Abstract
Background:
The trigeminocardiac reflex (TCR) is defined as sudden onset of parasympathetic dysrhythmias including hemodynamic irregularities, apnea, and gastric hypermotility during stimulation of sensory branches of the trigeminal nerve. Since the first description of the TCR 1999, there is an ongoing discussion about a more flexible than the existing clinical definition. Aim of this work was to create a clinical surrogate definition through a systematic review of the literature.
Methods:
In this meta-analysis study, literature about TCR occurrences was, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement, systematically identified through various search engines including PubMed (Medline), Embase (Ovid SP), and ISI Web of Sciences databases from January 2005 to August 2015. TCR was defined as a drop of heart rate (HR) below 60 bpm or 20% to the baseline. We extracted detailed data about hemodynamic changes and searched for connections between arterial blood pressure (BP) and HR changes during such episodes.
Results:
Overall 45 studies harboring 57 patients were included in the study but only 32 patients showed sufficient data for final analyze. HR showed a nonlinear behavior with a “tipping point” phenomena that differs in variance from the central/peripheral (20–30% drop) to ganglion (40–49% drop). BP showed a linear behavior with a “central limit” phenomena not differing in variance in the whole subgroup (30–39% drop). An analyzation of the correlation between BP and HR showed a trend to a linear correlation.
Conclusions:
We can show for the first time that HR is the dominant variable in the TCR and present a new surrogate definition model. This model and the role of BP must be better investigated in further studies.
Keywords: oculocardiac, reflex, TCR, trigeminocardiac
1. Introduction
The trigeminocardiac reflex (TCR) is a phylogenetic old, well-established brainstem reflex, that is triggered by the physical (traction, pressure) or chemical manipulation of the trigeminal nerve during its course and clinically manifests as changes in hemodynamic parameters such as heart rate (HR) and mean arterial blood pressure (MABP), apnea as well as gastric hypermotility. Clinically first examined by the senior author,[1] the reflex gained much interests during the last 2 decades[2–46] due to its high prevalence in certain surgical procedures (up to 60% prevalence in surgeries around the orbit and periorbit[47]) and due to its consecutive dramatical changes in hemodynamic stability of the patient (up to 30% asystole in light plain anesthesia).[18] Although the TCR is well known and daily seen in a clinically setting, there is still an ongoing discussion about its proper definition[47–58]; not at least fired by the notoriety of the phenomena. Nowadays, the most accepted definition requires a drop of HR and MABP of 20% as evaluated by Schaller et al in the year 1999.[1] Clinical practice suggested that this does not reflect all TCR subtypes that are described since that.
Nevertheless, the TCR is classically divided into the central (proximal) subtype with an intracranial trigger point proximal the Gasserian ganglion; the peripheral (distal) subtype, caused by stimulation upon the extra-cranial course of the trigeminal nerve; and the Gasserian ganglion subtypes.[49,59] Latest research implies a different manifestation of the TCR, according to the neuroanatomic/neuroembryolic events leading to a new classification of 5 key trigger points[47,60] around the 5th cranial nerve. The new classification model contains a subdivision of the peripheral subtype into the very peripheral diving reflex and the already known peripheral TCR, likewise the central TCR is now subdivided into a central and a further brainstem TCR. It has been observed that the main difference in the clinical manifestation is a different presentation of change in MABP whereas the HR decline is always observed.[49]
Several retrospective, and few prospective clinical investigations repeatedly showed and statistically proofed a substantial MABP decline of more than 20% during the central subtype of TCR.[1,3,61–66] Since most of these clinical studies examined the manifestation of the TCR provoked by an intracranial stimulation—the so-called central subtype of TCR—the nowadays accepted definition is mainly influenced by those results. While this central subtype shows an MABP decline,[1] the peripheral subtype (with the diving reflex as a subtype) seems to have less influence on blood pressure (BP) and can even manifest as an increase in MABP.[51,59] The ganglion subtype shows, according to previous research, a heterogeneous clinical manifestation with either increase or decrease of MABP.[47–49,51] This knowledge leads to a classification model after which the well-established subtypes (peripheral, ganglion, and central) of the TCR should be individually categorized.[48,49,51]
Therefore, the aim of this present study is to evaluate the specific changes in HR and MABP parameters according to the “classical” TCR classification,[1] which was developed only for the central subtype of the reflex. We choose an approach through a simplified classification with 3 TCR subtypes (central, peripheral, and Ganglion Gasseri)[56] to simplify data sampling as the updated categorization is just about to be introduced[49] and most of the current articles are still concerning the (older) simplified subdivision. Final goal is to develop a surrogate model of the definition of TCR that fits for every subtype in a clinically setting to ease its detection and therefore allows anesthesia providers to establish an adequate therapeutic management.
2. Methods
In this meta-analysis, a retrospective data collection was performed. The study design was thought to provide a complete, exhaustive summary of current literature relevant to our research question. By this objective approach, there can be achieved a synthesis with the aim of minimizing bias.
This systematic review was done in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.[67] The methodological quality of the included articles was assessed using the Cochrane Collaboration's domain-based evaluation tool for assessing risk of bias.[68]
2.1. Definition of the TCR
We defined the occurrence of a TCR episode for the nonrestrictive purpose of this study as bradycardia; a drop of HR below of 60/min or 20% or more from the baseline and/or asystole. Further, a TCR had to occur in a clinical setting during a surgery and fulfill at least one of the major criteria for a TCR as earlier defined by the authors (plausibility or reversibility).[1,49] There was a strong need for a detailed description and comprehensible cause–effect relationship, as earlier described,[1,49] for every included case. For the open character of this study and to consider the different clinical manifestation of the TCR subtypes, hypotension, thus a drop of BP below 90/60 mm Hg, 70 mm Hg MABP, respectively, was no criterion and not required for inclusion.
2.2. Identification of relevant data
For this study, we systematically collected data in a comprehensive literature research through various search engines including PubMed (Medline), Embase (Ovid SP), and the Institute for Scientific Information (ISI Web of Sciences) Database for the terms “Trigeminocardiac reflex,” “Trigemino-cardiac reflex,” “Trigeminal depressor response,” and “Oculocardiac reflex.” We included all publications released during January 2005 until August 2015. Also, reference lists of all included articles were reviewed to identify additional relevant articles. Contact was made with experts to identify other potentially relevant published and unpublished studies.
2.3. Inclusion and exclusion criteria
For this study, we analyzed all publications released in the recent 10 years (from January 1, 2005 to August 31, 2015) that presented a TCR manifestation as case report or a case series with patients age from 1 to 99 years old and that are published in English, German, or French. We included all case reports that reported a TCR in a clinical setting during surgery as defined above. If there was no link to a full-text version available through the various search engines: we tried to contact the author directly; if not successful, we excluded the article. Papers related to animal experiments were not included. All TCR cases were checked for double publication and if so, not included in this review.
Cases with hypertension during a TCR episode are excluded from this study. First, the few existing cases are ambivalent,[49] not clearly differentiating from a simple pain reaction. Second, it might play only a role in Ganglion subtype.
2.4. Data extraction
Data were collected and extracted by 2 independent reviewers (CM/BS) who selected all titles/abstracts. Articles that could not be excluded by title and/or abstract were assessed for defined eligibility criteria in full text. If there was no agreement, the article was read and checked for inclusion by a third reviewer (PE) independently, and the decision was made after thorough discussion.
2.5. Data synthesis and analysis
Collected data and results in the studies were also checked by 2 reviewers (CM/BS) independently, to find differences in the extracted data, if any. Following parameters were extracted: year of publication, gender, age, location,[4] changes in hemodynamic parameters as HR and MABP, calculated cerebral state index (CSI),[18] discussed risk factors, premedication, treatment of the TCR (e.g., stop of manipulation, atropine, cardio pulmonal resuscitation). If more than 1 episode of TCR was reported, the episode with the lowest values of HR or asystole was included. If an article showed missing data in 1 or more parameters, the corresponding author was contacted and asked to provide more detailed information. If the author was not reachable or did not respond, the article was rated with the available data and if reached a “well” rating, included in the study. If the case report was rated lower than well (more than 2 required parameters missing), the article was excluded from the analysis.
2.6. Parameters
2.6.1. Hemodynamic parameters
The definition of bradycardia is described earlier. MABP was calculated as diastolic BP + ½ (systolic BP − diastolic BP). Hypotension was defined as a systolic BP of 90 mm Hg and a diastolic BP of 60 mm Hg, thus a calculated MABP of 70 or lower.
2.6.2. Localization
The cases were sorted, according to the recently published literature,[47] into 3 different localizations: craniofacial skin, oral mucosa, orbit and periorbit as peripheral TCR; cavernous sinus plexus as a ganglion TCR; and middle fossa and posterior fossa as central TCR.
2.7. Rating of the extracted cases
We rated the cases, extracted from the studies that fulfill the inclusion criteria, by their information provided in the article. The list of necessary information was created according to the CARE[69] Guidelines and our inclusion criteria:
-
(a)
age, gender, and health status (American Society of Anesthesiologists Classification) of the patient
-
(b)
risk factors and premedication
-
(c)manifestation of TCR:
- (c1) surgical procedure that provoked TCR
- (c2) depth of anesthesia[18]
- (c3) change in HR and MABP and possible arrhythmias
- (c4) treatment of TCR
Out of this list, we defined 7 necessary data parameters: gender, age, localization, a drop of HR, a drop of MABP, CSI, and treatment. Parameters about risk factors and premedication were not included due to insufficient details in the literature available reports.
There was used a 3-level Likert scale for quality evaluation of the case report: a “very well” case report fulfilled the 7 rating criteria, while a “well” rated case report missed 1 or 2 of these 7 criteria. Case reports that showed a lack of more than 2 of our 7 rating criteria were not evaluated and excluded from the study.
2.8. Risk of bias
We analyzed the potential for different biases in our study and identified as most relevant biases for our systematic literature review as possible. These data were evaluated for biases using the “Cochrane Handbook for Systematic Reviews of Intervention.”[68]
2.9. Statistical analysis
Statistical analysis was performed using IBM SPSS 9.5.0.0 software and Microsoft Excel 14.4.2.
Means and standard deviations (SDs) were calculated for the continuous variables. Distributions of all variables were skewed; so, logarithm transformations were applied before further analyses. Unadjusted and partial Pearson correlations were obtained among all the variables. A P value of .05 was considered as statistically significant.
The nonlinear optimization problem was defined as a model in which the objective function and all of the constraints are smooth nonlinear functions of the decision variables. This nonconvex nonlinear optimization problem model is due to its multiple locally optimal points in multiple regions not simple to prove its feasibility or its limitlessness nor to find a “global optimum” that matches in all possible regions. Here the used Generalized Reduced Gradient method[70] can be seen as a nonlinear extension of the Simplex method, which selects a basis, determines a search direction, and performs a line search on each major iteration. This way, this approach solves nonlinear at each step to maintain feasibility.
2.10. Ethical statement
Due to the retrospective character of this research that includes only data from already published cases, an ethical approval is not needed. This article does not include potentially identifying characteristics and information.
3. Results
Altogether, 45 published articles, containing data about 57 TCR cases, fulfilled the inclusion criteria (see Table 1; Fig. 1). After rating all the sampled cases, 13 cases (23%) were rated as “very well,” 19 (33%) as “well” (see Table 2). Twenty-five cases’ (44%) reports did not contain enough data to be rated as “well” or “very well” and therefore excluded from further analyses. Finally, 26 articles, reporting about 32 patients were included for final analyses.
Table 1.
The patient's characteristics of included TCR cases.
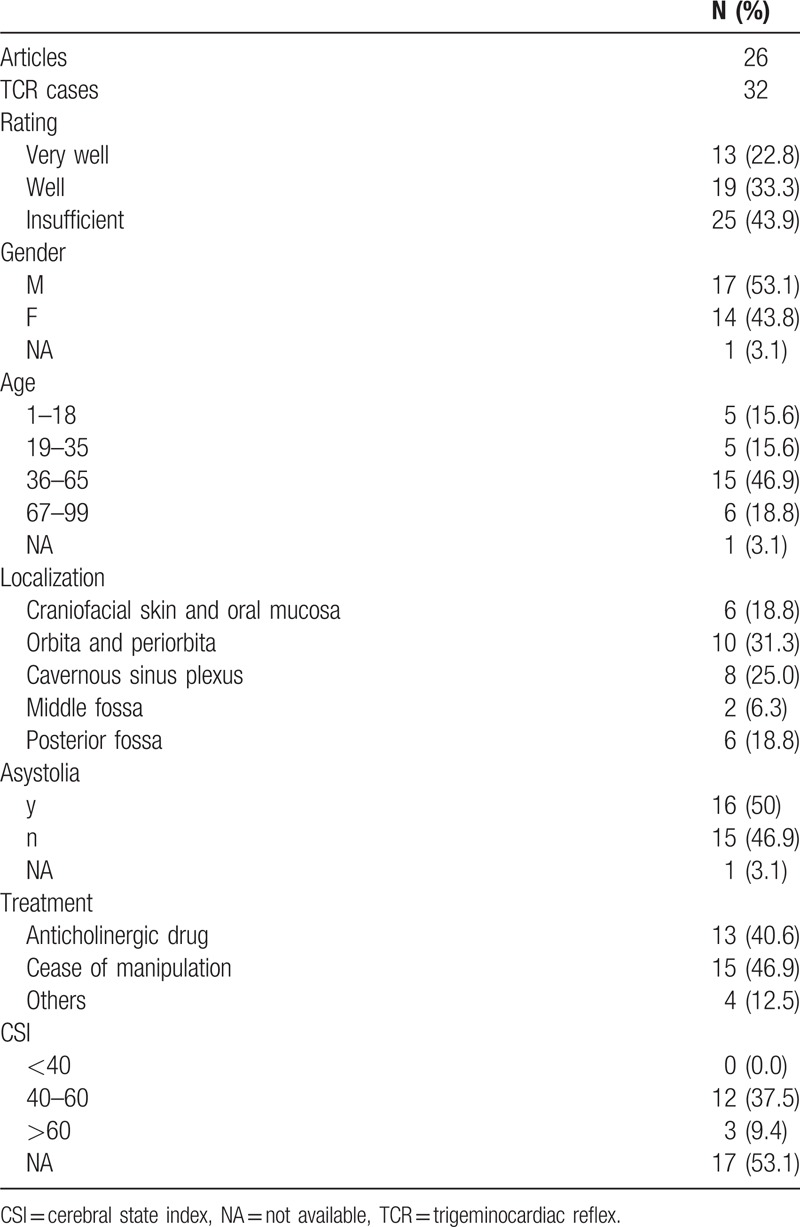
Figure 1.
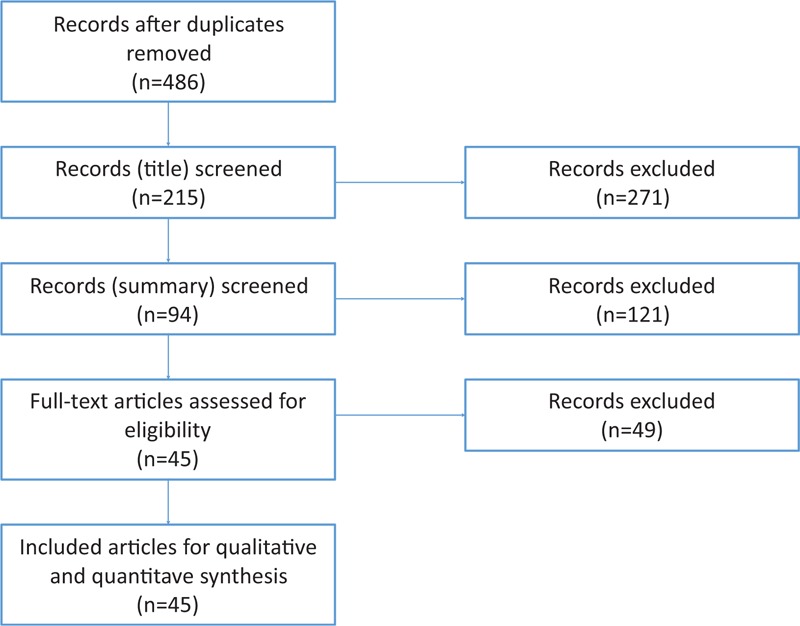
Flowchart of different phases of the review.
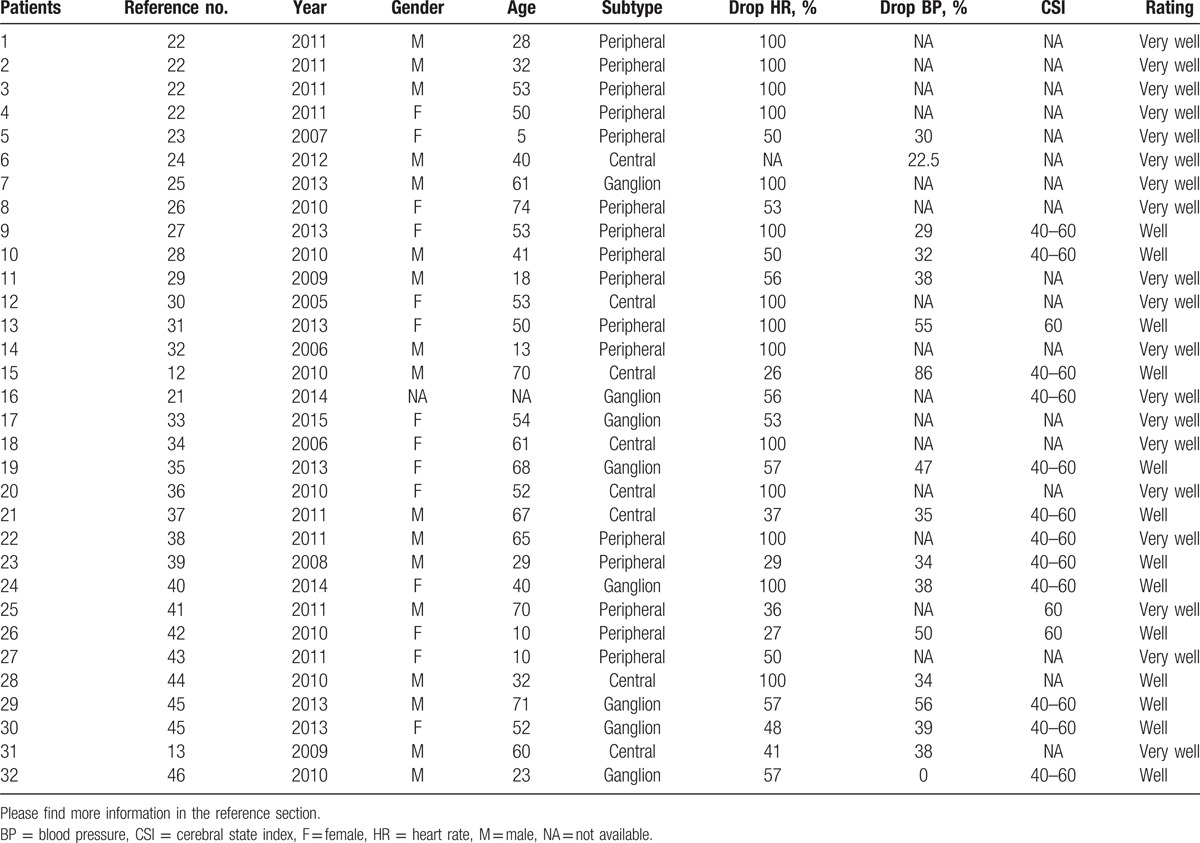
Table 2.
Listed cases included in this meta-analysis study.
3.1. Peripheral–central TCR
Regarding the reported cases, we were able to grade all included cases according to their anatomical location, thus the detailed position of the trigger points around the course of the trigeminal nerve.[49] Craniofacial skin, oral mucosa, as well as orbit and periorbit are locations of the peripheral course of the 5th cranial nerve; in our study, 16 (50%) included cases reported about the peripheral trigger point. The central part of the TCR is represented in the context of the trigeminal nerve through the middle and posterior fossa; in our study, 8 (25%) cases reported the central stimulation. The cavernous sinus plexus plays a multifaceted role in the occurrence and manifestation of the TCR[49,59] due to its various nerve fibers and internerve connections in and around the Gasserian ganglion.[48] This subtype has an special position in the classification of the trigemino-cardiac reflex because of its multifaceted clinical presentation; again, in our research, 8 (25%) cases described a trigger point, located around the cavernous sinus plexus.
3.2. Results regarding HR
In the peripheral subgroup, we had a mean drop of HR of 72% (SD of 30.01; interquartile range [IQR] of 50). In the ganglion subgroup, we had a mean drop of 66% (SD of 21.20 and an IQR of 12.5). In the central subgroup, we calculated a mean drop of 67% (SD of 36.12 and an IQR of 62).
Further, we analyzed the z-values of the mean drop of every subgroup in relation the mean drop of all included TCR cases. The z-value for the peripheral TCR was 0.054, for the ganglion subgroup −2.474 and for the central subgroup −0.109.
From the above findings, it can be demonstrated that we have a case of a nonlinear behavior (see Fig. 2). We have here a “tipping point” phenomenon[71] that differs from central/peripheral (20–30% drop) to Ganglion (40–49% drop).
Figure 2.
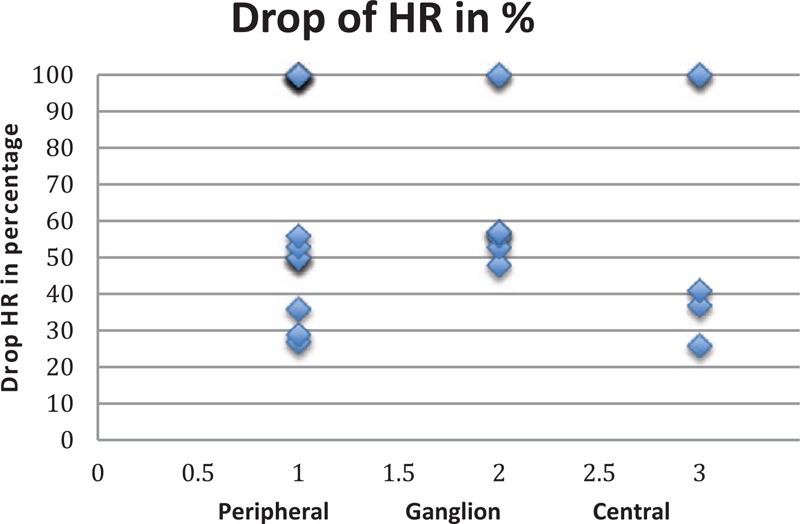
Drop of HR during trigeminocardiac reflex in the 3 main levels. HR = heart rate.
3.3. Results regarding MABP
Again, in the peripheral subgroup, we had a mean drop of MABP of 33.5% (SD of 16.5; IQR of 11.25). In the ganglion subgroup, we had a mean drop of 36% (SD of 21.38; IQR of 9). In the central subgroup, we had a mean drop of 43.1% (SD of 24.69; IQR of 4).
Analyzing the z-values of the mean drop in every subgroup, we can describe for the peripheral subgroup a z-value of −0.172, for the ganglion subgroup −0.044, and for the central group 0.319.
From the above findings, it can be demonstrated that we have a case of a linear behavior (see Fig. 3). We have here a “central limit” phenomenon[72] that does not differ from peripheral, ganglion to central (30–39% drop).
Figure 3.
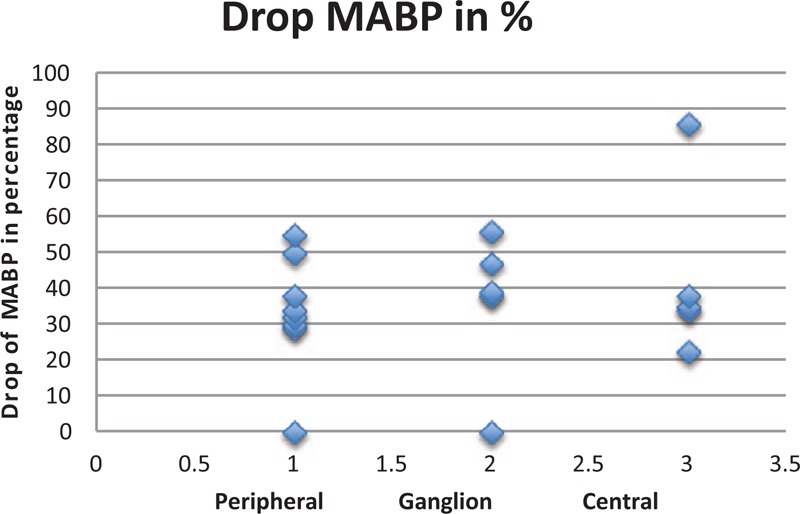
Drop of MABP during trigeminocardiac reflex in the 3 main levels. MABP = mean arterial blood pressure.
3.4. Changes in hemodynamic parameters
To further elucidate this “tipping point” (in HR)/central phenomenon (in MABP), we have searched for a connection between the hemodynamic parameters. As shown above, the HR corresponds to a nonlinear behavior while MABP shows a linear behavior related to the drop of the parameter (see Fig. 4).
Figure 4.
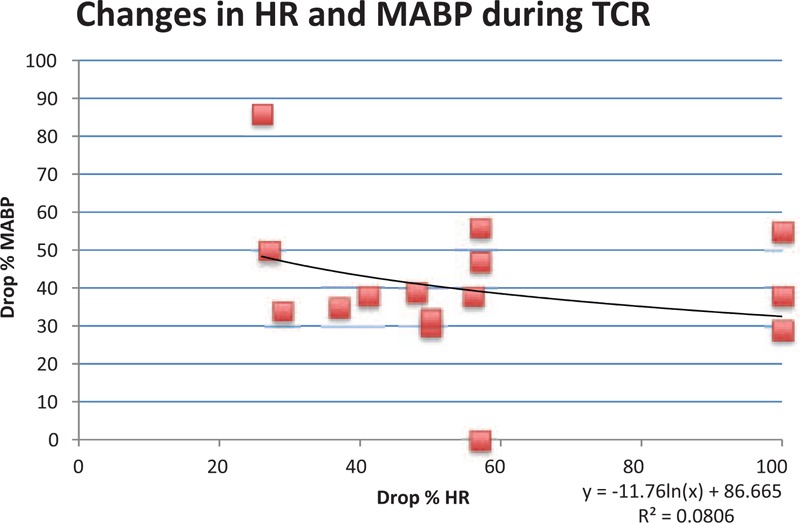
Hemodynamic changes of 16 cases with available data.
In our research, we found 16 cases only with properly described parameters such as HR and MABP. A single description of HR (without a description of MABP) was found in 15 cases. An only report about changes in MABP was found in 1 instance.
In this analysis, we found that depended on to the drop of HR, the decline of MABP in 86% is between 30% and 60%. Only 2 cases showed an extreme deviation to that baseline. The trend line underlines this finding with a good coefficient of determination (R2 = 0.080).
If we analyze all available values of HR and MABP separately without considering the anatomical location, we have data about 31 cases for HR with a median drop of HR of 57% (SD of 28.46; IQR of 50). The same with MABP, we have 17 values of MABP with a median drop of MABP of 36.5% (SD of 19.5; IQR of 15).
We have here a complex behavior that is dominated by the nonlinear HR behavior, confirming that our original arbitrary definition of a drop of more than 20% to the baseline is still valuable. However, in contrary to our initial suggestion in 1999,[1] the MABP changes seem to be Lyapunov function (constraint problem), for which a definition of % of the drop is not correct.
3.5. Optimization of the definition
According to our mathematical optimization model, the TCR definition of the HR should continue to be a decline of more than 20% of the baseline value. There is a strong causal relationship for the HR alone and occurrence of the TCR.
For the MABP it is more complex to find a TCR definition optimization: Mathematically the optimization is still a drop of more than 20%. However, there is no causal relationship (no temporal precedence, no covariation of the cause and effect, but no plausible other explanation) to a drop in MABP alone for the occurrence of the TCR.
Finally, the HR and MABP together show also here causal relations for the occurrence of the TCR as already described earlier.
4. Discussion
Our work demonstrates various interesting insights into the cardiovascular physiology and the TCR behavior, confirming the current clinical tendency and multiple studies that have shown the relative magnitudes of bradycardia in vagal reflexes.[73–77] The HR drop is dominant to the MABP drop and is following a nonlinear behavior with a tipping point around 20% drop. Such used definition that is based on an arbitrary definition that goes back to 1999[1] is now underlined by our simulation optimization and underlines the most often used definition of 20% (or more) HR drop. We are also able to demonstrate that there is no major difference between the “tipping-point” in the central and peripheral TCR so that it seems that they react similarly regarding HR. Strikingly, the ganglion type of TCR shows an exceptional strong drop in HR in our analyses. From our optimization analysis, there is not a clear cause–effect relationship between HR and MABP in all cases of TCR. This finding means, for the first time, that the MABP is no “conditio sine qua non” for all TCRs. Here is certainly further research needed to find the optimal definition.
The Lyapunov function of the MABP is interesting; as per definition externalities in a reasoning system do not go to equilibrium. Clinically this means, there must be external factors that have an influence on the MABP drop. From the current knowledge, this might be the location (peripheral, ganglion, central, etc.) of the stimulation of the trigeminal nerve. A possible explanation for the different behavior of HR and MABP in the TCR subtypes could be given by a different kinetics of the 2 autonomic divisions differ substantially.[74]
The vagal effects develop very rapidly, often within 1 heartbeat, and they decay quickly as well.[76] Hence, the vagus nerves can exert beat-by-beat control of cardiac function.[77] Conversely, the onset and decay of the sympathetic effects are much more gradual; only small changes are effected within the time of 1 cardiac cycle.[74] When both autonomic systems act concomitantly, the effects are not additive algebraically, but complex interactions prevail. Such interactions may be mediated either prejunctionally or postjunctionally with respect to the neuroeffector junction.[74]
If other factors, like for example the difference in pressure on the trigeminal nerve, have also influence or the location has an only influence on the power of the HR drop (with consequences on the control of MABP drop) must be the goal of further research.
This research gives, for the first time, interesting on the behavior of the TCR. The HR is the leading variable in the TCR. If such an HR drop of more than 20% does not exist, there is no TCR. The role of MABP in this reflex arc process is not yet clear. There must be external factors besides the HR that influence the MABP drop. It seems that the location of the trigeminal stimulation is this searched external factor, but we do not know yet in which relation it is to TCR occurrence. Another explanation could be that the TCR phenomenon influence more parasympathetic outputs[49] or the substantial influence of anesthetic drugs on the autonomous system[78] where most anesthetic drugs influence the HR less than MABP.[79,80] In addition, there are substantial differences in hemodynamic reaction on anesthetic drugs relating on gender, ages, and origin of the patient.[79] These different explanations fact may affect the manifestation of the TCR, but is still not explaining the differences in the subtypes of TCR. So it seems more reasonable that multifactorial reasons influence this phenomenon and that also depth of anesthesia[18] and gender[79] are associated hemodynamic changes.
In combination with the predefined major (plausibility and reversibility) and minor (repetition and prevention) criteria,[1,49] the findings from this research lead us to a new, more differentiated definition model of the TCR: As recommended before, a TCR should fulfill both major criteria. A strict definition depended on a steady change in the hemodynamic parameter is, according to the actual state of knowledge, only reasonable with a drop of HR. Out of this research, we can develop a definition model that requires the 2 major criteria and a 20% HR drop. A 20% MABP drop is, based on the here presented findings, only an additional criterion in combination with the 20% HR drop. Here the presented model opens the way to a new surrogate definition; that is valid for all TCR subgroups. There is a need for further studies to evaluate and further refine this model.
4.1. Limitation of this study
In this study, we worked with case reports only, as case reports offer an excellent possibility to create new insights.[81] However, due to the already predefined character of manifestation of TCR (a 20% drop in HR and MABP), there is a publication bias; cases with a drop of <20% could not be published or even not be interpreted as a TCR. This chosen procedure has the (positive) consequence that the TCR is underrepresented in this study, but there were probably no wrong positive cases included. In addition, a language bias exists as the research was only done in English, German, and French, even so all relevant journals publish today at least an abstract in English. Data extraction was performed by multiple reviewers and similar precautions to reduce the risk of reviewer error and bias were taken when assessing the studies for eligibility and validity.
Obviously, this is a descriptive analysis of quantitative data. The included studies were also quite limited in sample size. Given the differences in populations, interventions, and outcomes between the included studies, some narrative synthesis of the review data appeared appropriate. Therefore, detailed causative analysis cannot be done. Descriptive statistics, therefore, enable us to present the data in a more meaningful way, which allows simpler interpretation and commentary of the data.[82]
However, as there is a still ongoing discussion about this 20% drop and there are likewise many studies that applied to a definition of 10% or arrhythmia, this bias seems to be relativized and does not appear to be a major bias for our study.
5. Conclusion
We could for the first time show that the HR is the dominant variable in the TCR occurrence and presented a new surrogate definition model that includes our findings from our research. The new model is including all TCR subtypes into 1 definition to simplify the recognition of a manifest TCR in clinical setting. It allows an early recognition of an upcoming or manifest TCR and allows anesthesia providers to react promptly to prevent negative consequences caused by a persistent TCR. This model and the role of MABP must be better investigated in further studies.
Footnotes
Abbreviations: BP = blood pressure, CSI = cerebral state index, HR = heart rate, IQR = interquartile range, MABP = mean arterial blood pressure, SD = standard deviation, TCR = trigeminocardiac reflex.
CM and BS created the conception of the work, and performed the data collection and the draft. Together with TC they analyzed and interpreted the data. All authors helped with critical revision and final approval.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Schaller B, Probst R, Strebel S, et al. Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg 1999;90:215–20. [DOI] [PubMed] [Google Scholar]
- [2].Schaller B, Graf R. Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab 2004;24:351–71. [DOI] [PubMed] [Google Scholar]
- [3].Schaller B. Trigemino-cardiac reflex during transsphenoidal surgery for pituitary adenomas. Clin Neurol Neurosurg 2005;107:468–74. [DOI] [PubMed] [Google Scholar]
- [4].Schaller B. Trigemino-cardiac reflex during microvascular trigeminal decompression in cases of trigeminal neuralgia. J Neurosurg Anesthesiol 2005;17:45–8. [PubMed] [Google Scholar]
- [5].Schaller B, Buchfelder M. Delayed trigeminocardiac reflex induced by an intraorbital foreign body. Ophthalmologica 2006;220:348. [DOI] [PubMed] [Google Scholar]
- [6].Schaller BJ, Buchfelder M. Trigemino-cardiac reflex in skull base surgery: from a better understanding to a better outcome? Acta Neurochir (Wien) 2006;148:1029–31. [DOI] [PubMed] [Google Scholar]
- [7].Schaller BJ, Weigel D, Filis A, et al. Trigemino-cardiac reflex during transsphenoidal surgery for pituitary adenomas: methodological description of a prospective skull base study protocol. Brain Res 2007;1149:69–75. [DOI] [PubMed] [Google Scholar]
- [8].Schaller B, Sandu N, Filis A, et al. Peribulbar block or topical application of local anaesthesia combined for paediatric strabismus surgery. Anaesthesia 2008;63:1142–3. [DOI] [PubMed] [Google Scholar]
- [9].Schaller BJ. Ketamine and decrease of oculocardiac reflex. Acta Anaesthesiol Scand 2008;52:446. [DOI] [PubMed] [Google Scholar]
- [10].Schaller B, Sandu N, Ottoviani G, et al. Transient asystole during endoscopic transsphenoidal surgery: an example of trigeminocardiac reflex. Pituitary 2009;12:271–2. [DOI] [PubMed] [Google Scholar]
- [11].Cornelius J, Sandu N, Belachew A, et al. The trigemino-cardiac reflex: more than an intraoperative phenomenon. J Chinese Clin Med 2009;4:361–3. [Google Scholar]
- [12].Spiriev T, Sandu N, Arasho B, et al. A new predisposing factor for trigemino-cardiac reflex during subdural empyema drainage: a case report. J Med Case Rep 2010;4:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arasho B, Sandu N, Spiriev T, et al. Management of the trigeminocardiac reflex: facts and own experience. Neurol India 2009;57:375–80. [DOI] [PubMed] [Google Scholar]
- [14].Bohluli B, Schaller B, Sadr-Eshkevari P, et al. Trigeminal cardiac reflex: another all-or-none law? J Oral Maxillofac Surg 2010;68:2922–3. [DOI] [PubMed] [Google Scholar]
- [15].Spiriev T, Kondoff S, Schaller B. Trigeminocardiac reflex during temporary clipping in aneurismal surgery: first description. J Neurosurg Anesthesiol 2011;23:271–2. [DOI] [PubMed] [Google Scholar]
- [16].Schaller B, Filis A, Sandu N, et al. Trigemino-cardiac reflex may be refractory to conventional management in adults. Acta Neurochir (Wien) 2008;150:929–30. [DOI] [PubMed] [Google Scholar]
- [17].Meuwly C, Chowdhury T, Gelpi R, et al. The trigemino-cardiac reflex: is treatment with atropine still justified? J Neurosurg Anesthesiol 2017;29:372–3. [DOI] [PubMed] [Google Scholar]
- [18].Meuwly C, Chowdhury T, Sandu N, et al. Anesthetic influence on occurrence and treatment of the trigemino-cardiac reflex: a systematic literature review. Medicine (Baltimore) 2015;94:e807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chowdhury T, Ahuja N, Schaller B. Severe bradycardia during neurosurgical procedure: depth of anesthesia matters and leads to a new surrogate model of the trigeminocardiac reflex: a case report. Medicine (Baltimore) 2015;94:e2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chowdhury T, Schaller B. Key to prevention of bradycardia: be relax postoperatively: a case report. Medicine (Baltimore) 2016;95:e3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chowdhury T, Cappellani RB, Schaller B, et al. Retrogasserian glycerol rhizolysis: first description of occurrence trigeminocardiac reflex. J Neurosurg Anesthesiol 2014;26:86–7. [DOI] [PubMed] [Google Scholar]
- [22].Min SW, Hwang JM. Adjustment in patients with asystole during strabismus surgery. Graefes Arch Clin Exp Ophthalmol 2011;249:1889–92. [DOI] [PubMed] [Google Scholar]
- [23].Webb MD, Unkel JH. Anesthetic management of the trigeminocardiac reflex during mesiodens removal—a case report. Anesth Prog 2007;54:7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Goyal K, Philip FA, Rath GP, et al. Asystole during posterior fossa surgery: report of two cases. Asian J Neurosurg 2012;7:87–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Onodera Y, Takaoka S, Matuura Y, et al. A case report of cardiac arrest caused by the trigemino-cardiac reflex during endoscopic transsphenoidal pituitary surgery. J Neurosurg Anesthesiol 2013;25:509–10. [Google Scholar]
- [26].Lubbers HT, Zweifel D, Gratz KW, et al. Classification of potential risk factors for trigeminocardiac reflex in craniomaxillofacial surgery. J Oral Maxillofac Surg 2010;68:1317–21. [DOI] [PubMed] [Google Scholar]
- [27].Schipke JD, Cleveland S, Caspers C. Computer-assisted paranasal sinus operation induces diving bradycardia. Am J Otolaryngol 2013;34:617. [DOI] [PubMed] [Google Scholar]
- [28].Kroll HR, Arora V, Vangura D. Coronary artery spasm occurring in the setting of the oculocardiac reflex. J Anesth 2010;24:757–60. [DOI] [PubMed] [Google Scholar]
- [29].Khurana H, Dewan P, Ali Z, et al. Electrocardiographic changes due to vagosympathetic coactivation during the trigeminocardiac reflex. J Neurosurg Anesthesiol 2009;21:270. [DOI] [PubMed] [Google Scholar]
- [30].Bauer DF, Youkilis A, Schenck C, et al. The falcine trigeminocardiac reflex: case report and review of the literature. Surg Neurol 2005;63:143–8. [DOI] [PubMed] [Google Scholar]
- [31].Chowdhury T, West M. Intraoperative asystole in a patient undergoing craniotomy under monitored anesthesia care: is it TCR? J Neurosurg Anesthesiol 2013;25:92–3. [DOI] [PubMed] [Google Scholar]
- [32].Ghai B, Makkar JK, Arora S. Intraoperative cardiac arrest because of oculocardiac reflex and subsequent pulmonary edema in a patient with extraocular cysticercosis. Paediatr Anaesth 2006;16:1194–5. [DOI] [PubMed] [Google Scholar]
- [33].Gupta A, Thomas C, Gaikwad P. Slowdown during parotidectomy: a rare presentation of the trigeminocardiac reflex. Otolaryngol Head Neck Surg 2013;149:345–6. [DOI] [PubMed] [Google Scholar]
- [34].Prabhakar H, Anand N, Chouhan RS, et al. Sudden asystole during surgery in the cerebellopontine angle. Acta Neurochir (Wien) 2006;148:699–700. [DOI] [PubMed] [Google Scholar]
- [35].Chigurupati K, Vemuri NN, Velivela SR, et al. Topical lidocaine to suppress trigemino-cardiac reflex. Br J Anaesth 2013;110:145. [DOI] [PubMed] [Google Scholar]
- [36].Usami K, Kamada K, Kunii N, et al. Transient asystole during surgery for posterior fossa meningioma caused by activation of the trigeminocardiac reflex: three case reports. Neurol Med Chir (Tokyo) 2010;50:339–42. [DOI] [PubMed] [Google Scholar]
- [37].Spiriev T, Tzekov C, Kondoff S, et al. Trigemino-cardiac reflex during chronic subdural haematoma removal: report of chemical initiation of dural sensitization. JRSM Short Rep 2011;2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vasudev S, Reddy KS. Trigemino-cardiac reflex during orbital floor reconstruction: a case report and review. J Maxillofac Oral Surg 2015;14(suppl 1):32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schaller BJ, Filis A, Buchfelder M. Trigemino-cardiac reflex in humans initiated by peripheral stimulation during neurosurgical skull-base operations. Its first description. Acta Neurochir (Wien) 2008;150:715–7. [DOI] [PubMed] [Google Scholar]
- [40].Jeon DG, Kang BJ, Hur TW. Trigemino-cardiac reflex: occurrence of asystole during trans-sphenoidal adenomectomy: a case report. Korean J Anesthesiol 2014;67:209–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Holmes WD, Finch JJ, Snell D, et al. The trigeminocardiac reflex and dermatologic surgery. Dermatol Surg 2011;37:1795–7. [DOI] [PubMed] [Google Scholar]
- [42].Stavrinou P, Foroglou N, Patsalas I, et al. Trigeminocardiac reflex and ipsilateral mydriasis during stereotactic brain tumor biopsy: an insight into the anatomical and physiological pathways involved. Acta Neurochir (Wien) 2010;152:727–8. [DOI] [PubMed] [Google Scholar]
- [43].Puri AS, Thiex R, Zarzour H, et al. Trigeminocardiac reflex in a child during pre-Onyx DMSO injection for juvenile nasopharyngeal angiofibroma embolization: a case report. Interv Neuroradiol 2011;17:13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jaiswal AK, Gupta D, Verma N, et al. Trigeminocardiac reflex: a cause of sudden asystole during cerebellopontine angle surgery. J Clin Neurosci 2010;17:641–4. [DOI] [PubMed] [Google Scholar]
- [45].Amirjamshidi A, Abbasioun K, Etezadi F, et al. Trigeminocardiac reflex in neurosurgical practice: report of two new cases. Surg Neurol Int 2013;4:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chouwdhury T, Prabhakar H, Singh GP, et al. Oculocardiac reflex during endoscopic transsphenoidal removal of pituitary adenoma. Indian J Anaesth 2010;54:269–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Meuwly C, Chowdhury T, Sandu N, et al. Meta-areas of the trigeminocardiac reflex within the skull base: a neuroanatomic “thinking” model. J Neurosurg Anesthesiol 2016;28:437–8. [DOI] [PubMed] [Google Scholar]
- [48].Chowdhury T, Mendelowith D, Golanov E, et al. Trigeminocardiac reflex: the current clinical and physiological knowledge. J Neurosurg Anesthesiol 2015;27:136–47. [DOI] [PubMed] [Google Scholar]
- [49].Meuwly C, Golanov E, Chowdhury T, et al. Trigeminal cardiac reflex: new thinking model about the definition based on a literature review. Medicine (Baltimore) 2015;94:e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sandu N, Chowdhury T, Sadr-Eshkevari P, et al. Trigeminocardiac reflex during cerebellopontine angle surgery: anatomical location as a new risk factor. Future Neurol 2015;10:7–13. [Google Scholar]
- [51].Lemaitre F, Chowdhury T, Schaller B. The trigeminocardiac reflex—a comparison with the diving reflex in humans. Arch Med Sci 2015;11:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chowdhury T, Sandu N, Sadr-Eshkevari P, et al. Trigeminocardiac reflex: current trends. Expert Rev Cardiovasc Ther 2014;12:9–11. [DOI] [PubMed] [Google Scholar]
- [53].Sadr-Eshkevari P, Schaller BJ, Bohluli B. Trigeminocardiac reflex: some thought to the definition. Surg Neurol Int 2014;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sandu N, Schaller B. The trigemino-cardiac reflex: a view to the future. Arch Med Sci 2010;6:138–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schaller B, Filis A, Sandu N, et al. Peripheral trigemino-cardiac reflex. Acta Neurochir (Wien) 2009;151:1727. [DOI] [PubMed] [Google Scholar]
- [56].Schaller B, Cornelius JF, Prabhakar H, et al. The trigemino-cardiac reflex: an update of the current knowledge. J Neurosurg Anesthesiol 2009;21:187–95. [DOI] [PubMed] [Google Scholar]
- [57].Schaller B. Trigeminocardiac reflex. A clinical phenomenon or a new physiological entity? J Neurol 2004;251:658–65. [DOI] [PubMed] [Google Scholar]
- [58].Chowdhury T, Sandu TN, Schaller B, et al. Peripheral trigeminocardiac reflex. Am J Otolaryngol 2013;34:616. [DOI] [PubMed] [Google Scholar]
- [59].Chowdhury T, Sandu TN, Meuwly C, et al. Trigeminocardiac reflex: differential behavior and risk factors in the course of the trigeminal nerve. Future Neurol 2014;9:41–7. [Google Scholar]
- [60].Chowdhury T, Schaller B. The negative chronotropic effect during lumbar spine surgery: a systemic review and aggregation of an emerging model of spinal cardiac reflex. Medicine (Baltimore) 2017;96:e5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gharabaghi A, Koerbel A, Samii A, et al. The impact of hypotension due to the trigeminocardiac reflex on auditory function in vestibular schwannoma surgery. J Neurosurg 2006;104:369–75. [DOI] [PubMed] [Google Scholar]
- [62].Lv X, Jiang C, Li Y, et al. Results and complications of transarterial embolization of intracranial dural arteriovenous fistulas using Onyx-18. J Neurosurg 2008;109:1083–90. [DOI] [PubMed] [Google Scholar]
- [63].Acioly MA, Carvalho CH, Koerbel A, et al. Intraoperative brainstem auditory evoked potential observations after trigeminocardiac reflex during cerebellopontine angle surgery. J Neurosurg Anesthesiol 2010;22:347–53. [DOI] [PubMed] [Google Scholar]
- [64].Spiriev T, Kondoff S, Schaller B, et al. Cardiovascular changes after subarachnoid hemorrhage initiated by the trigeminocardiac reflex-first description of a case series. J Neurosurg Anesthesiol 2011;23:379–80. [DOI] [PubMed] [Google Scholar]
- [65].Koerbel A, Gharabaghi A, Samii A, et al. Trigeminocardiac reflex during skull base surgery: mechanism and management. Acta Neurochir (Wien) 2005;147:727–32. [DOI] [PubMed] [Google Scholar]
- [66].Meng Q, Yang Y, Zhou M, et al. Trigemino-cardiac reflex: the trigeminal depressor responses during skull base surgery. Clin Neurol Neurosurg 2008;110:662–6. [DOI] [PubMed] [Google Scholar]
- [67].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Oxford: The Cochrane Collaboration; 2011. [Google Scholar]
- [69].Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. J Clin Epidemiol 2014;67:46–51. [DOI] [PubMed] [Google Scholar]
- [70].Lasdon LS, Waren AD, Jain A, et al. Design and testing of a generalized reduced gradient code for nonlinear optimization. Technical Memorandum 1975. 353. [Google Scholar]
- [71].Schelling TC. Models of segregation. Am Econ Rev 1969;59:488–93. [Google Scholar]
- [72].Klartag B. A central limit theorem for convex sets. Invent Math 2007;168:91–131. [Google Scholar]
- [73].Michaels DC, Slenter VA, Salata JJ, et al. A model of dynamic vagus-sinoatrial node interactions. Am J Physiol 1983;245:H1043–53. [DOI] [PubMed] [Google Scholar]
- [74].Levy MN. Neural control of cardiac function. Baillieres Clin Neurol 1997;6:227–44. [PubMed] [Google Scholar]
- [75].Arnold RW, Dyer JA, Gould AB, Jr, et al. Sensitivity to vasovagal maneuvers in normal children and adults. Mayo Clin Proc 1991;66:797–804. [DOI] [PubMed] [Google Scholar]
- [76].Berk WA, Shea MJ, Crevey BJ. Bradycardic responses to vagally mediated bedside maneuvers in healthy volunteers. Am J Med 1991;90:725–9. [PubMed] [Google Scholar]
- [77].Arnold RW. The human heart rate response profiles to five vagal maneuvers. Yale J Biol Med 1999;72:237–44. [PMC free article] [PubMed] [Google Scholar]
- [78].Robson JG. Effects of anaesthetic drugs on the central nervous system. Proc R Soc Med 1971;64:211–3. [PMC free article] [PubMed] [Google Scholar]
- [79].Hug CC, Jr, McLeskey CH, Nahrwold ML, et al. Hemodynamic effects of propofol: data from over 25,000 patients. Anesth Analg 1993;77(suppl):S21–9. [PubMed] [Google Scholar]
- [80].Alwardt CM, Redford D, Larson DF. General anesthesia in cardiac surgery: a review of drugs and practices. J Extra Corpor Technol 2005;37:227–35. [PMC free article] [PubMed] [Google Scholar]
- [81].Sandu N, Chowdhury T, Schaller BJ, et al. How to apply case reports in clinical practice using surrogate models via example of the trigeminocardiac reflex. J Med Case Rep 2016;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Meuwly C, Chowdhury T, Sandu N, et al. Definition and diagnosis of the trigeminocardiac reflex: a grounded theory approach for an update. Front Neurol 2017;8:533. [DOI] [PMC free article] [PubMed] [Google Scholar]


