Supplemental Digital Content is available in the text
Keywords: bloodstream infection, Enterococcus faecium, mortality, multilocus sequence typing, vancomycin-resistant enterococci
Abstract
Bloodstream infections (BSIs) due to vancomycin-resistant Enterococcus faecium (VREfae) remain a therapeutic challenge. This study aimed to evaluate mortality from BSIs due to VREfae in Central Taiwan.
We retrospectively analyzed patients with significant VREfae BSIs in the Changhua Christian Hospital System between January 1, 2010 and December 31, 2014.
Of the 152 patients with Enterococcal BSI, 56 patients (36.8%) were admitted to intensive care units (ICUs) at the onset of BSI and 20 (13.2%) patients were associated with polymicrobial bacteremia. VREfae BSI was observed in 36 (23.7%) patients. Van A (100%) is the prevalence genotype, and ST 17 (41.7%) is the predominant ST type among 36 VREfae isolates during the study period. The 30-day mortality rate was 13.2% (20/152). The multivariate logistic regression analysis showed that the onset of VREfae BSI in the ICU (odds ratio [OR] = 4.2, 95% confidence interval [CI] = 1.7–10.0, P = .002) was a significant risk factor for 30-day mortality, whereas an appropriate antimicrobial therapy was a protective factor for 30-day mortality (OR = 0.33, 95% CI = 0.14–0.79, P = .013).
Our results underscore the need to assist patients who are admitted to ICUs with VREfae BSIs. We emphasize the use of an appropriate antimicrobial therapy for VREfae BSI with the aim to treat more patients with these infections.
1. Introduction
The management of bloodstream infections (BSIs) due to Enterococcus faecium, particularly those due to vancomycin-resistant enterococci (VRE), has become a therapeutic challenge. BSIs due to vancomycin-resistant E faecium (VREfae) were first reported in 1988, and a short time later, they were reported in the USA and European countries.[1] The first VRE BSI in Taiwan was reported in 1996.[2] In the USA, VRE has become an important nosocomial pathogen, and their ratio among all nosocomial pathogens has increased from 0.3% in 1989 to nearly 30% in 2003 according to data from the National Nosocomial Infections Surveillance System.[3] In addition, VRE accounts for 14% of the enterococcal isolates among patients in intensive care units (ICUs).[3] Although VRE represented <2% of the enterococci identified in an antimicrobial resistance surveillance program in Taiwan in 2000,[4] a rapid increase in vancomycin resistance from 12.4% in 2007 to 42.0% in 2016 among nosocomial enterococcal isolates in ICU was reported by the Taiwan Nosocomial Infection Surveillance System.[5] The persistence of E faecium is a cause for concern because the treatment options and infection control measures are limited, and there is a low clinical awareness. Therefore, BSIs due to VREfae are an important clinical issue.
Most studies on BSI due to enterococcal infections focus on the general infection, geography of the infection, and vancomycin resistance.[6,7] In addition, the majority of studies published to date have been conducted in the USA, where the epidemiological conditions differ from those occurring in Asia.[8] The risk factors associated with in-hospital mortality from BSIs due to E faecium have been reported,[9] but most of these factors are indicators in basic medical sciences, and the molecular biomarkers are not useful to physicians. Furthermore, information regarding BSIs due to VREfae in Central Taiwan is particularly scarce.[9–11] An increasing resistance rate to vancomycin among enterococcal isolates has been documented globally.[12] The clonal spread of certain epidemic VREfae strains belonging to clonal complex 17 (CC17) contributed to this increase.[13]
Therefore, the aim of the present study was to analyze the clinical features and microbiological characteristics of BSIs due to VREfae in Central Taiwan.
2. Materials and methods
2.1. Clinical setting and study population
The population living in the rural areas of Central Taiwan is mostly served by the Changhua Christian Hospital System (CCHS), which consists of 4000 beds. The Changhua Christian Hospital (CCH) is the largest hospital among 9 branch hospitals in the CCHS and is an 1800-bed tertiary referral medical center situated in Central Taiwan. This study was conducted in the CCHS and was approved by the institutional review board of CCH. Cases of BSIs due to VREfae were analyzed by reviewing the medical records, and a cross-sectional retrospective study was conducted between January 1, 2010 and December 31, 2014 in the CCHS.
2.2. Patient identification, patient analysis, and definition
The occurrence of symptomatic enterococcal BSI was defined as >2 blood cultures positive for Enterococcus sp. or a single blood culture positive for Enterococcus sp. combined with a significant source of infection. Patients with BSI due to VREfae were identified from the microbiological databases and medical records according to the ninth edition of the International Classification of Diseases, with some clinical modifications (ICD-9-CM) in the CCHS in Central Taiwan. We used computerized indices to identify cases with the following ICD-9-CM codes: V09.8, 041.04, 790.7, 038, and 038.9. All the patients documented with BSI due to VREfae were enrolled during the study period. The exclusion criteria included age <18 years, inadequate clinical data, inconsistency between the data from the ICD-9 code and the microbiological dataset, and misinterpretation of the microbiological examinations. A standardized case report form was used to collect the data contained in the medical records regarding the medical diagnoses, medical treatment, and other key information. The medical records of all cases involving BSI due to VREfae were manually reviewed by the primary investigator (CCH) to confirm the diagnosis (using CCHS resources). Clinically equivocal cases were discussed and decisions were made by the primary and the secondary investigator (CCY). Only the first episode of BSI due to VREfae in each patient during the study period was included in the statistical analysis.
The date of the onset of BSI was defined as the date on which the culture from the first blood sample was positive. The case group was defined as BSIs due to vancomycin-resistant E faecium (VREfae), and the control group was defined as enterococcal BSIs other than VREfae. Polymicrobial bacteremia was defined as the presence of >1 microorganism from the same blood culture specimen. The underlying severity was classified according to the McCabe and Jackson criteria.[14] During the initial 24 hours from the onset of BSI due to VREfae, the systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock were defined as previously described.[15] The source of infection was defined as the site of infection where it was microbiologically and clinically documented.[15] The outcome was evaluated 30 days after the onset of BSI due to VREfae in the CCHS. Death was considered associated with BSI when the patient died <2 weeks after the onset of BSI, and no other cause of death was identified. The all-cause 30-day mortality group was defined as patients with BSI due to VREfae who died within 30 days of diagnosis. BSI due to VREfae was classified as healthcare-onset or community-acquired according to a modified version of Horan's description.[16] An appropriate antimicrobial therapy was defined as patients who received effective antibiotics as evaluated by susceptibility tests during the period of effective empirical therapy.
2.3. Microbiological identification, susceptibility test, and molecular analysis
Between January 1, 2010 and December 31, 2014, all VREfae isolates cultured from blood samples were considered significant findings, and 36 clinical isolates were identified as VRE in the CCHS in Central Taiwan. Only the initial isolate from each patient was analyzed. The laboratory identification of Enterococcus spp. was performed using the 6.5% NaCl/bile-esculin agar test and confirmed with the Vitek-2 gram-positive identification system (bioMérieux, Marcy l’Etoile, France) at CCHS. Then, those isolates were sent to a reference laboratory (Research Laboratory, Division of Infectious Diseases, Department of Internal Medicine, National Taiwan University Hospital) for re-confirmation. The susceptibility to various antimicrobial agents by determination of MICs was performed with the Vitek-2 system (bioMérieux, Marcy l’Etoile, France).[17] The following drugs were tested: ampicillin, ciprofloxacin, erythromycin, linezolid, minocycline, penicillin, rifampin, teicoplanin, tigecycline, and vancomycin. The interpretive criteria of the Clinical and Laboratory Standards Institute (CLSI) were used to determine the isolate susceptibility.[17] Vancomycin resistance was defined as an minimum inhibitory concentration (MIC) ≥32 μg/mL.
The genotypes of VREfae were determined using polymerase chain reaction (PCR)-based methods as described previously.[18] Multilocus sequence typing (MLST) for VREfae was also performed as previously reported.[19]
2.4. Statistical analysis
Continuous variables were expressed as the mean ± standard deviation or the median and range if the distribution was not normal and were compared using Student's t-test or Mann–Whitney U test. Continuous data were expressed as the mean ± standard deviation or as the median and range. Univariate and multivariate analyses were performed to determine the independent risk factors for the all-cause 30-day mortality using logistic regression models. A P-value <.05 was considered statistically significant. All tests were performed using SPSS V.17.0 for Windows (SPSS Inc, Chicago, IL).
3. Results
3.1. Study population
Two hundred seventy-nine patients at CCHS were found to have a positive blood culture for enterococci. Among them, 177 patients were at CCH, and the other 102 patients were at other branches of CCHS. Symptomatic BSI was identified in 194 patients after excluding duplicates from 21 patients. Thirty-eight patients were excluded because 30 patients had incomplete data, and 8 patients were clinically determined to be without significant evidence of an infection; therefore, 152 patients were analyzed (Fig. 1).
Figure 1.
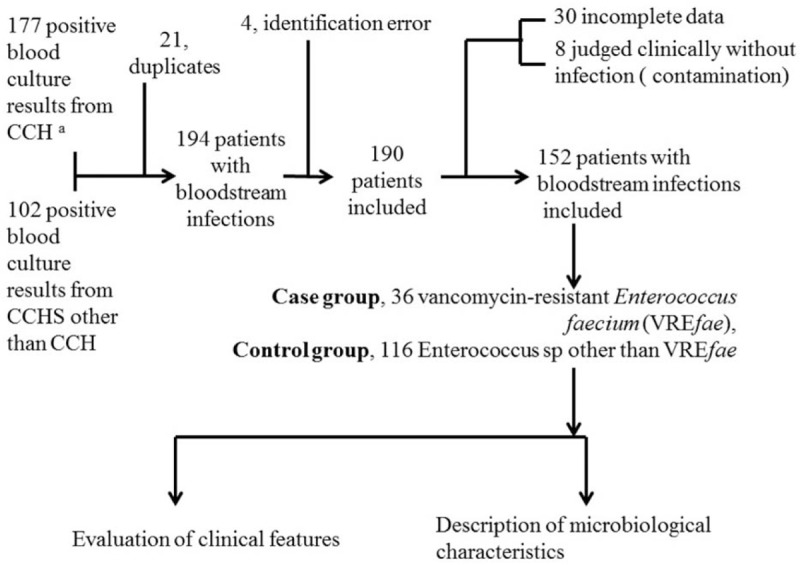
Concept frame of this study. CCH = Changhua Christian Hospital, CCHS = Changhua Christian Hospital System, VREfae = vancomycin-resistant Enterococcus faecium. aPartial data were reported at Rev Chilena Infectol (Chang-Hua C, Li-Chen L, Yu-Jun C, Chih-Yen C.[Mortality analysis of Enterococcus faecium bloodstream infection in central Taiwan]. Rev Chilena Infectol 2016 Aug;33(4):395–402.[Article in Spanish]).
3.2. Clinical features of the case group (BSI due to VREfae) and control group (BSI other than VREfae)
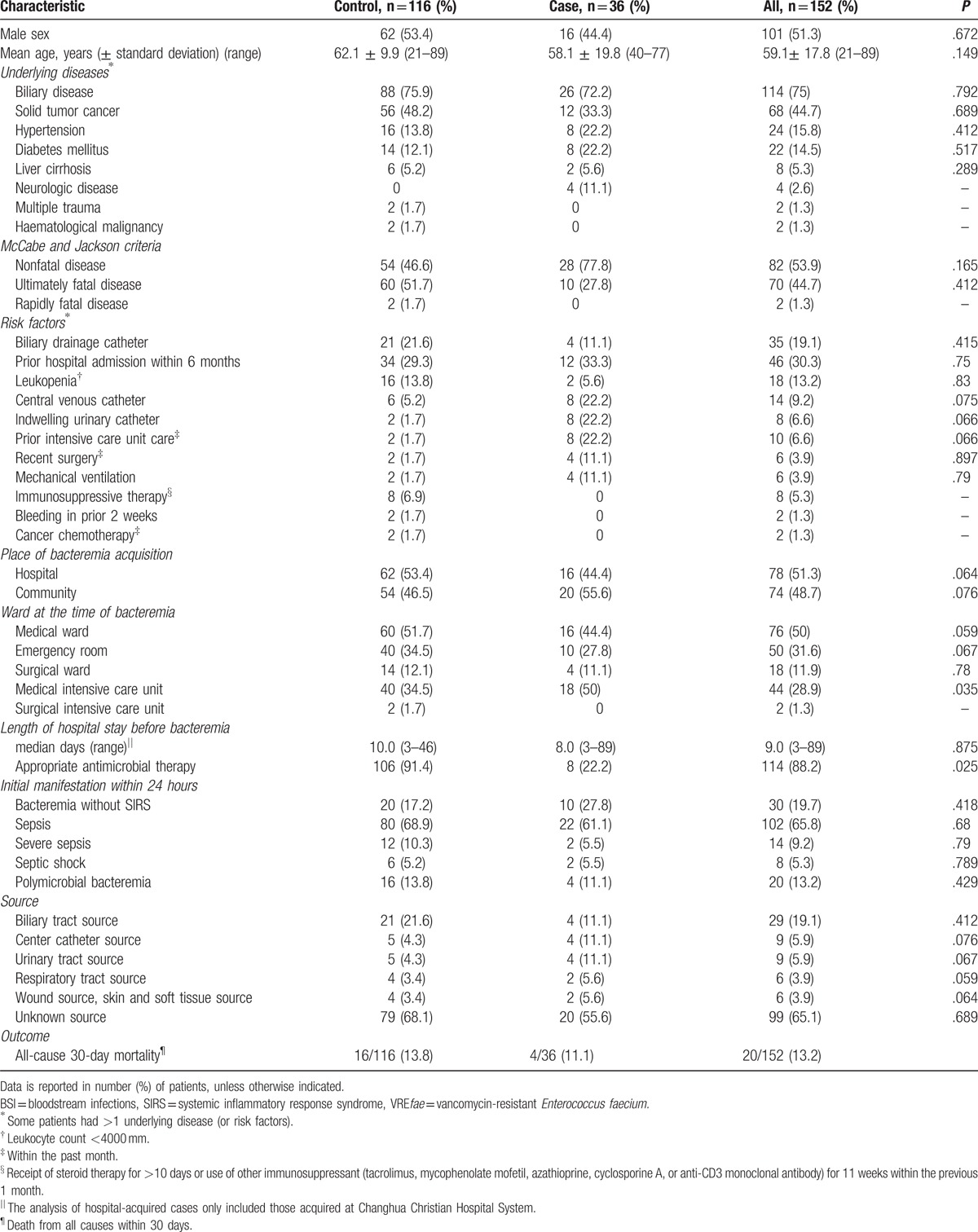
The epidemiological characteristics and underlying conditions of the 152 patients are shown in Table 1. The distribution of all analyzed characteristics was not significantly different between the case group (36/152, 23.7%) and the control group (116/152, 76.3%). Thirty-nine patients (51.8%) were men, and the median age was 59.1 years (range, 21–89 years). The most common underlying disease was biliary disease (75.0%), followed by solid cancer (44.7%). According to the McCabe and Jackson criteria, the underlying diseases were nonfatal in 82 patients (53.9%), ultimately fatal in 68 patients (44.7%), and rapidly fatal in 2 patients (1.3%). Forty-six (30.3%) patients had a history of hospital admissions, and 29 patients (19.1%) had biliary drainage catheters in place. Thirty-nine (51.8%) BSI episodes occurred in the hospital with a median admission period of 9.0 days (standard deviation, 6.8 days; range, 3–89 days) before the onset of BSI. Fifty-two patients (36.8%) were in ICUs at the onset of BSI. Of the enterococcal isolates recovered from the 152 patients, 36 (23.7%) were VREfae. Twenty (13.2%) patients had polymicrobial bacteremia with microorganisms, including Escherichia coli (8 patients), Klebsiella pneumoniae (4 patients), Pseudomonas aeruginosa, Enterobacter aerogenes, Citrobacter freundii, and Acinetobacter baumannii (2 patients each). Of the 20 patients with polymicrobial bacteremia, 6 (30%) involved VREfae (30.0%). Of the 152 patients evaluated, only 6 patients received parenteral vancomycin before developing BSI due to VREfae, and none received oral vancomycin. The 30-day mortality rate was 13.2% (20 of 152 patients). There were significant statistical differences between VREfae BSI and enterococcal BSI other than VREfae BSI upon admission at the medical ICU in terms of the onset of BSI (P = .035) and the appropriate antimicrobial therapy (P = .025). The multivariate logistic regression analysis, including a univariate analysis of variables with P <.05, showed that the onset of VREfae BSI in ICUs (odds ratio [OR] = 4.2, 95% confidence interval [CI] = 1.7–10, P = .002) was the only significant risk factor for 30-day mortality. An appropriate antimicrobial therapy was a protective factor for 30-day mortality (OR = 0.33, 95% CI = 0.14–0.79, P = .013).
Table 1.
Clinical features of patients in the case group (VREfae BSI) and control group (enterococcal BSI other than VREfae).
3.3. Microbiological characteristics of 36 VREfae
The antibiotic susceptibility of the 36 VREfae isolates is shown in Table 2. The genotypic testing for 36 VREfae showed that all isolates carried the van A gene. The results of the MLST and their relationships with antibiotic susceptibilities for the 36 VREfae isolates are shown in Table 2. Overall, all isolates were susceptible to linezolid. All 36 VREfae isolates were resistant to ampicillin, penicillin, ciprofloxacin, and vancomycin. All isolates were resistant to erythromycin except for 4 ST-17 isolates. Two isolates (5.6%) were susceptible to teicoplanin, and both were of the van A genotype. The resistance rates to minocycline and tigecycline varied with different STs. Among the VREfae isolates, there were 2 major STs as follows: ST-17 and ST-78. Both ST-17 and ST-78 accounted for 59.6% of all VREfae isolates tested. Before 2010, ST-414 and ST-18 were the 2 predominant STs, accounting for 79.6% of isolates (Appendix 1). However, ST-17 (41.7%) and ST-78 (18.0%) were the predominant STs during this study period (Fig. 2).
Table 2.
Microbiological characteristics of the 36 VREfae isolates.
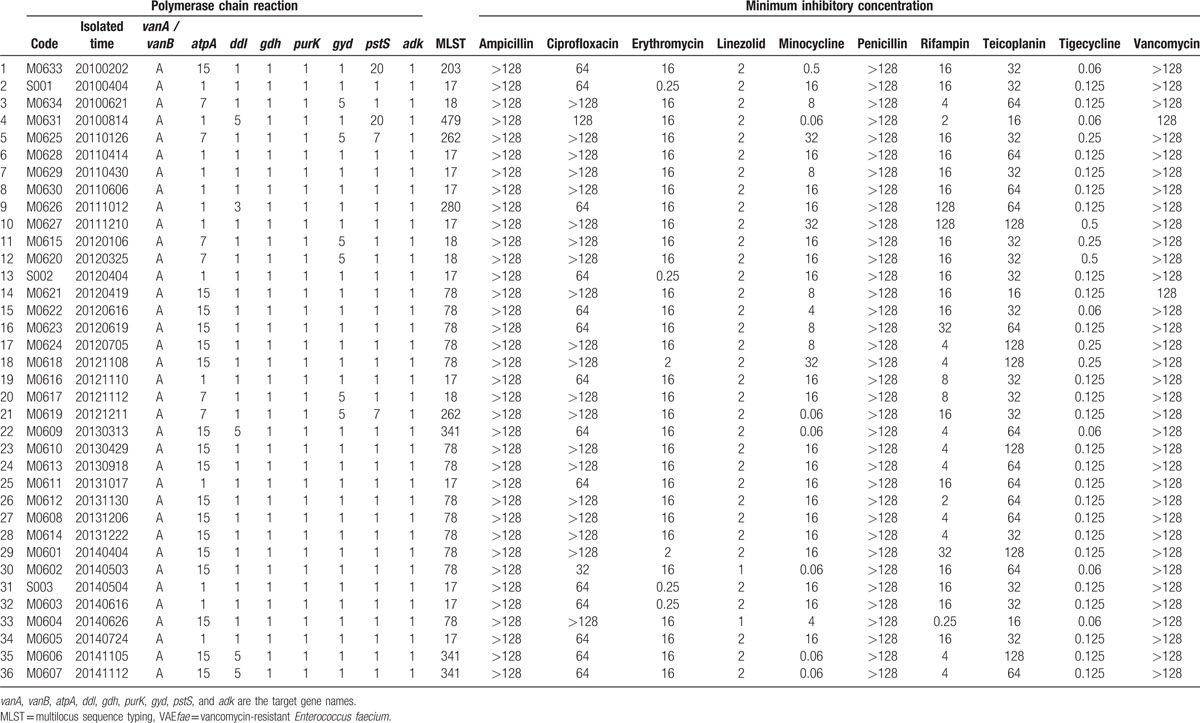
Figure 2.
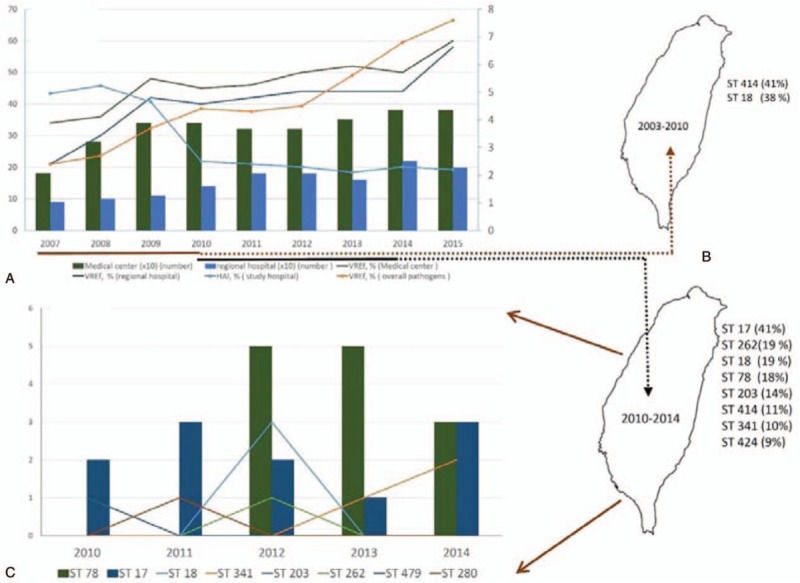
Nine-year trend of the rate of vancomycin-resistant Enterococcus faecium isolates. (A) A rapid increase in vancomycin resistance from 12.4% in 2007 to 39.9% in 2015 among enterococcal isolates was reported in Taiwan Nosocomial Infection Surveillance System (TNIS), and an increase of Enterococcus faecium from 2.4% in 2007 to 8.6% in 2015 among Enterococcal isolates was noted in TNIS (data from Taiwan Centers for Disease Control). (B) Before 2010, ST-414 and ST-18 of vancomycin-resistant Enterococcus faecium isolates were the 2 predominant STs, accounting for 79.6% of the isolates (data from Appendix 1). (C) ST-17 (41.7%) and ST-78 (18.0%) vancomycin-resistant Enterococcus faecium isolates were the predominant STs during the study period (data from this study). HAI = healthcare-associated infection, TNIS = Taiwan Nosocomial Infection Surveillance System, VREfae = vancomycin-resistant Enterococcus faecium.
4. Discussion
This is the first study to describe the clinical features of BSI due to VREfae and identify the microbiological characteristics of VREfae in Central Taiwan. After comparing our results with those of other recent studies on BSI due to Enterococcus sp., we noted that the proportion (7.6%) of enterococcal BSI was similar to that of the total BSI episodes (5.0%–7.1%).[20,21] The proportion (23.7%) of VREfae BSI was in good agreement with previous results.[9,22,23] VREfae infections in food-producing animals derived from broiler production have also been reported.[24]
We observed that the 30-day mortality rate (20/152, 13.2%) from VREfae BSI among our patients was lower than that observed in previous studies.[20,25] The lower mortality rate observed may be due to multiple factors, including patient populations, variations in the portal of entry, serious underlying conditions, and lack of lethal clonal spread. However, further experiments are needed to elucidate these factors. The emergence of antimicrobial-resistant bacteria, including VREfae, poses a difficult task for physicians who have limited therapeutic options. Critically ill patients admitted to ICUs are at a major risk of being infected with resistant bacteria that will have an adverse impact on mortality.[26] Jung's study showed that VREfae colonization was associated with increased mortality,[27] and the results of our study showed that the onset of VREfae BSI in the ICU was another significant risk factor for 30-day mortality (OR = 4.2, 95% CI = 1.7–10.0, P = .002). The length of ICU stay, which may reflect the severity of BSI, was also strongly associated with the odds of death from BSI due to VREfae.[27] Certainly, critically ill patients admitted to the ICUs are at a major risk of mortality, and VREfae can play an important role as the causative agent. In addition, an appropriate antimicrobial therapy for VREfae BSI was a protective factor for 30-day mortality (OR = 0.33, 95% CI = 0.14–0.79, P = .013). The results of our study were similar to the results of other studies.[27] Importantly, the results of the present study suggest that an appropriate antimicrobial therapy results in lower odds of all-cause 30-day mortality. The international guidelines for the management of severe sepsis and septic shock recommend the administration of broad-spectrum antimicrobials within 1 hour of the diagnosis of septic shock (1B) and severe sepsis without septic shock (1C) as the goal of therapy.[28] Therefore, we highlight the need to address the patients admitted to the ICU with VREfae BSI and use appropriate antimicrobial therapy for VREfae BSI with the aim to treat more patients with these infections.
Among the VREfae isolates, there were 2 major STs, that is, ST-17 and ST-78. These 2 STs accounted for 59.6% of all VREfae isolates tested. Before 2010, ST-414 and ST-18 were the 2 predominant STs, accounting for 79.6% of the isolates (Appendix 1). However, ST-17 (41.7%) and ST-78 (18.0%) were the predominant STs during the study period (Fig. 2).
An increasing resistance rate to vancomycin among enterococcal isolates has been documented globally.[12] The clonal spread of certain epidemic VREfae strains belonging to CC17 contributed to this increase.[13] The 2 major STs among the VREfae isolates tested in our study, ST-17 and ST-78, all belong to CC17, which might explain the same increasing trend of vancomycin resistance in Taiwan. A previous study demonstrated that ST-78 was the epidemic strain causing VRE infections in Taiwan in 2007.[29,30] Our study showed that the VREfae isolates of ST-18 and ST-414 were first noted before 2010. However, in 2010 and 2014, ST-17 and ST-78 became the predominant STs, which temporally correlated with the rapid increase in VREfae. Because of the small sample size of individual STs in this study, an accurate correlation between STs and mortality rate cannot be calculated. Among the blood enterococcal isolates, 23.7% (36/152) were VREfae, and all 36 VREfae remained highly susceptible to linezolid and tigecycline. ST-17 and ST-78 were the 2 predominant STs during the study period. The pathogenicity and virulence of predominant VREfae STs warrants further study. We assumed that no predominant clonal spread occurred in the study year due to the absence of outbreaks.
The present study has several strengths. Most importantly, we accumulated a complete longitudinal dataset over 4 years. We had access to excellent data to evaluate the demographic features of VREfae BSI. In addition, the present findings provide invaluable epidemiological information about BSI due to VREfae in Central Taiwan.
Our study has several limitations. The first limitation was that our facility was unable to perform the molecular typing of the isolates; therefore, the clonality and genotypes of the isolates could not be evaluated. Second, we could described the correlation between ICU stay and VREfae BSI for all-cause 30-day mortality, but the causality analysis between ICU stay and VREfae BSI could not be clearly established due to retrospective analysis. We suggested each episode of BSI at ICU should serve patient aggressively and carefully. Third, our microbiological laboratory did not evaluate the microbial susceptibility to daptomycin. We assessed the adequacy of the antibiotic therapy using the in vitro breakpoint method reported by CLSI.[17] Vancomycin therapy for strains with MIC ≤32 μg/mL was regarded as adequate. Lastly, some data suggest that resistance may be inducible in some strains.[29] Therefore, vancomycin therapy against infection due to vanC-VRE has been regarded as inadequate. Furthermore, vanC genotype was not included in this study, but we can assume that a similar phenomenon has occurred in our institute, as previously reported.[23]
In conclusion, our results suggest that onset of VREfae BSI at ICU was the only significant risk factors for all-cause 30-day mortality. An appropriate antimicrobial therapy was a protective factor for 30-day mortality. Therefore, we highlight the need to use appropriate antimicrobial therapy in patients admitted to the ICUs with VREfae BSIs with the aim to treat more patients with these infections.
Supplementary Material
Acknowledgments
All authors thank Doctor Jann-Tay Wang and his research assistant Duckling Chen for their help in performing drug susceptibilities testing and molecular studies. All authors thank the assistant of the Clinical Microbiology Laboratory of Changhua Christian Hospital. This research project would not have been possible without the support of many people. The authors wish to express their gratitude to staffs of Division of Infectious Diseases and Division of Critical Care Medicine of Changhua Christian Hospital who were offered patient care and invaluable support.
Footnotes
Abbreviations: BSI = bloodstream infections, CC = clonal complex, CCH = Changhua Christian Hospital, CCHS = Changhua Christian Hospital System, CI = confidence interval, ICUs = intensive care units, MIC = minimum inhibitory concentration, MLST = multilocus sequence typing, OR = odds ratio, PCR = polymerase chain reaction, VRE = vancomycin-resistant enterococci, VREfae = vancomycin-resistant Enterococcus faecium.
Funding: Changhua Christian Hospital (grant 104-CCH-IPR-001 and 105-CCH-IPR-001).
Ethical approval: The study was approved by the institutional review board of Changhua Christian Hospital (CCH IRB No. 140304).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Uttley AH, Collins CH, Naidoo J, et al. Vancomycin-resistant enterococci. Lancet 1988;103:57–8. [DOI] [PubMed] [Google Scholar]
- [2].Ben RJ, Lu JJ, Young TG, et al. Clinical isolation of vancomycin-resistant enterococcus faecalis in Taiwan. J Formos Med Assoc 1996;95:946–9. [PubMed] [Google Scholar]
- [3].National Nosocomial Infections Surveillance System. National nosocomial infections surveillance (NNIS) system report data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32:470–85. [DOI] [PubMed] [Google Scholar]
- [4].McDonald LC, Lauderdale TL, Shiau YR, et al. TSAR Participating Hospitals. The status of antimicrobial resistance in Taiwan among gram-positive pathogens: the Taiwan surveillance of antimicrobial resistance (TSAR) programme, 2000. Int J Antimicrob Agents 2004;23:362–70. [DOI] [PubMed] [Google Scholar]
- [5].Taiwan Centers for Disease Control. Statistics of communicable diseases and surveillance report in Taiwan 2015. Taiwan. 2016. [in English]. [Google Scholar]
- [6].Conde-Estévez D, Grau S, Albanell J, et al. Clinical characteristics and outcomes of patients with vancomycin-susceptible Enterococcus faecalis and Enterococcus faecium bacteremia in cancer patients. Eur J Clin Microbiol Infect Dis 2011;30:103–8. [DOI] [PubMed] [Google Scholar]
- [7].McBride SJ, Upton A, Roberts SA. Characteristics and outcomes of patients with vancomycin-susceptible Enterococcus faecalis and Enterococcus faecium bacteraemia—a five-year retrospective review. Eur J Clin Microbiol Infect Dis0 2010;29:107–14. [DOI] [PubMed] [Google Scholar]
- [8].Arias CA, Murray BE. The rise of the enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chang CM, Wang LR, Lee HC, et al. Characterisation of vancomycin-resistant enterococci from hospitalised patients at a tertiary centre over a seven-year period. J Hosp Infect 2010;74:377–84. [DOI] [PubMed] [Google Scholar]
- [10].Wang J, Chen YC, Chang SC, et al. Control of vancomycin-resistant enterococci in a hospital: a five-year experience in a Taiwanese teaching hospital. J Hosp Infect 2004;58:97–103. [DOI] [PubMed] [Google Scholar]
- [11].Tan C, Lai CC, Wang JY, et al. Bacteremia caused by non-faecalis and non-faecium enterococcus species at a medical center in Taiwan, 2000 to 2008. J Infect 2010;61:34–43. [DOI] [PubMed] [Google Scholar]
- [12].Werner G, Coque TM, Hammerum AM, et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Euro Surveill 2008;13:pii:19046. [PubMed] [Google Scholar]
- [13].Willems RJ, Top J, van Santen M, et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis 2005;11:821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McCabe WR, Jackson G. Gram-negative bacteremia. I. etiology and ecology. Arch Intern Med 1962;110:847–55. [Google Scholar]
- [15].Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 1992;101:1481–3. [DOI] [PubMed] [Google Scholar]
- [16].Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–32. [DOI] [PubMed] [Google Scholar]
- [17].Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-sixth Informational Supplement M100-S26. Wayne, PA, USA, 2016. [Google Scholar]
- [18].Dutka-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol 1995;33:1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Homan WL, Homan WL, Tribe D, et al. Multilocus sequence typing scheme for Enterococcus faecium. J Clin Microbiol 2002;40:1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Caballero-Granado FJ, Becerril B, Cuberos L, et al. Attributable mortality rate and duration of hospital stay associated with Enterococcal bacteraemia. Clin Infect Dis 2001;32:587–94. [DOI] [PubMed] [Google Scholar]
- [21].Peset V, Tallón P, Sola C, et al. Epidemiological, microbiological, clinical, and prognostic factors of bacteremia caused by high-level vancomycin-resistant enterococcus species. Eur J Clin Microbiol Infect Dis 2000;19:742–9. [DOI] [PubMed] [Google Scholar]
- [22].Zhao C, Sun H, Wang H, et al. Antimicrobial resistance trends among 5608 clinical Gram-positive isolates in china: results from the Gram-positive cocci resistance surveillance program (2005–2010). Diagn Microbiol Infect Dis 2012;73:174–81. [DOI] [PubMed] [Google Scholar]
- [23].Lu CL, Chuang YC, Chang HC, et al. Microbiological and clinical characteristics of vancomycin-resistant Enterococcus faecium bacteraemia in Taiwan: Implication of sequence type for prognosis. J Antimicrob Chemother 2012;67:2243–9. [DOI] [PubMed] [Google Scholar]
- [24].Nilsson O. Ncomycin resistant enterococci in farm animals—occurrence and importance. Infect Ecol Epidemiol 2012;20:3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Suppli M, Aabenhus R, Harboe ZB, et al. Mortality in Enterococcal bloodstream infections increases with inappropriate antimicrobial therapy. Clin Microbiol Infect 2011;17:1078–83. [DOI] [PubMed] [Google Scholar]
- [26].Cornejo-Juárez P, Vilar-Compte D, Pérez-Jiménez C, et al. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. Int J Infect Dis 2015;31:31–4. [DOI] [PubMed] [Google Scholar]
- [27].Cheah AL, Spelman T, Liew D, et al. Enterococcal bacteraemia: factors influencing mortality, length of stay and costs of hospitalization. Clin Microbiol Infect 2013;19:e181–189. [DOI] [PubMed] [Google Scholar]
- [28].Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hsieh YC, Lee WS, Ou TY, et al. Clonal spread of CC17 vancomycin-resistant Enterococcus faecium with multilocus sequence type 78 (ST78) and a novel ST444 in Taiwan. Eur J Clin Microbiol Infect Dis 2010;29:25–30. [DOI] [PubMed] [Google Scholar]
- [30].Hsueh PR, Teng LJ, Pan HJ, et al. Emergence of vancomycin-resistant enterococci at a university hospital in Taiwan: persistence of multiple species and multiple clones. Infect Control Hosp Epidemiol 1999;20:828–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


