetic-conserving device (ACD) with IV sedation in terms of awakening and extubation times, lengths of ICU and hospital stay, and pharmacologic end-organ effects.
Results:
Thirteen trials with a total of 1027 patients were included. Volatile sedation (sevoflurane or isoflurane) administered through an ACD shortened the awakening time [mean difference (MD), −80.0 minutes; 95% confidence intervals (95% CIs), −134.5 to −25.6; P = .004] and extubation time (MD, −196.0 minutes; 95% CIs, −305.2 to −86.8; P < .001) compared with IV sedation (midazolam or propofol). No differences in the lengths of ICU and hospital stay were noted between the 2 groups. In the analysis of cardiac effects of sedation from 5 studies, patients who received volatile sedation showed lower serum troponin levels 6 hours after ICU admission than patients who received IV sedation (P < .05). The effect size of troponin was largest between 12 and 24 hours after ICU admission (MD, −0.27 μg/L; 95% CIs, −0.44 to −0.09; P = .003).
Conclusion:
Compared with IV sedation, volatile sedation administered through an ACD in the ICU shortened the awakening and extubation times. Considering the difference in serum troponin levels between both arms, volatile anesthetics might have a myocardial protective effect after cardiac surgery even at a subanesthetic dose. Because the included studies used small sample sizes with high heterogeneity, further large, high-quality prospective clinical trials are needed to confirm our findings.
1. Introduction
Suboptimal sedation in critically ill patients is associated with adverse events, high costs, and increases in morbidity and mortality.[1–3] The current sedation guidelines, which are updated periodically, are based on intravenous (IV) agents.[2] However, the updated sedation practices with IV agents are problematic due to adverse effects such as accumulation, tolerance, withdrawal, delirium, and hemodynamic instability.[4–9]
Volatile anesthetic agents used in general anesthesia have also been used as sedatives due to favorable pharmacokinetics such as rapid elimination via pulmonary exhalation, limited hepatic metabolism, and no accumulation.[10–12] Moreover, the perioperative organ protective effects of volatile anesthetic agents, especially on the heart, have been confirmed through the mechanisms of ischemic pre- and post-conditioning.[13–17] Nevertheless, the use of volatile sedation in the intensive care unit (ICU) has been limited due to intensivists’ lack of familiarity with these agents, emergence agitation, postoperative nausea and vomiting (PONV), and nephrotoxicity from inorganic fluoride.[18–22] Most importantly, volatile sedation in the ICU has been limited by technical problems, including the wasting of volatile agents by high-flow ICU ventilators and atmospheric contamination by open ventilator circuits.[23]
Volatile sedation in the ICU is becoming increasingly popular due to fewer technical problems since the development of anesthetic reflectors, such as AnaConDa (SEDANA Medical, Uppsala, Sweden) and Mirus (Pall Medical, Dreieich, Germany), which reduce volatile agent wasting.[24,25] Once these anesthetic reflectors were commercially available, several small randomized controlled trials were published comparing the effects of volatile and conventional IV sedative agents in the ICU.[26–38] Therefore, we performed a systematic review and meta-analysis of randomized controlled trials (RCTs) using these new anesthetic reflectors (AnaConDa and Mirus) to evaluate whether volatile sedation is associated with improved outcomes compared with IV sedation in adult ICU patients.
2. Materials and methods
2.1. Literature search
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews and meta-analyses of RCTs.[39] This study did not require ethical approval because it was an analysis of previously published studies. Two independent reviewers (JMK and HYK) separately searched the PubMed, Embase, Cochrane Central Register, and Web of Science of Controlled Trials databases for all studies, regardless of language, published before May 31, 2017. The search terms used were: (“sevoflurane” OR “isoflurane” OR “desflurane” OR “anesthetic conserving device” OR “AnaConDa” OR “Mirus”) AND ‘sedation” AND (“critical care” OR “intensive care”). Additional studies were identified by manually searching the references of the original studies.
2.2. Study selection
We included RCTs and quasi-RCTs of patients who underwent sedation in the ICU. The inclusion criteria, based on the Patient, Intervention, Comparator, Outcomes, and Study (PICOS) design criteria, were as follows: patient: adult patients (≥18 years) who underwent sedation in the ICU; intervention: patients sedated with volatile sedatives (sevoflurane, isoflurane, or desflurane) via an AnaConDa or Mirus reflector; comparator: patients sedated with IV sedatives; at least 1 primary outcome [awakening time, extubation time, length of stay (LOS) in the ICU, or LOS in the hospital] or secondary outcomes (myocardial effects, renal effects, incidence of delirium, or incidence of PONV); and study design: RCT or quasi-RCT. Observational studies, retrospective studies, case reports, letters, reviews, and abstracts were excluded.
2.3. Data extraction and outcome measurement
The 2 reviewers (JMK and HYK) selected all datasets for this study. Disagreements were resolved by discussion and consensus. Authors of potentially relevant studies were contacted for further information if the relevant data were not published. Among the primary outcomes, awakening time was defined as the time (in minutes) from the termination of sedative administration to awakening. Extubation time was defined as the time (in minutes) from the termination of sedative administration to extubation. The LOS in the ICU and hospital were defined as the hours and the days from admission to discharge. Among the secondary outcomes, myocardial effects were determined by examining serum troponin (μg/L) and serum N-terminal prohormone of brain natriuretic peptide (NT-proBNP) (pg/mL) levels after ICU admission. The serum creatinine (mg/dL) level on the first postoperative day was used as a measure of the renal effects. The incidences of delirium and PONV were recorded as the number of patients who experienced these effects during the post-sedation period. If studies had more than 1 volatile or IV sedation arm, the arms were combined such that there was only 1 volatile and 1 sedation arm.
2.4. Quality assessment
Two reviewers assessed the articles and investigated the risk of bias for RCTs using the Risk of Bias tool from the Cochrane Collaboration.[40] The 7 different domains were as follows: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, selective reporting, and other bias. The risk of bias for each trial was reported as “low,” “unclear,” or “high.” In the allocation concealment domain, we considered the difficulty in ensuring complete blinding of a caregiver when administering sedation to a patient via anesthetic reflectors or IV. The primary outcomes, such as awakening and extubation times and LOS in the ICU and hospital, were estimated according to the robustness of the study protocol. If the trial had objective criteria, such as a targeted sedation level or plans for stopping sedation and starting ventilator weaning, the risk of bias was rated as low despite the lack of blindness. For PONV and delirium outcomes, we also evaluated whether the method of measurement was objective. In the selective reporting domain, we evaluated bias based on protocols from http://www.clinicaltrials.gov and outcomes that were expressed in the methods. For other bias domains, we considered the influence of sponsors. If the trials received financial assistance from a medical instrument or pharmaceutical company, the risk of bias was rated as “unclear.” Review Manager software (RevMan; version 5.3) was used to present the risk of bias.
2.5. Data synthesis and statistical analysis
Data that were reported as median and range were changed to mean and standard deviation.[41] Data that were not reported numerically in the original articles were extracted from the figures. Measurement units were standardized. Troponin I levels were converted to troponin T levels using a conversion factor of 0.65/2, based on the ratio of the upper limit and previous literature.[16] Units of serum creatinine levels were converted to mg/dL. Meta-analyses were performed to calculate the pooled mean difference (MD) for continuous data or the odds ratio (OR) for dichotomous data with 95% confidence intervals (95% CIs) using either a fixed effects or random effects model. Heterogeneity was assessed using the Cochrane Q test and I2 statistics.[42] The fixed effects model was used for meta-analysis unless at least 4 studies were included and the I2 exceeded 50, at which point the random effects model was used. In addition, subgroup analyses were performed in primary outcomes showing substantial heterogeneity to identify the influence of sedation duration, patient type, financial support, and type of IV agents. Differences in effect size between subgroups were analyzed with a meta-regression model. Publication bias was evaluated using the Egger regression test and a funnel plot.[43] If the outcomes showed significant publication bias, then the trim and fill method was used for additional analyses. All statistical analyses were performed using the meta-analysis package for R ver. 3.3.2 (metaphor; Vienna, Austria; http://www.R-project.org).[44]
3. Results
3.1. Study selection
A flow chart illustrating the study selection process is shown in Fig. 1. We retrieved 1532 records in our initial search. After removing 459 duplicates, we excluded 1023 other records for the following reasons: non-ICU or nonvolatile sedation studies (n = 547), pediatric patients (n = 242), reviews or meta-analyses (n = 70), case reports, comments, letters, or conference papers (n = 68), abstracts only (n = 61), and animal studies (n = 35). Of the 50 potentially eligible studies, we excluded 37 because they did not meet the PICOS criteria. Ultimately, 13 RCTs published between November 2004 and May 2017 were included in the meta-analysis.
Figure 1.
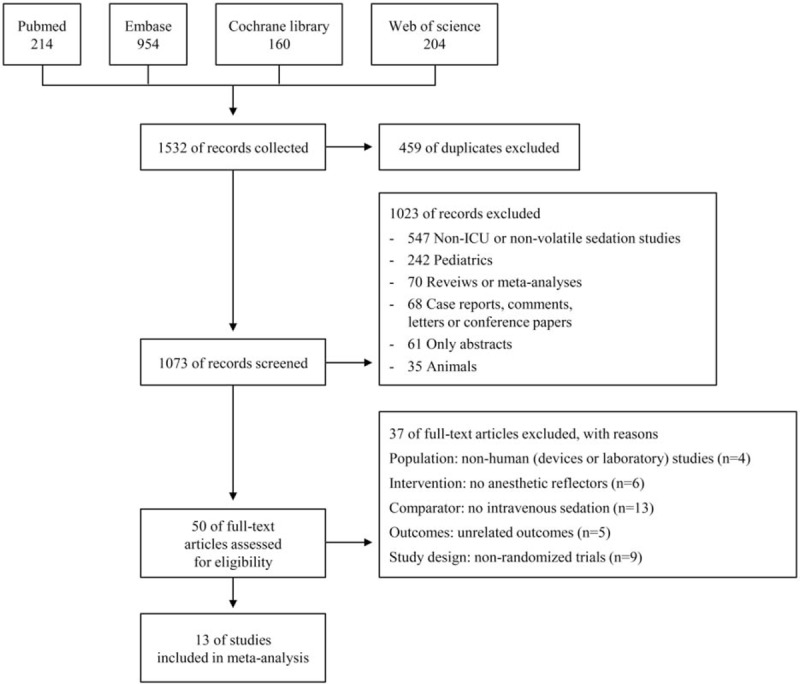
Flow diagram depicting the study selection process.
3.2. Characteristics of the included studies
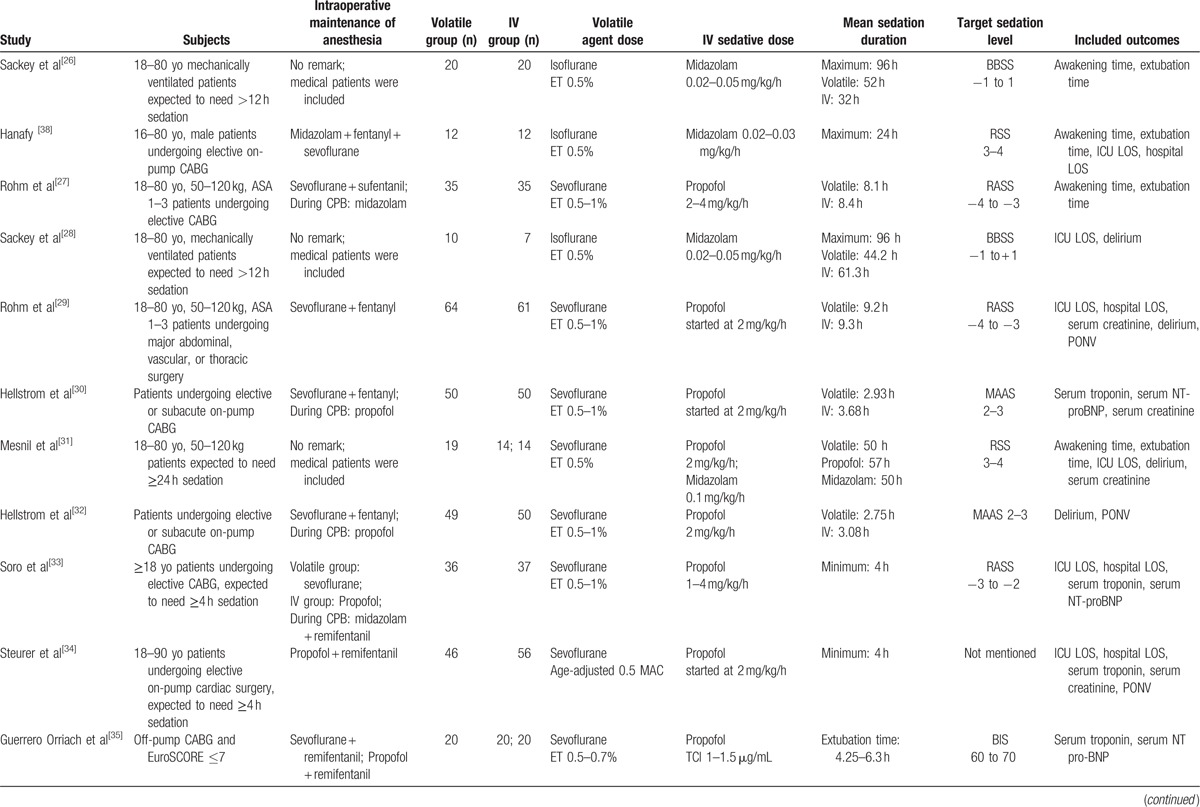
Thirteen studies[26–38] were included in the analysis. Two sets of studies ([27,29] and [30,32]) were assumed to be the same trials based on their clinical trial numbers (http://www.clinicaltrials.gov). Because the outcomes overlapped in 2 of these studies,[27,29] outcomes were extracted from the study with the larger sample size.[29] Because 1 study[32] represented outcomes of continuous variables as medians and interquartile ranges without the first and third quartiles, these outcomes were excluded and only outcomes with categorical variables, such as the incidences of delirium and PONV, were included in our meta-analysis. Three studies were performed in mixed medical-surgical ICUs,[26,28,31] while the remaining 10 studies were performed in surgical ICUs consisting of only postsurgical patients.[27,29,30,32–38] Of the 10 studies performed in surgical ICUs, 1 study included patients who underwent major abdominal, vascular, or thoracic surgery,[29] while the other 9 studies only enrolled patients who underwent cardiac surgery.[27,30,32–38] All included studies used the AnaConDa device in the volatile sedation arm; there was no RCT using the Mirus device. Among the 13 included studies, 9 compared sevoflurane with propofol,[27,29,30,32–37] 3 compared isoflurane with midazolam,[26,28,38] and 1 compared sevoflurane with propofol and midazolam.[31] We did not consider the type of anesthetics used intraoperatively. The characteristics of the included studies are summarized in Table 1 . The details on the sedation scales used in the included studies are listed in Table S1, Supplemental Content.
Table 1.
Characteristics of the included studies.
3.3. Quality assessment
The 13 included studies were evaluated using the Risk of Bias tool (Fig. 2). Although 2 sets of studies were identified as being the same trials ([27,29] and [30,32]), the risk of bias in these studies was assessed independently because the included outcomes did not coincide. Detailed information on the risk of bias assessment is presented Table 2 .
Figure 2.
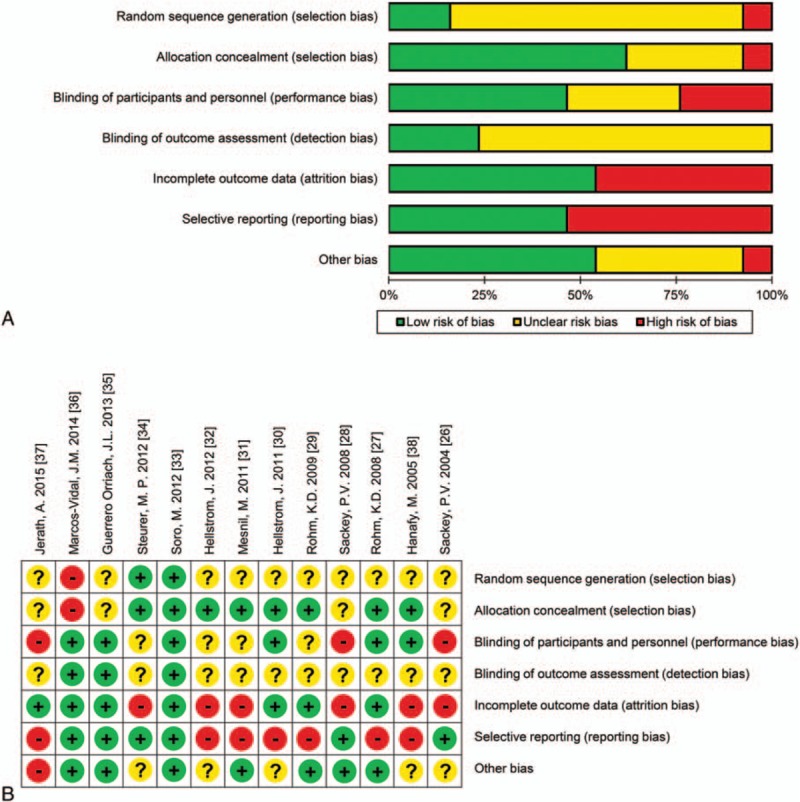
Risk of bias graph (A) and summary (B) of the included studies. + indicates a low risk of bias, − indicates a high risk of bias, and? indicates an unclear risk of bias.
Table 1 (Continued).
Characteristics of the included studies.
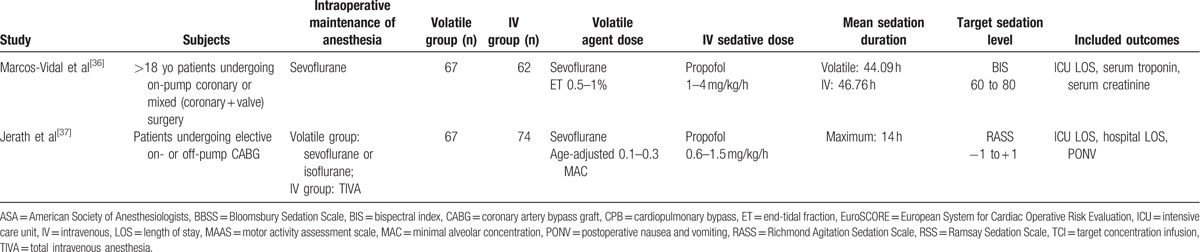
Table 2.
Assessment of risk of bias.
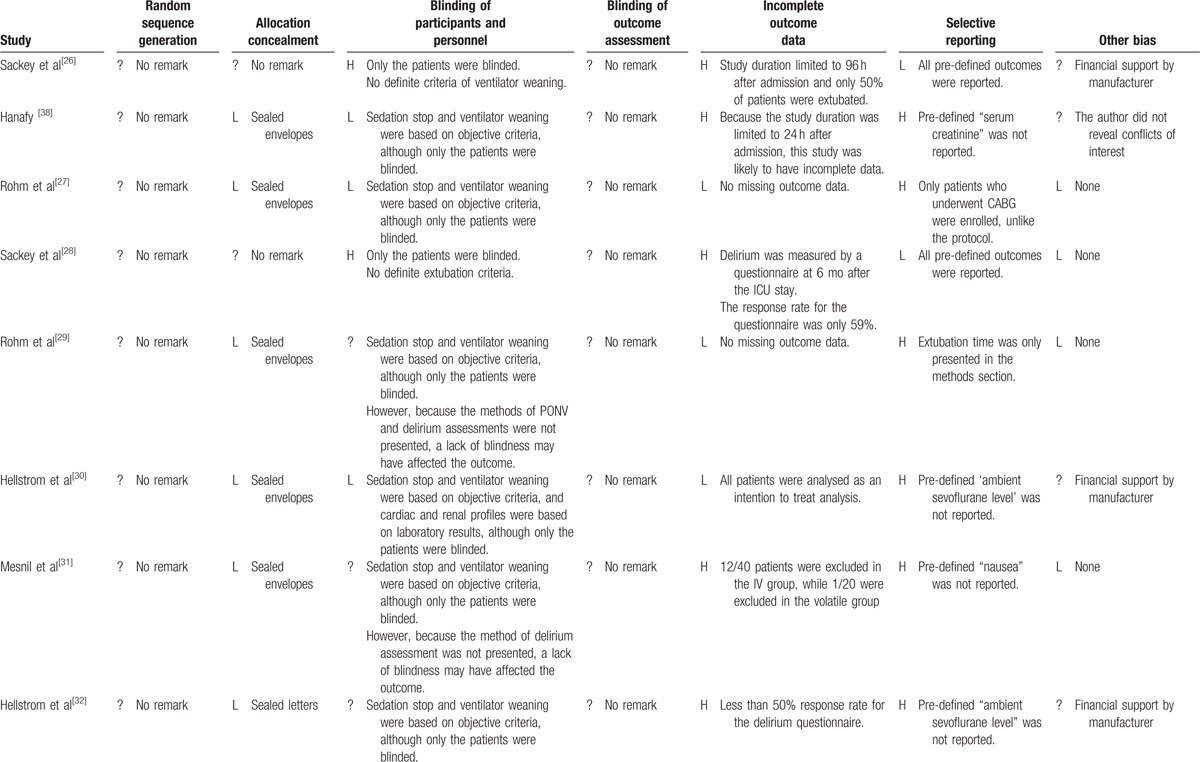
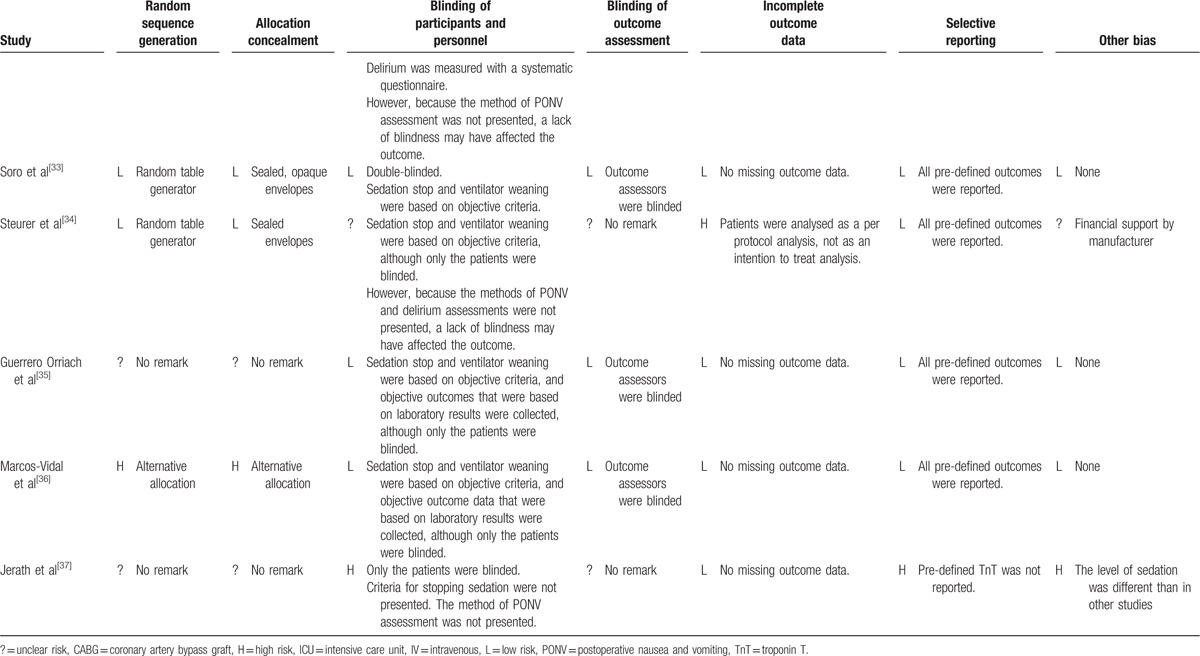
Table 2 (Continued).
Assessment of risk of bias.
3.4. Primary outcomes
3.4.1. Awakening time
The 4 studies that examined awakening time included a total of 181 patients, with 86 in the volatile sedation arm and 95 in the IV sedation arm.[26,27,31,38] Our analysis using the random effects model showed that the awakening time was significantly shorter for volatile sedation than for IV sedation (MD, −80.1 minutes; 95% CIs, −134.5 to −25.6; P = .004; I2 = 95%; Fig. 3). Subgroup analyses were performed to explore possible sources of heterogeneity. In subgroup analyses, the pooled effect sizes were smaller in the short-term (≤24 hours) sedation group[29,38] (MD, −41.7 minutes, 95% CIs, −51.2 to −32.1; P < .001; I2 = 0%) than the long-term (>24 hours) sedation group[26,31] (MD, −133.1 minutes, 95% CIs, −170.7 to −95.5; P < .001; I2 = 54%) (Figure S1, Supplemental Content). The pooled effect sizes between subgroups were significantly different (P < .001) using meta-regression.
Figure 3.
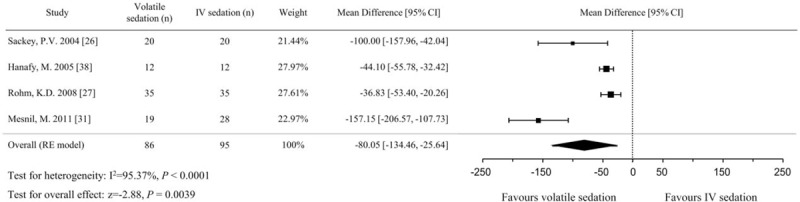
Forest plot of the mean differences and 95% confidence intervals (CIs) for awakening time (in min) in the volatile and IV sedation groups. Data were analyzed using a random effects model.
3.4.2. Extubation time
Although 6 studies presented the extubation time as an outcome,[26,27,31,32,37,38] 2 studies were excluded due to ambiguous start time measurements[37] and different data representation.[32] There were 181 patients (86 in the volatile sedation arm and 95 in the IV sedation arm) in the 4 included studies.[26,27,31,38] In the pooled analysis using the random effects model, volatile sedation significantly shortened the extubation time compared with IV sedation (MD, −196.0 minutes; 95% CIs, −305.2 to −86.8; P < .001; I2 = 90%; Fig. 4). In subgroup analyses, the short-term (≤24 hours) sedation group[29,38] showed a smaller effect on extubation time (MD, −108.5 minutes, 95% CIs, −125.2 to −91.9; P < .001; I2 = 52%) than the long-term (>24 h) sedation group[26,31] (MD, −284.4 minutes, 95% CIs, −388.9 to −179.9; P < .001; I2 = 54%) (Figure S2, Supplemental Content). The pooled effect sizes between subgroups were also significantly different (P = .006) using meta-regression. Additional subgroup analyses according to whether or not the studies received financial support from a medical instrument or pharmaceutical company (P = .911) or which IV agent was used (propofol vs midazolam) (P = .542) did not show any significant differences using meta-regression, and heterogeneity remained high at >80%.
Figure 4.
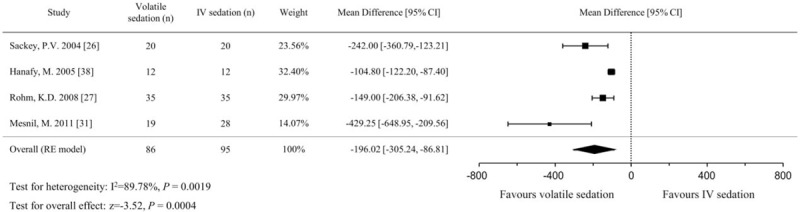
Forest plot of the mean differences and 95% confidence intervals (CIs) for extubation time (in min) in the volatile and IV sedation groups. Data were analyzed using a random effects model.
3.4.3. LOS in the ICU and hospital
A total of 658 patients (321 in the volatile sedation arm and 337 in the IV sedation arm) from 8 studies[28,29,31,33,34,36–38] were included in the analysis of LOS in the ICU. The pooled analysis using the fixed effects model did not show a significant difference between volatile and IV sedation in terms of LOS in the ICU (MD, −0.9 hours; 95% CIs, −3.6 to 1.8; P = .513; I2 = 0%; Fig. 5). For the analysis of LOS in the hospital, 465 patients (225 in the volatile sedation arm and 240 in the IV sedation arm) from 5 studies were identified.[29,33,34,37,38] The pooled effect sizes were comparable between both arms using the fixed effects model (MD, −0.5 hours; 95% CIs, −1.0 to 0.0; P = .059; I2 = 0%; Fig. 6).
Figure 5.
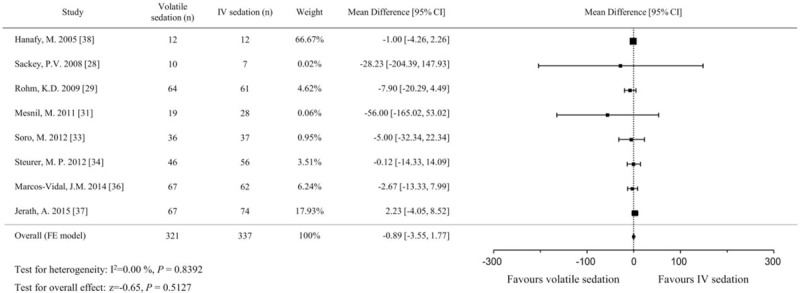
Forest plot of the mean differences and 95% confidence intervals (CIs) for length of stay (in h) in the intensive care unit in the volatile and IV sedation groups. Data were analyzed using a fixed effects model.
Figure 6.
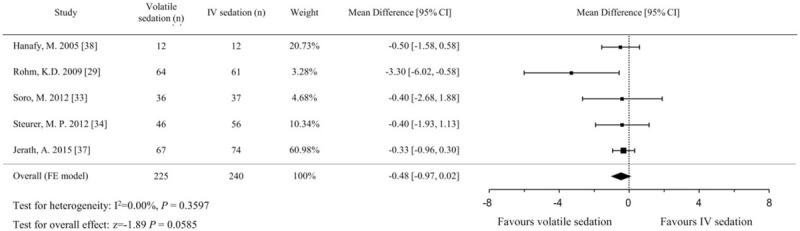
Forest plot of the mean differences and 95% confidence intervals (CIs) for length of stay (in d) in the hospital in the volatile and IV sedation groups. Data were analyzed using a fixed effects model.
3.5. Secondary outcomes
3.5.1. Myocardial and renal effects
Five studies with a total 464 patients used serum troponin T[30,34,36] or troponin I[33,35] levels as a marker of cardiac injury. Serum troponin I levels were converted to troponin T levels according to a predefined formula (Troponin T = Troponin I ∗ 0.65/2). All of the patients in the eligible studies underwent cardiac surgery and were sedated with low-dose sevoflurane (end-tidal concentration 0.5–1%) or propofol (1–4 mg/kg/h) after admission to the ICU. Because each trial measured the serum troponin at different time points after ICU admission, we analyzed the data by dividing them into time intervals as follows: 0 to 6, 6 to 12, 12 to 24, and 24 to 48 hours after ICU admission. The serum troponin levels were significantly lower in the volatile sedation arm than the IV sedation arm at the 6 to 12, 12 to 24, and 24 to 48-hour intervals, but not at the 0 to 6-hour time interval (Fig. 7). The effect size was largest in the 12 to 24-hour time interval (MD, −0.27 μg/L; 95% CIs, −0.44 to −0.09; P = .003; I2 = 73%). Serum NT-proBNP on the first postoperative day was recorded in 3 studies[30,33,35] and was significantly lower in the volatile sedation arm than the IV sedation arm (MD, −711.6 pg/mL; 95% CIs, −904.9 to −518.3; P < .001; I2 = 90%, Fig. 8).
Figure 7.
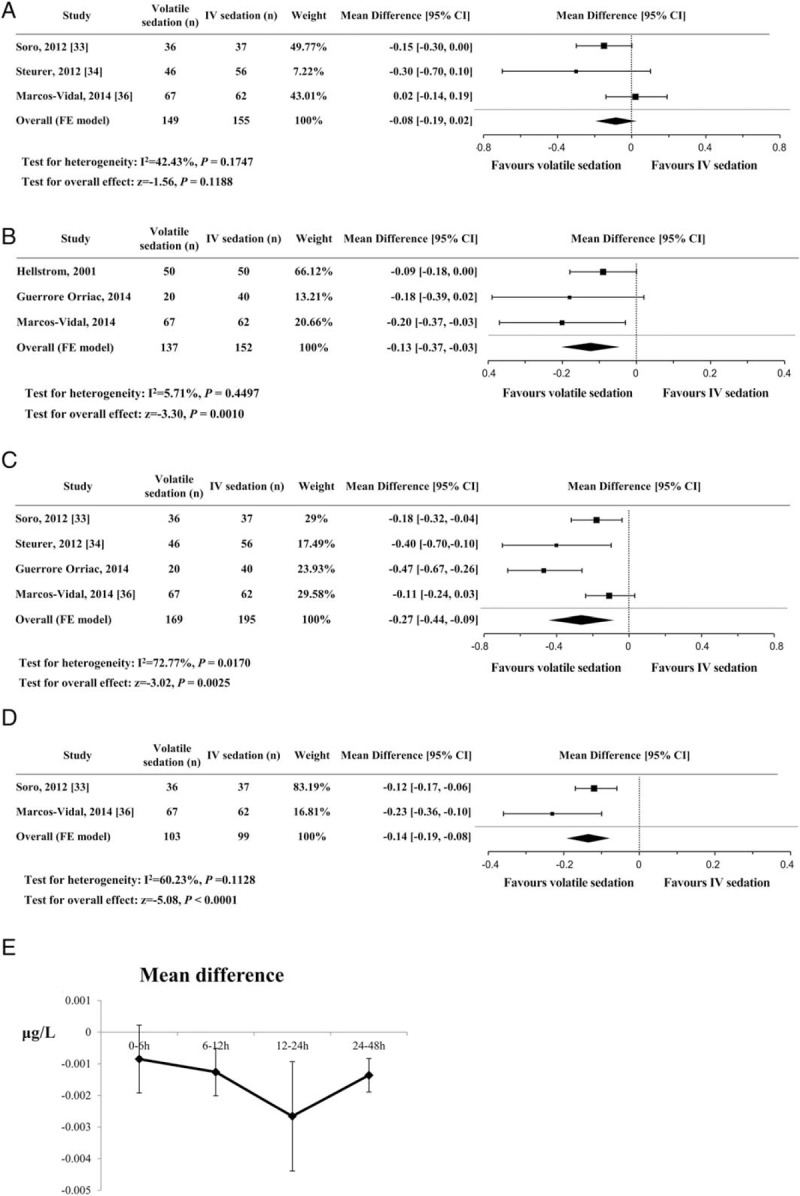
Forest plot of the mean differences and 95% confidence intervals (CIs) for serum troponin levels (μg/L) at different time points after ICU admission. The data were analyzed by dividing them into time intervals as follows: (A) 0–6 h, (B) 6–12 h, (C) 12–24 h, and (D) 24–48 h after ICU admission. (E) The line represents the difference in means and the vertical bar represents 95% confidence intervals for serum troponin levels (vertical axis) at different time points after ICU admission (horizontal axis).
Figure 8.
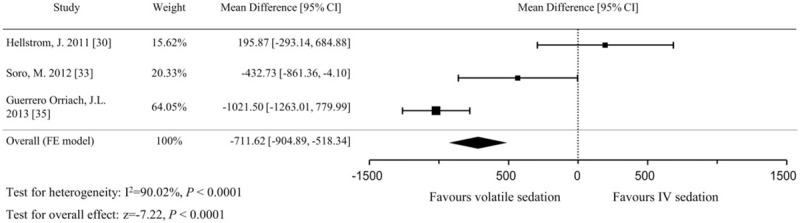
Forest plot of the mean differences and 95% confidence intervals (CIs) for serum N-terminal prohormone of brain natriuretic peptide levels (pg/mL) on the first postoperative day. Data were analyzed using a fixed effects model.
Renal effects of sedatives were assessed by measuring serum creatinine levels on the first postoperative day. The 5 included studies[29–31,34,36] consisted of 489 patients with 246 in the sevoflurane arm and 243 in the propofol arm. Although no study showed a significant difference, the pooled analysis showed a lower serum creatinine level in the sevoflurane arm compared with the propofol arm (MD, −0.05 mg/dL; 95% CIs, −0.10 to −0.002; P = .043; I2 = 44%, Fig. 9).
Figure 9.
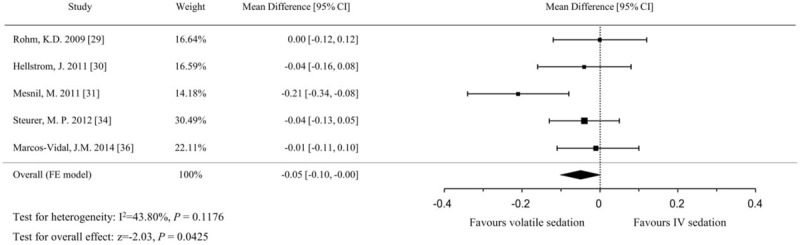
Forest plot of the mean differences and 95% confidence intervals (CIs) for serum creatinine levels (mg/dL) on the first postoperative day. Data were analyzed using a fixed effects model.
3.5.2. Delirium and PONV
Four studies evaluated the incidence of delirium by clinician observations[29,31] or patient questionnaires.[28,32] A significantly lower incidence of delirium was identified in the volatile sedation arm (OR, 0.47; 95% CIs, 0.23–0.94; P = .033, I2 = 0%) compared with the IV sedation arm (Fig. 10). The incidence of PONV from 4 studies,[29,32,34,37] which compared sevoflurane sedation with propofol sedation in postsurgical patients, was comparable between the volatile and IV sedation arms (OR, 1.58; 95% CIs, 0.97–2.58; P = .068; I2 = 0%, Fig. 11).
Figure 10.
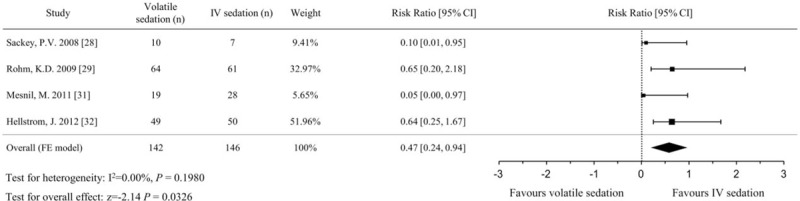
Forest plot of the risk ratio and 95% confidence intervals (CIs) for the incidence of delirium in the volatile and IV sedation groups. Data were analyzed using a fixed effects model.
Figure 11.
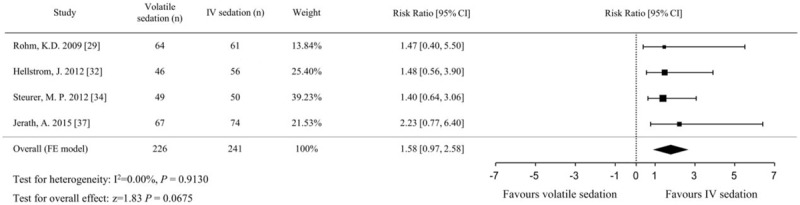
Forest plot of the risk ratio and 95% confidence intervals (CIs) for the incidence of postoperative nausea and vomiting in the volatile and IV sedation groups. Data were analyzed using a fixed effects model.
3.6. Publication bias
Although the number of included studies for each outcome was small, we evaluated publication bias using the Egger regression test and a funnel plot. Adjustment using the trim and fill method was performed for the extubation time and incidence of delirium, which showed a positive publication bias. The extubation time after including 2 imputed studies to improve asymmetry still showed a significant reduction in the volatile sedation arm compared with the IV sedation arm (MD, −108.5 minutes; 95% CIs, −124.8 to −92.3; P < .001). The incidence of delirium after including 1 imputed study did not show any difference between the 2 arms (OR, 0.54; 95% CIs, 0.27–1.05; P = .070) after adjustment.
4. Discussion
Our systematic review and meta-analysis of 13 RCTs revealed that sedation in the ICU with volatile anesthetic agents compared with conventional IV sedatives, such as propofol or midazolam, shortened the awakening time by 80 minutes and the extubation time by 196 minutes. Despite these reductions in awakening and extubation times with volatile sedation, no reductions in the LOS in the ICU or hospital were noted. Compared with IV sedation, volatile sedation showed lower serum troponin and NT-proBNP levels, beginning around 6 hours after ICU admission, although cardiac function was not directly evaluated.
After the introduction of volatile sedation, there have been several recent meta-analyses in ICU patients[45,46] and postcardiac surgical patients.[47] However, these meta-analyses included volatile sedation using a conventional vaporizer, which had significantly slow anesthetic wash-out times compared with the new anesthetic reflectors using the same fresh gas flow rates; this is because the conventional vaporizer could not be removed from the breathing circuit.[48,49] In addition, the time difference of more than 5 years between studies using conventional vaporizers versus the new anesthetic reflectors might have been influenced by changes in the sedation guidelines. Therefore, we selected only studies that used the new anesthetic reflectors. Because no RCT used the Mirus device, our meta-analysis included only RCTs using the AnaConDa device.
The results of our pooled analysis, as well as each included study, showed significantly shorter awakening and extubation times in the volatile sedation arm than in the IV sedation arm, regardless of whether propofol or midazolam was used as the conventional IV sedative. The rapid elimination of volatile anesthetics via pulmonary exhalation, lack of accumulation, and increased control of the drug concentration by monitoring end-tidal fractions are likely explanations for these results.[10,11] For the volatile agents, analgesic effects induced by N-methyl-D-aspartate antagonist activity[31] may have contributed to opioid-sparing effects and shorter awakening and extubation times. However, there was a lack of criteria for controlling pain. In addition, various types of analgesics such as acetaminophen, morphine, remifentanil, and sufentanil were used in this study. Therefore, further evaluations are needed to elucidate opioid-sparing effects of volatile agents.
Meanwhile, our pooled effect size in extubation time (196 minutes) was larger than was seen in previous meta-analyses, which showed pooled effect sizes of 52.7 minutes[45] and 76 minutes.[47] This result may be explained by the fact that we only included studies using the AnaConDa device and excluded 2 studies of extubation time from our analysis due to ambiguous time measurements[37] and different data representations.[30] Despite the more consistent selection of studies, however, substantial heterogeneity (I2 = 90%) remained. To identify sources of heterogeneity, we performed additional subgroup analyses in awakening and extubation times according to the sedation duration [short-term (≤24 hours) vs long-term (>24 h)], patient type (cardiac surgical patients vs noncardiac surgical or medical patients), financial support (supported vs unsupported studies from a medical instrument or pharmaceutical company), and which IV agent was used (midazolam vs propofol). The subgroup analyses according to sedation duration and patient type showed reduced heterogeneity (I2 = 50–52%), and the MD was greater in the long-term sedation (> 24 h) and noncardiac surgery groups than the short-term sedation (≤24 hours) and cardiac surgery groups. Other subgroup analyses according to financial support and IV agents were comparable between the 2 sedation groups. However, all subgroup analyses should be cautiously interpreted due to the small number of included studies.
The correlation between the duration of mechanical ventilation (MV), LOS in the ICU, and complications such as increased ventilator dependency, ventilator-associated pneumonia, and ventilator-induced lung injury has been previously established.[50,51] A meta-analysis comparing ICU sedatives by Fraser et al[52] showed that non-benzodiazepine based sedation shortened the MV duration and LOS in the ICU compared with benzodiazepine-based sedation. Although volatile sedation shortened the MV duration in the present study, our meta-analysis did not indicate that volatile sedation shortened the LOS in the ICU. Before interpreting the results for LOS, we examined the length of sedation and MV duration. Here, the mean MV duration in all of the studies was within 3 days; this was different from the mean MV duration in the studies examined by Fraser et al,[52] which ranged from 3.7 to 8.4 days. Relatively short sedation periods in these studies that we included might be insufficient to reveal differences in LOS in the ICU. Therefore, additional studies with longer sedation periods and controlled conditions should be performed to examine the link between type of sedation and LOS in the ICU.
The end-organ protective effects of halogenated volatile agents have also been examined previously by numerous studies.[15–17,53–57] Among these, the most extensive studies focused on cardiac effects. Such studies confirmed that volatile agents reduce myocardial damage when administered immediately before an ischemic event (pre-conditioning) or during the early reperfusion period after an ischemic event (post-conditioning).[54,58] Several receptors and chemical mediators have been shown to play roles in the reduction of ischemia/reperfusion injury in hibernating and stunned tissue.[15] Because the optimal length of volatile agent administration for maximizing the post-conditioning effect is unknown, several studies investigated the cardioprotective effects using volatile agents as sedatives in the ICU.[30,33–36] Our pooled effect sizes from 5 cardiac surgical populations were 0.27 μg/L in troponin T (at the largest time interval; 12–24 hours after ICU admission) and 711 pg/mL in NT-proBNP. Considering the upper reference limits (0.014 μg/L in troponin T and 300 pg/mL in NT-proBNP) for diagnosing myocardial infarction and heart failure,[59,60] the pooled results suggest that even a late (postoperative) and subanesthetic dose (one-third of the dose used for general anesthesia) may have cardioprotective effects (Fig. 6). However, there were differences in intraoperative management and postoperative sedation durations. Unfortunately, we were unable to perform an analysis to calibrate the sedation duration, as not every study reported the exact sedation duration. Thus, further studies that can adjust for sedation duration are needed.
Several previous studies have also reported that volatile agents are renoprotective.[53,56,61] However, the risk of nephrotoxicity from inorganic fluoride, which forms when volatile agents such as sevoflurane are metabolized, is still a concern. Although our analysis identified differences in the serum creatinine levels on the first postoperative day, the pooled effect size of 0.05 mg/dL was too small to assess the effect on renal function if significant renal dysfunction was defined as an increase of 0.3 mg/dL over baseline.[62,63] Similarly, whether volatile agents have neuroprotective effects[55,64] or are neurotoxic and induce cognitive dysfunction[65,66] remains controversial. There was a difference in the incidence of delirium between the volatile and IV sedation arms; however, after adjusting for publication bias, both arms were comparable. Furthermore, delirium, which was used as a marker of cognitive dysfunction, was not measured using currently recommended tools (i.e., the Confusion Assessment Method for ICU or the Intensive Care Delirium Screening Checklist). Therefore, the data regarding the effects of sedative agents on the kidney and brain should be interpreted with caution.
One of the major concerns of using volatile agents is PONV.[18,67,68] When administering general anesthesia, volatile agents are known to be a potent risk factor of PONV.[19] In our analysis, the pooled OR of PONV did not show a significant difference between the volatile and IV sedation arms. However, the interpretation was limited due to variance among studies in terms of adjuvant opioid and anti-emetic usage.
The present study has several limitations. First, the number of included studies and sample sizes were small and the study durations were short. The largest study included only 141 subjects, and the mean sedation duration in all of the studies was less than 3 days. Thus, several outcomes may have been underpowered. Second, none of the included studies, except for one,[33] were double-blind; it is likely that this lack of blindness affected the observed findings. Third, these studies had multiple heterogeneities, including the group of patients examined (medical or surgical, cardiac or noncardiac surgical patients), intraoperative anesthesia (volatile anesthesia or total IV anesthesia), patient management (different methods of assessing sedation), and outcome measurement (different time points for measuring outcomes). These heterogeneities might have influenced outcomes by introducing many potential confounders. Fourth, all studies included were conducted in Europe except 1 Egyptian study.[38] Therefore, it is uncertain whether intercontinental differences in sedation practices might have affected outcomes. Fifth, we used laboratory values to evaluate end-organ protective effects and did not to directly measure organ function. Finally, we could not analyze other important factors such as cost-effectiveness, hemodynamic stability, or the effects of increased respiratory dead space and work of breathing.
Despite several limiting factors, our study provides the following new knowledge. First, volatile sedation using the only new anesthetic reflector had more reduction in awakening and extubation times than previous meta-analyses,[45–47] including studies using conventional ventilator and new anesthetic reflector together. In addition, the effect size was greater in long-term sedation (>24 hours) than short-term sedation (≤24 hours). Second, subanesthetic dose (one-third of the dose used for general anesthesia) of volatile sedation administered after cardiac surgery might have cardioprotective effects. Third, major concerns about volatile anesthetics (nephrotoxicity, nausea, and vomiting) were not proven at the sedation dose used in the present study.
A strength of our meta-analysis was that we only included RCTs; before-and-after and retrospective studies were excluded to minimize biases such as drug hangover effects. The fact that we only included studies where the AnaConDa device was used also reduced bias. To the best of our knowledge, this is the first meta-analysis to compare volatile sedation via the AnaConDa device with IV sedation in the ICU. Our results demonstrate that volatile sedation supports the current sedation practice emphasizing daily awakening and early extubation.[2] Especially, ICU patients requiring long-term sedation may benefit from volatile sedation due to rapid elimination of volatile anesthetics. In addition, postsurgical sedation after cardiac surgery may benefit from volatile sedation in term of myocardial protection.
In conclusion, the present meta-analysis found that volatile sedatives administered through the AnaConDa device in the ICU reduced awakening and extubation times compared with IV sedatives. Moreover, subanesthetic doses of volatile sedation administered after cardiac surgery might have cardioprotective effects. Given the technological advancements in volatile vaporizers, it is possible that volatile sedation will become the new standard of care in ICU sedation. However, because the included studies were small with high heterogeneity, additional large, high-quality prospective clinical trials are needed to validate these findings.
Supplementary Material
Footnotes
Abbreviations: ACD = anesthetic conserving device, CI = confidence interval, ICU = intensive care unit, IV = intravenous, LOS = length of stay, MD = mean difference, MV = mechanical ventilation, NT-proBNP = serum N-terminal prohormone of brain natriuretic peptide, OR = odds ratio, PICOS = the Patient, Intervention, Comparator, Outcomes, and Study, PONV = postoperative nausea and vomiting, PRISMA = the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized controlled trial.
No external funding and no competing interests were declared by the authors.
Supplemental Digital Content is available for this article.
References
- [1].Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 2002;30:119–41. [DOI] [PubMed] [Google Scholar]
- [2].Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306. [DOI] [PubMed] [Google Scholar]
- [3].Sydow M, Neumann P. Sedation for the critically ill. Intensive Care Med 1999;25:634–6. [DOI] [PubMed] [Google Scholar]
- [4].Ahmed S, Murugan R. Dexmedetomidine use in the ICU: are we there yet? Crit Care 2013;17:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301:489–99. [DOI] [PubMed] [Google Scholar]
- [6].Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med 2010;36:926–39. [DOI] [PubMed] [Google Scholar]
- [7].Holliday SF, Kane-Gill SL, Empey PE, et al. Interpatient variability in dexmedetomidine response: a survey of the literature. Sci World J 2014;2014:805013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Page VJ, McAuley DF. Sedation/drugs used in intensive care sedation. Curr Opin Anaesthesiol 2015;28:139–44. [DOI] [PubMed] [Google Scholar]
- [9].Mirrakhimov AE, Voore P, Halytskyy O, et al. Propofol infusion syndrome in adults: a clinical update. Crit Care Res Pract 2015;2015:260385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Whalen FX, Bacon DR, Smith HM. Inhaled anesthetics: an historical overview. Best Pract Res Clin Anaesthesiol 2005;19:323–30. [DOI] [PubMed] [Google Scholar]
- [11].Preckel B, Bolten J. Pharmacology of modern volatile anaesthetics. Best Pract Res Clin Anaesthesiol 2005;19:331–48. [DOI] [PubMed] [Google Scholar]
- [12].Patel SS, Goa KL. Sevoflurane. A review of its pharmacodynamic and pharmacokinetic properties and its clinical use in general anaesthesia. Drugs 1996;51:658–700. [DOI] [PubMed] [Google Scholar]
- [13].Orriach JL, Aliaga MR, Ortega MG, et al. Sevoflurane in intraoperative and postoperative cardiac surgery patients. Our experience in intensive care unit with sevoflurane sedation. Curr Pharm Des 2013;19:3996–4002. [DOI] [PubMed] [Google Scholar]
- [14].Guarracino F, Landoni G, Tritapepe L, et al. Myocardial damage prevented by volatile anesthetics: a multicenter randomized controlled study. J Cardiothorac Vasc Anesth 2006;20:477–83. [DOI] [PubMed] [Google Scholar]
- [15].Landoni G, Fochi O, Tritapepe L, et al. Cardiac protection by volatile anesthetics. A review. Minerva Anestesiol 2009;75:269–73. [PubMed] [Google Scholar]
- [16].Symons JA, Myles PS. Myocardial protection with volatile anaesthetic agents during coronary artery bypass surgery: a meta-analysis. Br J Anaesth 2006;97:127–36. [DOI] [PubMed] [Google Scholar]
- [17].Burchell SR, Dixon BJ, Tang J, et al. Isoflurane provides neuroprotection in neonatal hypoxic ischemic brain injury. J Investig Med 2013;61:1078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Apfel CC, Kranke P, Eberhart LH, et al. Comparison of predictive models for postoperative nausea and vomiting. Br J Anaesth 2002;88:234–40. [DOI] [PubMed] [Google Scholar]
- [19].Apfel CC, Kranke P, Katz MH, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth 2002;88:659–68. [DOI] [PubMed] [Google Scholar]
- [20].Apfel CC, Stoecklein K, Lipfert P. PONV: a problem of inhalational anaesthesia? Best Pract Res Clin Anaesthesiol 2005;19:485–500. [DOI] [PubMed] [Google Scholar]
- [21].Welborn LG, Hannallah RS, Norden JM, et al. Comparison of emergence and recovery characteristics of sevoflurane, desflurane, and halothane in pediatric ambulatory patients. Anesth Analg 1996;83:917–20. [DOI] [PubMed] [Google Scholar]
- [22].Cousins MJ, Mazze RI. Methoxyflurane nephrotoxicity. A study of dose response in man. JAMA 1973;225:1611–6. [DOI] [PubMed] [Google Scholar]
- [23].Meiser A, Laubenthal H. Inhalational anaesthetics in the ICU: theory and practice of inhalational sedation in the ICU, economics, risk-benefit. Best Pract Res Clin Anaesthesiol 2005;19:523–38. [DOI] [PubMed] [Google Scholar]
- [24].Enlund M, Wiklund L, Lambert H. A new device to reduce the consumption of a halogenated anaesthetic agent. Anaesthesia 2001;56:429–32. [DOI] [PubMed] [Google Scholar]
- [25].Bomberg H, Glas M, Groesdonk VH, et al. A novel device for target controlled administration and reflection of desflurane: the Mirus (TM). Anaesthesia 2014;69:1241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sackey PV, Martling CR, Granath F, et al. Prolonged isoflurane sedation of intensive care unit patients with the Anesthetic Conserving Device. Crit Care Med 2004;32:2241–6. [DOI] [PubMed] [Google Scholar]
- [27].Rohm KD, Wolf MW, Schollhorn T, et al. Short-term sevoflurane sedation using the Anaesthetic Conserving Device after cardiothoracic surgery. Intensive Care Med 2008;34:1683–9. [DOI] [PubMed] [Google Scholar]
- [28].Sackey PV, Martling CR, Carlsward C, et al. Short- and long-term follow-up of intensive care unit patients after sedation with isoflurane and midazolam: a pilot study. Crit Care Med 2008;36:801–6. [DOI] [PubMed] [Google Scholar]
- [29].Rohm KD, Mengistu A, Boldt J, et al. Renal integrity in sevoflurane sedation in the intensive care unit with the anesthetic-conserving device: a comparison with intravenous propofol sedation. Anesth Analg 2009;108:1848–54. [DOI] [PubMed] [Google Scholar]
- [30].Hellstrom J, Owall A, Bergstrom J, et al. Cardiac outcome after sevoflurane versus propofol sedation following coronary bypass surgery: a pilot study. Acta Anaesthesiol Scand 2011;55:460–7. [DOI] [PubMed] [Google Scholar]
- [31].Mesnil M, Capdevila X, Bringuier S, et al. Long-term sedation in intensive care unit: a randomized comparison between inhaled sevoflurane and intravenous propofol or midazolam. Intensive Care Med 2011;37:933–41. [DOI] [PubMed] [Google Scholar]
- [32].Hellstrom J, Owall A, Sackey PV. Wake-up times following sedation with sevoflurane versus propofol after cardiac surgery. Scand Cardiovasc J 2012;46:262–8. [DOI] [PubMed] [Google Scholar]
- [33].Soro M, Gallego L, Silva V, et al. Cardioprotective effect of sevoflurane and propofol during anaesthesia and the postoperative period in coronary bypass graft surgery: a double-blind randomised study. Eur J Anaesthesiol 2012;29:561–9. [DOI] [PubMed] [Google Scholar]
- [34].Steurer MP, Steurer MA, Baulig W, et al. Late pharmacologic conditioning with volatile anesthetics after cardiac surgery. Crit Care 2012;16:R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guerrero Orriach JL, Galan Ortega M, Ramirez Aliaga M, et al. Prolonged sevoflurane administration in the off-pump coronary artery bypass graft surgery: beneficial effects. J Crit Care 2013;28:879.e13–8. [DOI] [PubMed] [Google Scholar]
- [36].Marcos-Vidal JM, Gonzalez R, Garcia C, et al. Sedation with sevoflurane in postoperative cardiac surgery: influence on troponin T and creatinine values. Heart Lung Vessel 2014;6:33–42. [PMC free article] [PubMed] [Google Scholar]
- [37].Jerath A, Beattie SW, Chandy T, et al. Volatile-based short-term sedation in cardiac surgical patients: a prospective randomized controlled trial. Crit Care Med 2015;43:1062–9. [DOI] [PubMed] [Google Scholar]
- [38].Hanafy M. Clinical evaluation of inhalation sedation following coronary artery bypass grafting. Eg J Anaesth 2005;21:237–42. [Google Scholar]
- [39].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [40].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software 2010;36:1–48. [Google Scholar]
- [45].Jerath A, Panckhurst J, Parotto M, et al. Safety and efficacy of volatile anesthetic agents compared with standard intravenous midazolam/propofol sedation in ventilated critical care patients: a meta-analysis and systematic review of prospective trials. Anesth Analg 2017;124:1190–9. [DOI] [PubMed] [Google Scholar]
- [46].Landoni G, Pasin L, Cabrini L, et al. Volatile agents in medical and surgical intensive care units: a meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth 2016;30:1005–14. [DOI] [PubMed] [Google Scholar]
- [47].Spence J, Belley-Cote E, Ma HK, et al. Efficacy and safety of inhaled anaesthetic for postoperative sedation during mechanical ventilation in adult cardiac surgery patients: a systematic review and meta-analysis. Br J Anaesth 2017;118:658–69. [DOI] [PubMed] [Google Scholar]
- [48].Tempia A, Olivei MC, Calza E, et al. The anesthetic conserving device compared with conventional circle system used under different flow conditions for inhaled anesthesia. Anesth Analg 2003;96:1056–61. table of contents. [DOI] [PubMed] [Google Scholar]
- [49].Nishiyama T. Saving sevoflurane and hastening emergence from anaesthesia using an anaesthetic-conserving device. Eur J Anaesthesiol 2009;26:35–8. [DOI] [PubMed] [Google Scholar]
- [50].Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000;342:1471–7. [DOI] [PubMed] [Google Scholar]
- [51].Lonardo NW, Mone MC, Nirula R, et al. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. Am J Respir Crit Care Med 2014;189:1383–94. [DOI] [PubMed] [Google Scholar]
- [52].Fraser GL, Devlin JW, Worby CP, et al. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and meta-analysis of randomized trials. Crit Care Med 2013;41:S30–8. [DOI] [PubMed] [Google Scholar]
- [53].Fukazawa K, Lee HT. Volatile anesthetics and AKI: risks, mechanisms, and a potential therapeutic window. J Am Soc Nephrol 2014;25:884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].De Hert SG, Turani F, Mathur S, et al. Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth Analg 2005;100:1584–93. [DOI] [PubMed] [Google Scholar]
- [55].Zhao P, Peng L, Li L, et al. Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology 2007;107:963–70. [DOI] [PubMed] [Google Scholar]
- [56].Kong HY, Zhu SM, Wang LQ, et al. Sevoflurane protects against acute kidney injury in a small-size liver transplantation model. Am J Nephrol 2010;32:347–55. [DOI] [PubMed] [Google Scholar]
- [57].De Conno E, Steurer MP, Wittlinger M, et al. Anesthetic-induced improvement of the inflammatory response to one-lung ventilation. Anesthesiology 2009;110:1316–26. [DOI] [PubMed] [Google Scholar]
- [58].Pagel PS. Postconditioning by volatile anesthetics: salvaging ischemic myocardium at reperfusion by activation of prosurvival signaling. J Cardiothorac Vasc Anesth 2008;22:753–65. [DOI] [PubMed] [Google Scholar]
- [59].Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients. The International Collaborative of NT-proBNP Study. Eur Heart J 2006;27:330–7. [DOI] [PubMed] [Google Scholar]
- [60].Kimenai DM, Henry RM, van der Kallen CJ, et al. Direct comparison of clinical decision limits for cardiac troponin T and I. Heart 2016;102:610–6. [DOI] [PubMed] [Google Scholar]
- [61].Cai J, Xu R, Yu X, et al. Volatile anesthetics in preventing acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2014;148:3127–36. [DOI] [PubMed] [Google Scholar]
- [62].Grynberg K, Polkinghorne KR, Ford S, et al. Early serum creatinine accurately predicts acute kidney injury post cardiac surgery. BMC Nephrol 2017;18:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Karkouti K, Rao V, Chan CT, et al. Early rise in postoperative creatinine for identification of acute kidney injury after cardiac surgery. Can J Anaesth 2017;64:801–9. [DOI] [PubMed] [Google Scholar]
- [64].McAuliffe JJ, Joseph B, Vorhees CV. Isoflurane-delayed preconditioning reduces immediate mortality and improves striatal function in adult mice after neonatal hypoxia-ischemia. Anesth Analg 2007;104:1066–77. tables of contents. [DOI] [PubMed] [Google Scholar]
- [65].Acharya NK, Goldwaser EL, Forsberg MM, et al. Sevoflurane and isoflurane induce structural changes in brain vascular endothelial cells and increase blood-brain barrier permeability: possible link to postoperative delirium and cognitive decline. Brain Res 2015;1620:29–41. [DOI] [PubMed] [Google Scholar]
- [66].Yang CW, Fuh JL. Exposure to general anesthesia and the risk of dementia. J Pain Res 2015;8:711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chui J, Mariappan R, Mehta J, et al. Comparison of propofol and volatile agents for maintenance of anesthesia during elective craniotomy procedures: systematic review and meta-analysis. Can J Anaesth 2014;61:347–56. [DOI] [PubMed] [Google Scholar]
- [68].Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg 2006;102:1884–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


