Abstract
Rationale:
Occupational burn injuries can be detrimental and difficult to manage. The majority of complex cases are referred and managed at regional burn centers where access to specialized care is available. As an alternative to hospitalization with staged surgical procedures, placental products may be used for outpatient medical management of these common burn injuries, especially if access to a regional burn center is limited or restricted.
Fresh amnion has been a treatment of choice in burns for more than 100 years. As a biological covering with a broad scope of potential uses, human placental membranes represent a dressing that is particularly advantageous for burn therapy. Recent advances in tissue-preservation technology have allowed for the commercialization of placental amnion products.
Patient concerns:
To address several complications associated with burn injuries—contractures, scar formation, and pain—a viable cryopreserved placental membrane (vCPM) (Grafix—PRIME, Osiris Therapeutics, Inc., MD) retaining the anti-inflammatory, anti-fibrotic, and antimicrobial properties of fresh placental tissues was chosen for clinical use in the 2 cases reported, where both patients had restricted access to the regional burn center.
Diagnoses:
Two cases of work-related extremity burns presented to a local rural hospital for immediate post-injury assessment. The 1st case was of a man who sustained a 55.4 cm2 full-thickness 3rd degree thermal burn with exposed bone and tendon, to the left dorsal forefoot after having an industrial pressure washer caught on his work boot. The 2nd case was of a female who sustained a 4.7 cm2 full-thickness 3rd degree crush burn to the dorsum extensor surface of her dominant hand's index finger after applying 80-pounds per square inch of heated pressure from a hydraulic press.
Interventions:
Both burn patients elected to continue their care at the outpatient-based wound and hyperbaric center, receiving a combination of weekly ad libitum debridement, applications of vCPM, and occupational therapy.
Outcomes:
Both burns reached timely wound closure, and patients regained full range of motion of the affected limb, allowing for early return to work. The average number of allograft applications was 7.5, allowing both patients to return to work in an average of 63.5 days without adverse events or post-treatment complications.
Lessons:
The incorporation of this product in the treatment of these complex burns prevented amputation in one patient, and skin autografting and potential index finger contracture-formation in the second patient. The incorporation of vCPM in burn management may offer a new approach to outpatient burn management and may mitigate several of the complications seen post burn injury, leading to favorable patient outcomes.
Keywords: amniotic membrane, full-thickness wound, occupational injury, thermal burn, viable cryopreserved placental allograft
1. Introduction
More than 40% of all medically treated burns are a result of occupationally based accidents.[1] Work-related hazards give way to nonfatal combination injuries in which the affected limb may suffer both thermal- and crush- or high-pressure injection-related tissue damage.[2] Partial and full-thickness burn injuries to the upper and lower extremities are the most common injuries and require early post-injury care to avoid functional impairments resulting from contracture or amputation.[1,2] Individuals without geographic access to a regional burn center may rely on emergency department assessments with outpatient referral for definitive treatment and rehabilitation.[3] In this setting, as an alternative to hospitalization with staged xenografting or dermal substitutes followed by autografting, initial surgical debridement alongside biological dressings such as human placental membranes (HPMs) may provide a viable option for outpatient medical management of these common burn injuries.
HPMs—mostly fresh human amnion—have been a treatment of choice in burn management for >100 years.[4–7] Specifically, in the last 2 decades, the clinical benefits of amnion have been documented for burn management, infection control, pain reduction, and thermoregulation maintenance, and numerous preservation methods of amnion have been explored.[7–10] Advances in tissue-preservation technology have led to the development of various point-of-care amniotic biological coverings that, when applied, serve as an adherent dressing that protects the wound while maintaining a moist environment, reduce pain, and support epithelialization.[11] Amniotic membranes also provide antimicrobial activity that protects the area from secondary infection, a major contributor to complication, and poor burn-wound resolution.[12,13]
The antimicrobial, analgesic, proangiogenic, and anti-inflammatory properties of HPMs are mediated by components in the tissue that may be altered or destroyed during tissue processing and preservation.[12] Viable cryopreserved placental membrane (vCPM) (Grafix—PRIME, Osiris Therapeutics, Inc., MD) is a commercially available allograft processed with an aseptic cryopreservation technology that retains the native HPM components: a structural 3-D ECM, resident growth factors and cytokines (PDGF, bFGF, EGF, KGF, VEGF, TGF-β3, EGF), and viable cells, including neonatal MSCs, epithelial cells, and fibroblasts. vCPM retains the anti-inflammatory, anti-fibrotic, chemoattractive, pro-angiogenic, and antimicrobial properties of fresh tissue that are known to be beneficial for deep thermal-burn treatment.[14–16] This point-of-care allograft is stored frozen at –80 °C with 2-years shelf life.[17] The membranes retain their structure after thawing and can be easily handled and applied (Fig. 1).
Figure 1.
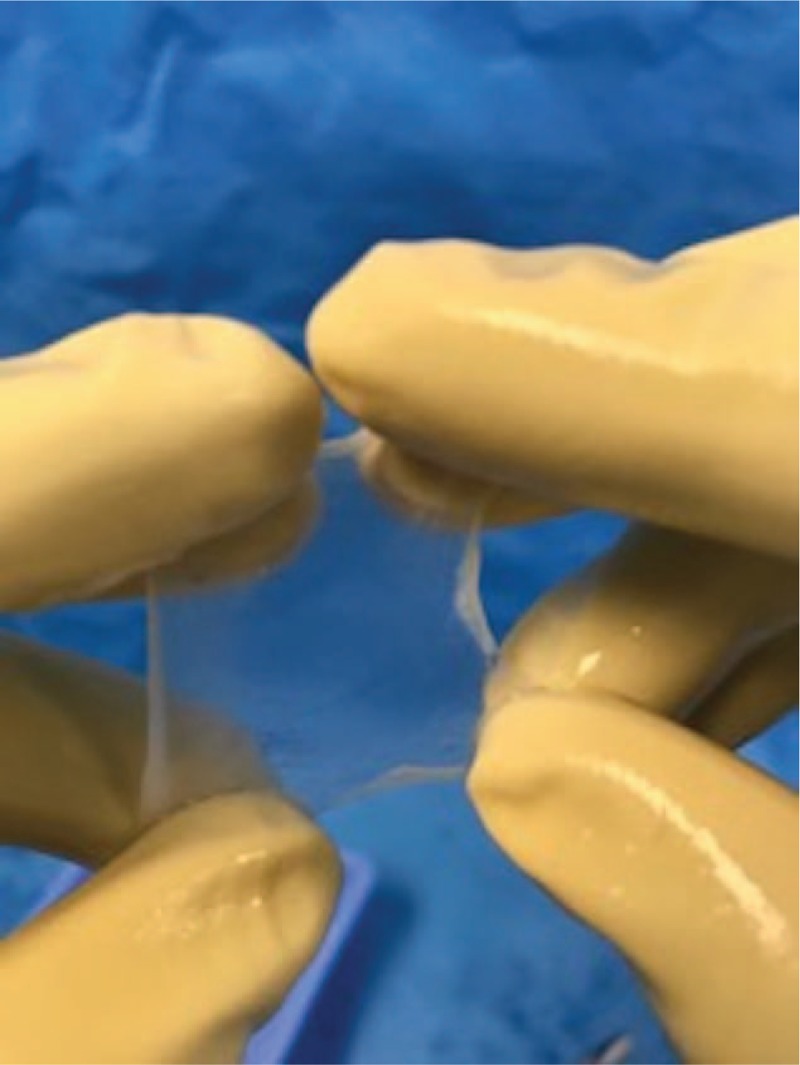
Thawed vCPM (3 × 4 cm) ready for point-of-care application to burn-wound bed. vCPM = viable cryopreserved placental membrane.
Here, we present 2 cases of work-related extremity burns presenting to a local rural hospital for immediate post-injury assessment. The nearest burn care facility exceeded 6 hours of travel time by car with a distance of 412 miles. Both patients elected to continue their care at the outpatient-based wound and hyperbaric center, receiving a combination of weekly ad libitum debridement, applications of vCPM, and occupational therapy (OT).
2. Case reports
2.1. Patient 1: Full-thickness 3rd degree burn via high-pressure heated water injection to the foot
A healthy 23-year-old man presented to the emergency department after an industrial pressure washer was caught on his work boot, resulting in a 1% total body-surface area high-pressure heated water injection injury to the lower extremity. Upon initial assessment, the dorsal aspect of the left forefoot was warm to the touch with evident cellulitis. The area of burned tissue was demarcated by white discoloration and blistering with clear drainage (Fig. 2A). Patient was negative for fever or chills and reported a pain score of 5/10 upon admission. After intravenous administration of cefazolin (2 g), the patient was transferred to the operating room for surgical irrigation and debridement.
Figure 2.
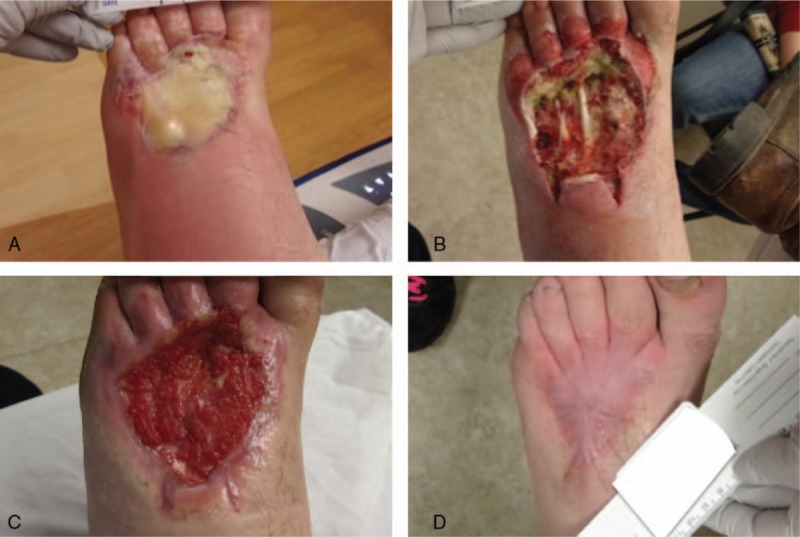
Full-thickness burn via high-pressure heated water injection to the left dorsal forefoot at (A) emergency room evaluation immediately following injury, (B) postsurgical debridement, (C) after demonstration of progressive granulation, and (D) full burn-wound closure.
In the operating room, copious pulse lavage was performed with 2 L of sterile saline containing 100,000 units of bacitracin to irrigate the wound. After careful excision of devitalized and burned tissues, wound-surface area was 55.4 cm2 with exposed bone and extensor tendons of the left dorsal forefoot (Fig. 2B). Initial wound dressings over exposed vital structures included bacitracin and a nonadherent layer (Adaptic, Acelity, TX) secured with a negative-pressure dressing and supportive elastic bandage wrap. Purulent discharge from the wound was cultured positive for bacillus at postoperative day 4. The patient was prescribed cephalexin (500 mg 4 times per day) for the infection and received surgeon referral to the hospital-based wound and hyperbaric center for continuation of care.
Beginning post burn-injury day 15, the patient began receiving weekly outpatient evaluation and care that included episodic debridement and compression and serial grafting with vCPM. When necessary, sharp excision of nonviable tissue was performed with tissue forceps and scissors after administration of 4% topical lidocaine. Placental grafts were covered with a nonadherent layer (Mepitel, Mölnlycke Health Care, Sweden) followed by a negative-pressure bolster. Negative-pressure dressings were discontinued at day 44 and transitioned to a 2-layer bandage compression system (Comperm, Hartmann USA, Inc., SC) with every-other-day secondary dressing changes using bacitracin ointment, collagen, and petroleum jelly gauze. Ongoing vCPM applications contributed to progressive granulation over exposed structures (Fig. 2C). There were no further episodes of secondary-burn infection. Complete burn-wound resolution resulted in lower limb salvage after a total of 10 vCPM applications (Fig. 2D). The patient returned to work at post-injury day 93 with a pain score of 0/10.
2.2. Patient 2: Full-thickness 3rd degree crush burn to the finger via heated hydraulic press
A healthy 30-year-old woman presented to the emergency department after a digit of her dominant hand was caught in hydraulic high-temperature machinery where 80-pounds per square inch (psi) of heated pressure were applied for nearly 10 seconds. The machinery caused a <1% total body surface area 4.7 cm2 full-thickness burn injury to the dorsum extensor surface of the left index finger with involvement of the middle interphalangeal joint and a portion of the proximal nailbed. Approximately, 50% of the region was blanched and surrounded by erythema without drainage (Fig. 3A). A consulting surgeon performed bedside-emergency sharp debridement of the nonviable burned tissue (Fig. 3B). Silver sulfadiazine (Silvadene, Pfizer, Inc., NY) was applied to the burn and a basic primary nonadherent bandage was applied.
Figure 3.
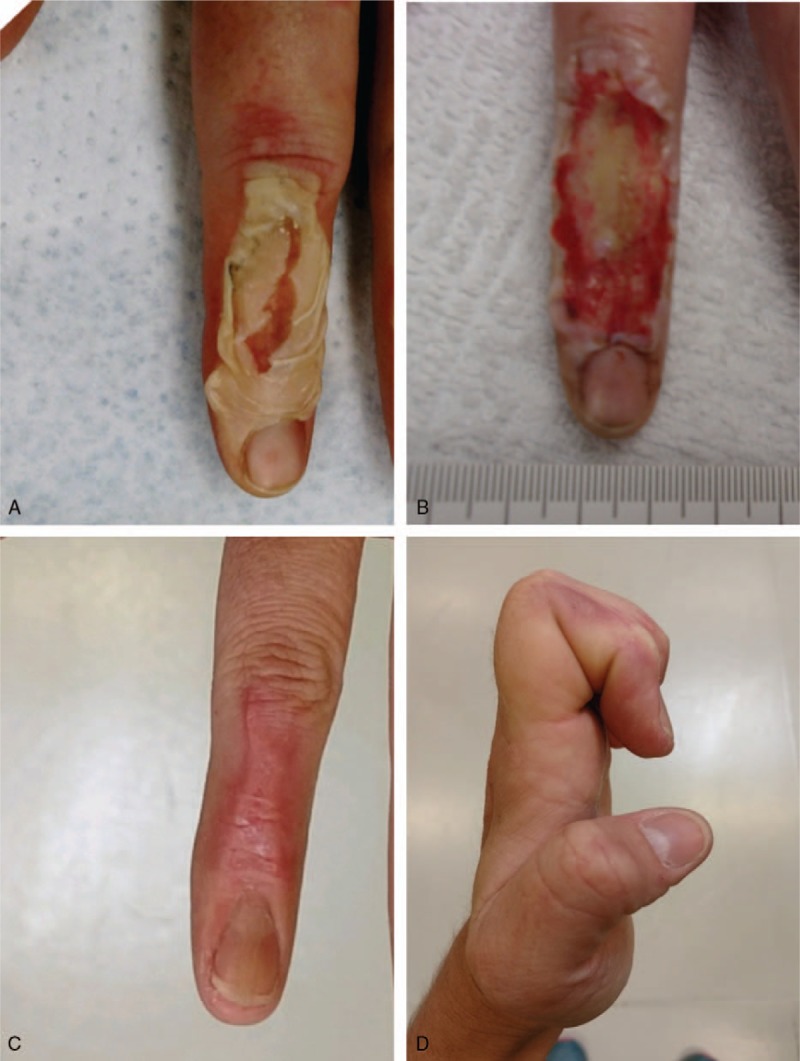
Full-thickness crush burn to the left index finger via heated hydraulic press following compression with 80 psi at (A) emergency room evaluation immediately following injury, (B) post bedside debridement, (C) complete reepithelialization, and (D) during follow-up demonstrating functional range of motion.
At 2 days post injury, the patient received evaluation at the outpatient wound and hyperbaric center where a telemedicine consultation with the nearest regional burn facility was held. Burn surgeon recommendation consisted of staged surgical care with application of a dermal regeneration template followed by split thickness skin autografting. Alternatively, the patient chose to receive serial outpatient grafting with vCPM in addition to sessions of hyperbaric oxygen therapy at 2.4 atmospheres absolute (ATA). Grafts were covered with a nonadherent layer (Mepitel, Mölnlycke Health Care, Sweden) followed by a light compressive bandage. As the burn resolution progressed, OT was initiated where active range of motion (ROM) values were obtained and tracked, demonstrating progressive gains in functional mobility over time. The patient-reported level of pain with initiation of OT was 4/10, which then decreased to 0/10 prior to returning to work at day 34 post injury. A total of 5 vCPM applications contributed to burn-wound resolution without debilitating contracture of the digit (Fig. 3C). There were no incidents of burn infection reported. Upon follow-up, the patient expressed gratitude after regaining full ROM of her interphalangeal joint and was able to resume playing the guitar (Fig. 3D).
3. Discussion
Burn injuries presenting to the emergency department can be managed at the local level, yet more extensive burn injuries that involve the hand, foot, surface over a joint, or affect >5% total body surface area are generally referred and managed at the nearest burn center with access to specialized care.[18–20] In these 2 cases, despite referral to a burn unit, treatment was successfully conducted at the local wound clinic with the use of HPMs. Both patients attained burn-wound closure with an average 7.5 vCPM applications and ultimately returned to work in a mean time of 63.5 days.
Research indicates that, on average, only 66% of patients return to work following burn injury.[21] Schneider et al[22] reported that impaired mobility, defined as the presence of contractures or amputation, was the only statistically significant predictor of unemployment post burn injury at the 12-month mark. Initially, given the severity of the burn injury with exposed bone and tendon, amputation of the foot was considered for patient 1. However, the use of vCPM offered an alternative to amputation allowing the patient to regain lower limb function and normal ambulation. This is consistent with previously published data where similarly complex wounds were treated with vCPM and subsequently healed, avoiding the need for amputation.[23,24] In the case of patient 2, the severity of the crush-burn injury placed the patient at high risk for long-term functional loss of the index finger due to contracture over the joint. A telemedicine consultation with a regional burn center included recommendations for staged autografting with a dermal regeneration template. Poor ROM was demonstrated during the initial assessment of all index finger joints. Following treatment with vCPM and OT, significantly improved active ROM values were obtained for patient 2. Final ROM measurements were comparable with the uninjured finger allowing full flexion of the joint (Fig. 3D).
Several studies have also indicated that a patient's return to work time is prolonged in the presence of ongoing pain.[22,25,26] Following treatment with vCPM, both patients in this report demonstrated a measureable reduction in pain scores from the time of admittance to the time of returning to work. This observation is consistent with other published data indicating pain reduction after treatment with amnion.[27–29] Both patients were prescribed narcotics, a practice often seen in burn-pain management; however, neither patient required use of narcotics during the course of medical care.[30] Instead, over-the-counter ibuprofen (200–600 mg every 4–6 hours) sufficiently managed any injury-related discomfort. Additionally, there were no signs of treatment-related infection or adverse events documented with the use of placental allograft in either patient, which confirms literature demonstrating reduction in rates of infection with amnion application.[27–29]
The results presented in the aforementioned cases detailing the incorporation of HPMs in burn management may offer a new approach to outpatient burn management. The ease of application and associated clinical benefits of vCPM use may mitigate several of the complications seen post burn injury leading to favorable patient outcomes, even in the outpatient clinical setting.
Footnotes
Abbreviations: 3-D = 3 dimensional, ATA = atmospheres absolute, ECM = extracellular matrix, HPM = human placental membrane, MSCs = mesenchymal stem cells, OT = occupational therapy, psi = pounds per square inch, ROM = range of motion, vCPM = viable cryopreserved placental membrane.
The authors have no conflicts of interest to disclose.
References
- [1].Reichard AA, Konda S, Jackson LL. Occupational burns treated in emergency departments. Am J Ind Med 2015;58:290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hultman CS, Erfanian K, Fraser J, et al. Comprehensive management of hot-press hand injuries: long-term outcomes following reconstruction and rehabilitation. Ann Plast Surg 2010;64:553–8. [DOI] [PubMed] [Google Scholar]
- [3].Klein MB, Kramer CB, Nelson J, et al. Geographic access to burn center hospitals. JAMA 2009;302:1774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J 1910;15:307. [Google Scholar]
- [5].Ganatra MA, Durrani KM. Method of obtaining and preparation of fresh human amniotic membrane for clinical use. J Pak Med Assoc 1996;46:126–8. [PubMed] [Google Scholar]
- [6].Magliacani G. The surgical treatment of burns: skin substitutes. Ann Med Burn Club 1990;3:145–9. [Google Scholar]
- [7].Ramakrishnan KM, Jayaraman V. Management of partial-thickness burn wounds by amniotic membrane: a cost-effective treatment in developing countries. Burns 1997;23(Suppl):S33–6. [DOI] [PubMed] [Google Scholar]
- [8].Sawhney CP. Amniotic membrane as a biological dressing in the management of burns. Burns 1989;15:339–42. [DOI] [PubMed] [Google Scholar]
- [9].Gajiwala K, Lobo GA. Use of banked tissue in plastic surgery. Cell Tissue Bank 2003;4:141–6. [DOI] [PubMed] [Google Scholar]
- [10].Branski LK, Herndon DN, Celis MM, et al. Amnion in the treatment of pediatric partial-thickness facial burns. Burns 2008;34:393–9. [DOI] [PubMed] [Google Scholar]
- [11].Ravishanker R, Bath AS, Roy R. “Amnion Bank”—the use of long term glycerol preserved amniotic membranes in the management of superficial and superficial partial thickness burns. Burns 2003;29:369–74. [DOI] [PubMed] [Google Scholar]
- [12].Kesting MR, Wolff K, Hohlweg-Majert B, et al. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res 2008;29:907–16. [DOI] [PubMed] [Google Scholar]
- [13].Adly OA, Moghazy AM, Abbas AH, et al. Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns 2010;36:703–10. [DOI] [PubMed] [Google Scholar]
- [14].Duan-Arnold Y, Gyurdieva A, Johnson A, et al. Soluble factors released by endogenous viable cells enhance the antioxidant and chemoattractive activities of cryopreserved amniotic membrane. Adv Wound Care (New Rochelle) 2015;4:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Duan-Arnold Y, Gyurdieva A, Johnson A, et al. Retention of endogenous viable cells enhances the anti-inflammatory activity of cryopreserved amnion. Adv Wound Care (New Rochelle) 2015;4:523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Duan-Arnold Y, Uveges TE, Gyurdieva A, et al. Angiogenic potential of cryopreserved amniotic membrane is enhanced through retention of all tissue components in their native state. Adv Wound Care (New Rochelle) 2015;4:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gibbons GW. Grafix®, a cryopreserved placental membrane, for the treatment of chronic/stalled wounds. Adv Wound Care (New Rochelle) 2015;4:534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dries DJ, Marini JJ. Management of critical burn injuries: recent developments. Korean J Crit Care Med 2017;32:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Singer AJ, Brebbia J, Soroff HH. Management of local burn wounds in the ED. Am J Emerg Med 2007;25:666–71. [DOI] [PubMed] [Google Scholar]
- [20].Rowley-Conwy G. Management of major burns in the emergency department. Nurs Stand 2013;27:62–8. [DOI] [PubMed] [Google Scholar]
- [21].Quinn T, Wasiak J, Cleland H. An examination of factors that affect return to work following burns: a systematic review of the literature. Burns 2010;36:1021–6. [DOI] [PubMed] [Google Scholar]
- [22].Schneider JC, Bassi S, Ryan CM. Barriers impacting employment after burn injury. J Burn Care Res 2009;30:294–300. [DOI] [PubMed] [Google Scholar]
- [23].Suzuki K, Michael G, Tamire Y. Viable intact cryopreserved human placental membrane for a non-surgical approach to closure in complex wounds. J Wound Care 2016;25(Suppl):S25–31. [DOI] [PubMed] [Google Scholar]
- [24].Frykberg RG, Gibbons GW, Walters JL, et al. A prospective, multicentre, open-label, single-arm clinical trial for treatment of chronic complex diabetic foot wounds with exposed tendon and/or bone: positive clinical outcomes of viable cryopreserved human placental membrane. Int Wound J 2017;14:569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schneider JC, Bassi S, Ryan CM. Employment outcomes after burn injury: a comparison of those burned at work and those burned outside of work. J Burn Care Res 2011;32:294–301. [DOI] [PubMed] [Google Scholar]
- [26].Tang D, Li-Tsang CW, Au RK, et al. Predictors of functional independence, quality of life, and return to work in patients with burn injuries in mainland China. Burns Trauma 2016;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ley-Chavez E, et al. Application of biological dressings from radiosterilized amnios with cobalt 60 and serologic studies on the handling of burns in pediatric patients. Ann Transplant 2003;8:46–9. [PubMed] [Google Scholar]
- [28].Ugar N, Haberal M. Comparison of various dressing materials used for out-patient burn treatment at our centre. Ann Med Burn Club 1994;7:147–9. [Google Scholar]
- [29].Lorusso R, Geraci V, Masellis M. The treatment of superficial burns with biological and synthetic material: frozen amnion and biobrane. Ann Med Burn Club 1989;2:79–84. [Google Scholar]
- [30].Gamst-Jensen H, Vedel PN, Lindberg-Larsen VO, et al. Acute pain management in burn patients: appraisal and thematic analysis of four clinical guidelines. Burns 2014;40:1463–9. [DOI] [PubMed] [Google Scholar]


