Abstract
Transjugular intrahepatic portosystemic shunt (TIPS) reduces the portal venous pressure of patients with hepatopulmonary syndrome (HPS).
To describe the patients who underwent TIPS for the treatment of HPS.
A retrospective study was performed on 81 patients with HPS and gastrointestinal hemorrhage treated with TIPS. Thirty patients underwent TIPS through the main portal vein (group A), 24 through the left branch of the portal vein (group B), and 27 through the right branch of the portal vein (group C). The partial pressure of arterial oxygen (PaO2), alveolar-to-arterial oxygen partial pressure gradient (A–aPO2), oxygen saturation (SO2), and complications were recorded and compared. The survival rate for each group was calculated.
The technical success rate was 100% in the 3 groups. Preoperative portal vein pressure showed no significant differences between the 3 groups, which was decreased post-TIPS operation. In group A, PaO2 and SO2 were higher in 15 days and 3 months postoperative than preoperative (P < .05), whereas A–aPO2 was lower (P < .05). No difference occurred between 12 months post- and preoperative group. In group C, PaO2 and SO2 did not alter significantly at each time point after operation (P > .05), whereas A–aPO2 decreased at 3 months (P = .041) than preoperative. In group B, all indicators at each follow-up time point after TIPS were improved significantly as compared with the preoperative group (P < .05), which showed an excellent effect on hypoxemia treatment. Although the 1-year survival rate of 3 groups of patients was 92.85%, 90.90%, and 91.67%, respectively, the rate of hepatic encephalopathy and hepatic myelopathy was 33.33% (10/30), 16.67% (4/24), and 33.33% (9/27) after TIPS.
TIPS reduced the pressure of the portal vein effectively and alleviated hypoxemia in most HPS patients successfully. Thus, the left branch of the portal vein is optimal for TIPS owing to fewer complications and efficacy in improving PaO2 as compared with the main portal vein and right branch.
Keywords: arterial/alveolar tension ratio, HPS, oxygen partial pressure, oxygen saturation, portal hypertension, portal vein shunt, TIPS
1. Introduction
Hepatopulmonary syndrome (HPS) is a clinical syndrome resulting from the complications of hepatic dysfunction and/or portal hypertension, characterized by widened alveolar–arterial oxygen gradient with or without hypoxemia induced by intrapulmonary vascular dilation.[1–4] HPS primarily affects patients with liver disease, irrespective of age and sex.[1,3] The prevalence of HPS among patients with cirrhosis and advanced liver disease is 15% to 30%.[5,6] The pathogenic hallmark of HPS is microvascular dilation within the pulmonary arterial circulation.[4] In the setting of liver disease, arterial oxygenation defect may occur due to ventilation–perfusion mismatch and putative right-to-left intrapulmonary shunting.[1,2] The most specific presentation of HPS is liver disease-related severe hypoxemia.[1–4]
Currently, a specific treatment for HPS is not available; however, liver transplantation has been considered as the most effective radical treatment.[7,8] The currently used treatments for HPS include general treatment that includes improving liver damage, preventing infection, and reducing portal pressure to reduce the volume of intrapulmonary and portopulmonary shunt; oxygen inhalation, which is only suitable for patients with early or mild HPS. In the case of patients with chronic liver disease combined with portal hypertension, the risk of upper gastrointestinal hemorrhage and refractory ascites could increase with the progression of the disease. Therefore, transjugular intrahepatic portosystemic shunt (TIPS) can be used to reduce the portal venous pressure in the event of failure of conservative treatments.[9] TIPS was first described by Josef Rösch in 1969. In 1988, the first successful TIPS was performed by Rössle et al, to decrease the portal resistance by shunting. Since then, the procedure has become widely accepted as the preferred method for treating portal hypertension that is refractory to medical therapy, replacing the surgical portocaval shunt in that role. The common indications of TIPS include ineffectual medical treatment, unsuitable or unwilling to undergo surgical treatment of upper gastrointestinal hemorrhage caused by portal hypertension; patients with a high risk of bleeding caused by portal hypertension (prophylactic TIPS); patients with recurrent gastrointestinal bleeding after treatment of endoscopic sclerosis and/or ligation; patients with refractory ascites and/or pleural effusion caused by portal hypertension; patients with portal vein thrombosis or BCS (Budd-Chiari syndrome) along with recurrent gastrointestinal bleeding, refractory ascites, and/or pleural effusion.
Previous studies showed that TIPS could improve the symptoms of hypoxemia in patients with HPS by increasing the arterial oxygen pressure, inducing the redistribution of blood flow and reducing the effects of neurokinesis and humoral factors on pulmonary blood vessel dilation. In addition, TIPS could also reduce the incidence of complications such as hemorrhage and ascites. Therefore, the approach could have a significant treatment efficacy in patients with HPS, especially those waiting for liver transplantation. Previous studies have shown that TIPS could decrease mortality and increase the success rate of liver transplantation.[9–12] Nevertheless, accumulating evidence demonstrated that increase in portosystemic shunt and cardiac output could be observed post-TIPS, which increases the incidence of hepatic encephalopathy. In addition, some studies have shown that the partial pressure of arterial oxygen (PaO2) increased by 20 mm Hg 6 months post-TIPS.[9] Furthermore, radionuclide pulmonary perfusion imaging suggested the existence of persistent intrapulmonary shunt accompanied by an increased cardiac output,[13] and thus oxygenation could be improved by mechanisms other than reversing the intrapulmonary shunt. Taken together, the effects of TIPS in HPS are yet to be elucidated.
The present study was to examine a series of patients with HPS who underwent TIPS in a single institution. Relevant clinical indicators and complications were recorded and analyzed to evaluate the effect of TIPS on oxygenation improvement. The results could also help on selecting right procedure of TIPS in HPS patients.
2. Materials and methods
2.1. Study design and patients
This was a retrospective study of patients with HPS and gastrointestinal hemorrhage, who underwent TIPS in our hospital from July 2008 to June 2015. HPS was diagnosed according to the 2004 criteria of the European Association for the Study of Respiratory Diseases: the presence of an increased alveolar–arterial oxygen difference (A–aPO2 >15 mm Hg for patients <64 years old or A–aPO2 >20 mm Hg for patients >64 years old); patients with chronic liver disease; patients with chronic lung disease diagnosed using high-resolution computed tomography (CT) scan (Fig. 5) and pulmonary function tests; evidence of pulmonary vascular dilatation by contrast-enhanced echocardiography.[2,14,15] The exclusion criteria were as follows: cardiovascular and cerebrovascular diseases; portal thrombosis; malignant liver tumors or malignant tumors of any organ. A complete clinical history and physical examination, abdominal CT scan, chest CT scan, ultrasound, magnetic resonance imaging (MRI) of the portal vein (MRPV), gastroscopy and/or esophagography, and chest X-ray data were collected to evaluate the baseline characteristics of the patients.
Figure 5.
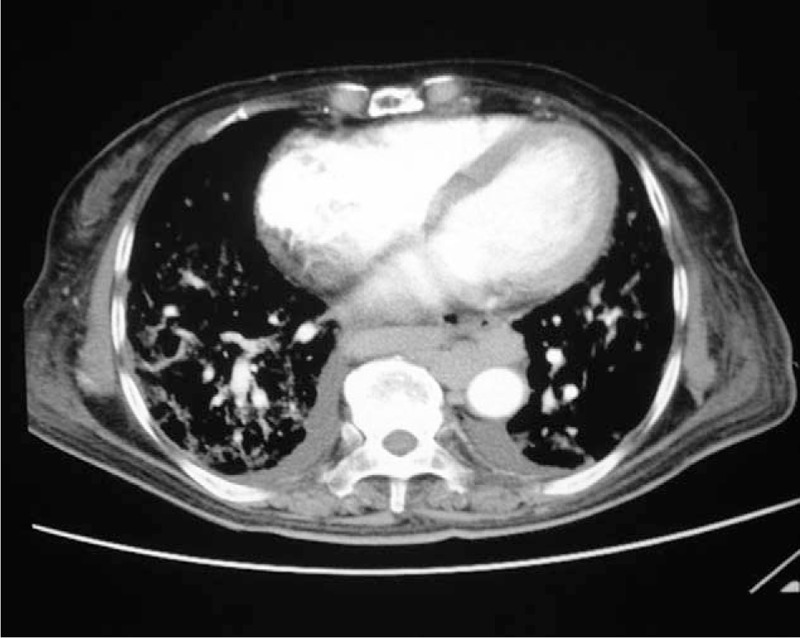
Chest CT showing the clubbed enlargement and honeycomb-shaped expansion of small arteries at the base of the lung, which is merged with the adjacent pleura to form “spider angioma.”
Subsequently, 81 eligible patients were divided into 3 groups based on different operation methods. Among them, 30 patients underwent TIPS through the main portal vein (group A), as for right and left branch converged outside the liver (Fig. 1B) due to evident liver atrophy led by severe cirrhosis (Fig. 1A). Therefore, in group A, the main portal vein was punctured directly to establish the shunt (Fig. 2). Other patients were randomly assigned to groups B and C. Twenty-four patients underwent TIPS through the left branch of the portal vein (group B), and 27 patients underwent TIPS through the right branch of the portal vein (group C).
Figure 1.
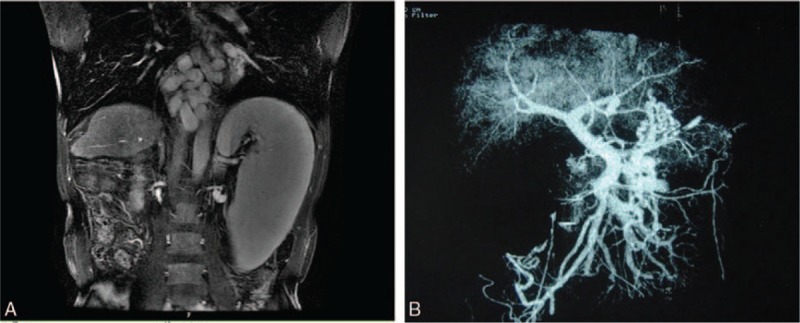
(A) MRI showing liver atrophy and spleen enlargement. (B) Preoperative imaging showing liver atrophy. The right and left portal vein are merging outside the liver.
Figure 2.
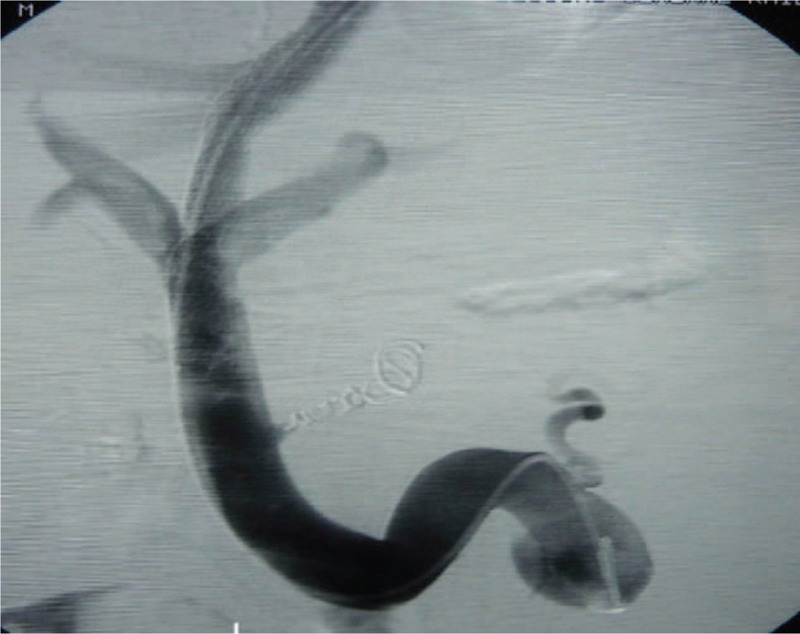
TIPS through the main portal vein.
2.2. Ethics statement
This study was approved by the Ethics Committees of our hospital. The need for individual consent was waived due to the retrospective design of the study.
2.3. TIPS procedure
After the TIPS placement was selected, local disinfection was performed, followed by topical anesthesia with 1% lidocaine. Subsequently, different groups were subjected to various procedures. In group A, the metal guiding tube of the RUPS-100 (Cook Inc., Bloomington, IL) was bent >45°. In some cases, almost 90° manual bending was required. The stent was placed in the main portal vein. In group B, the metal guidance tube of the RUPS-100 was bent >45° (or almost 90° heavy manual bending in some cases), then turned 60° anticlockwise such that the puncture needle could reach the left branch of the portal vein from the left or the middle hepatic vein (Fig. 3). In group C, a straight puncture from the right or the middle hepatic vein to the right branch of the portal vein was common (Fig. 4); the metal cannula of the RUPS-100 was prebend to 45° and displayed sufficient torque control. The Fluency Plus Endovascular Stent Graft (C.R. Bard Inc., Murray Hill, NJ), 8 mm in diameter and 60 to 120 mm in length, was implanted.
Figure 3.
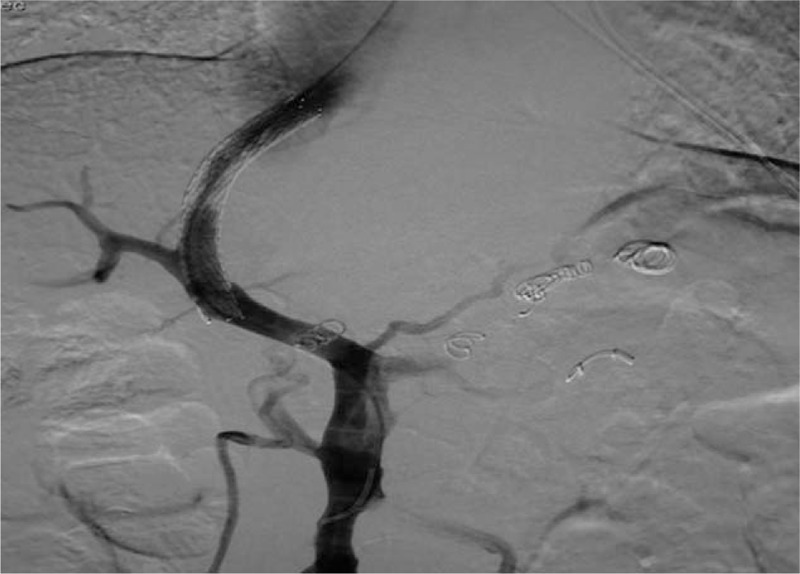
TIPS through the left branch of the portal vein.
Figure 4.
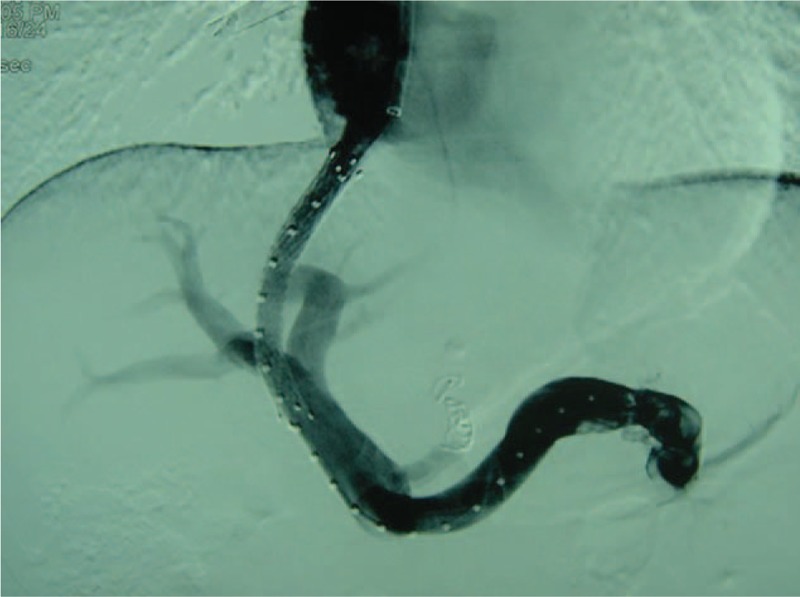
TIPS through the right branch of the portal vein.
During the TIPS operation, a pigtail catheter was placed in the main portal vein to measure the portal vein pressure before and after shunting. If the stent expanded completely, the TIPS procedure was considered successful, and the technical success rate was recorded.
2.4. Arterial blood gas analysis indicators
The recorded indexes included A–aPO2, PaO2, and SaO2. Blood samples were routinely collected using a PE-10 femoral artery catheter (Intramedic; BD Diagnostic Systems, Sparks, MD) and blood gas was measured (Rapidlab 348; Bayer, Etobicoke, Ontario, Canada) before and at 15 days, 3 months, and 12 months post-TIPS.
During the peroration assessment, all patients were examined in both upright and supine positions. Hypoxemia was evaluated by the formula of (a−b)/a, where a is PaO2 in the supine position and b is PaO2 in the standing position. Patients were considered as suffering from hypoxemia when the ratio was >0.1.
2.5. Adverse events evaluation
The observed adverse events included intraoperative thoracic or abdominal hemorrhage, hepatic encephalopathy, hepatic myelopathy, stress gastrointestinal bleeding (hematemesis, black stool), and death caused by liver function failure and stent dysfunction.
2.6. Statistical analysis
Categorical data were expressed as frequency and compared using the χ2 test or the Fisher exact test. Continuous variables were presented as mean ± standard deviation or median and compared by one-way analyses of variance (ANOVA) or nonparametric tests. SPSS 16.0 software (SPSS Inc., Chicago, IL) was used for all the statistical analyses. Two-sided P values <.05 were considered statistically significant.
3. Results
3.1. General characteristics of the patients
A total of 81 patients were included in the study. The baseline characteristics are summarized in Table 1. The mean age of the patients was 56.4 ± 17.6 years; the cohort included 52 males. The disease duration of portal hypertension was 3.2 to 15.4 years. The primary causes of cirrhosis were hepatitis virus cirrhosis (n = 33), alcoholic cirrhosis (n = 17), veno-occlusive cirrhosis (n = 13), cholestasis cirrhosis (n = 9), and others (n = 9). Symptoms and signs included hematemesis (n = 60), black stools (n = 59), ascites (n = 35), spider nevus (n = 53), liver palms (n = 51), dyspnea (n = 51), cyanosis (n = 26), and clubbing (n = 35). The majority of patients were categorized as Child-Pugh stage C (n = 44). However, no significant differences were observed in the demographic and clinical data among the 3 groups (P > .05).
Table 1.
Demographic and clinical characteristics of the patients.
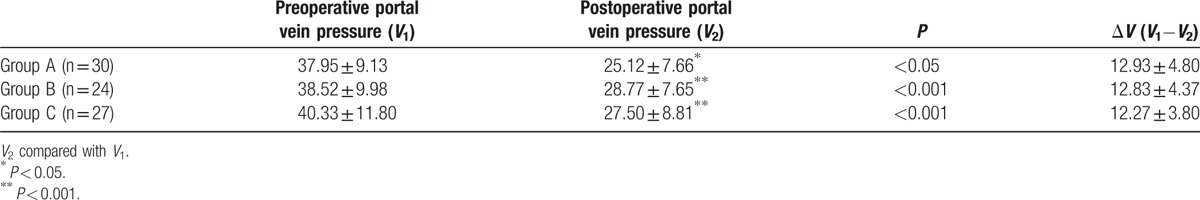
3.2. Technical success rate and changes in portal vein pressure after TIPS
The stents expanded completely in all the patients, and the technical success rate was 100% in the 3 groups. As shown in Table 2, the portal vein pressure decreased significantly after TIPS operation: ΔV was 12.93 ± 4.80, 12.83 ± 4.37, and 12.27 ± 3.80 mm Hg for groups A, B, and C, respectively. No significant difference was observed among the 3 groups with respect to preoperative portal vein pressure, postoperative portal vein pressure, and change in the portal vein pressure.
Table 2.
Changes of portal vein pressure (mm Hg) after TIPS.
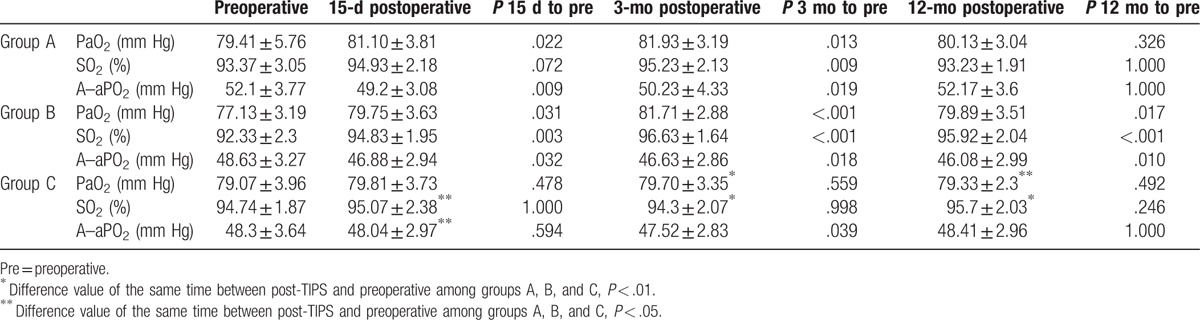
3.3. Comparison of arterial blood gas analysis indicators
In group A, PaO2 (81.10 ± 3.81 and 81.93 ± 3.04 mm Hg) and SO2 (94.93 ± 2.18 and 95.23 ± 2.13) at 15 days and 3 months postoperatively, respectively, were higher than preoperative (79.41 ± 5.76 and 93.37 ± 3.05 mm Hg, P < .05) (Table 3). On the other hand, A–aPO2 (49.2 ± 3.08and 50.23 ± 4.33 mm Hg) was lower postoperatively than preoperative (52.1 ± 3.77 mm Hg, P < .05). No significant difference was observed between 12 months postoperative and preoperative group.
Table 3.
Changes of PaO2, SO2, and A–sPO2 for different TIPS procedures.
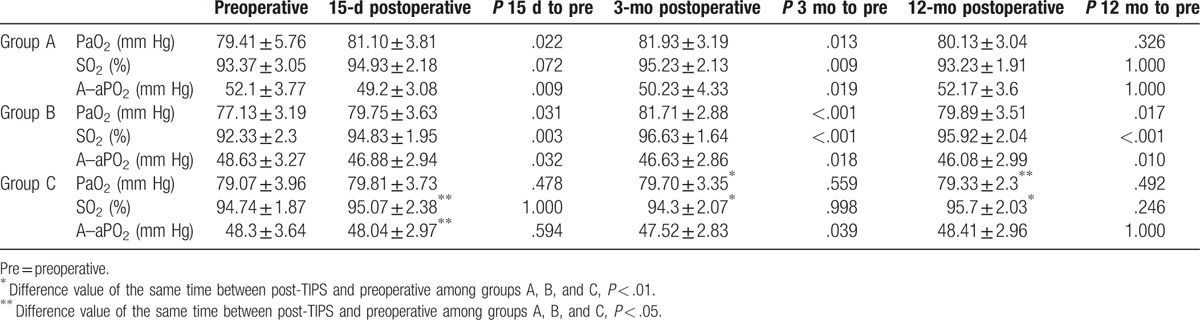
In group C, no significant change in PaO2 and SO2 was noted at each point after operation (P > .05). Only A–aPO2 decreased at 3 months (47.52 ± 2.83, P = .041) as compared with preoperative.
In group B, all indicators at each follow-up time after TIPS were significantly improved as compared preoperatively (P < .05), thereby displaying an excellent effect on the hypoxemia treatment.
3.4. Adverse events and mortality
In group A, 3 patients presented black stool, 2 patients had hematemesis, whereas 10 patients suffered from hepatic encephalopathy and/or hepatic myelopathy. Two patients died of liver function failure 7 and 9 months after the operation, respectively (Table 4). Considering that 2 patients underwent liver transplantation 4 and 11 months after TIPS, respectively, the 1-year survival rate was 92.85%.
Table 4.
Adverse events for different TIPS approaches.
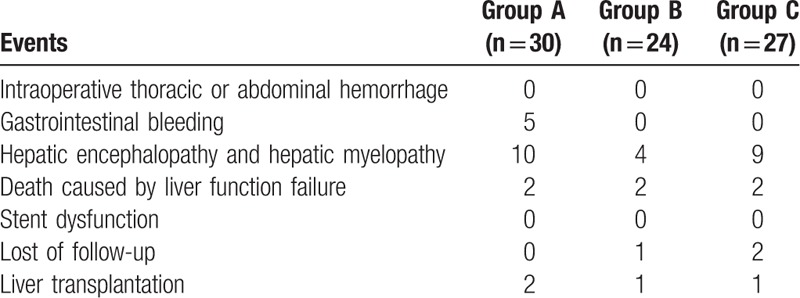
In group B, only 4 cases exhibited hepatic encephalopathy and/or hepatic myelopathy. Two patients died due to the failure of liver function 4.5 and 6 months postoperation, respectively. As 1 patient was lost to follow-up 1 month after TIPS and 1 patient underwent liver transplantation, the 1-year survival rate was 90.90%.
In group C, 9 patients exhibited hepatic encephalopathy and/or hepatic myelopathy, and 2 deaths were noted due to liver function failure 3 and 5 months postoperation, respectively. One patient underwent liver transplantation 5 months after the operation, and 2 patients were lost to follow-up 6 months after the operation. The 1-year survival rate was 91.67% without any significant difference between the 3 groups.
No intraoperative thoracic or abdominal hemorrhage and stent dysfunction was found in either of the groups in the present study.
4. Discussion
HPS is a syndrome exhibiting shortness of breath and hypoxemia (low oxygen levels in the blood of the arteries) caused by vasodilation (broadening of the blood vessels) in the lungs of patients with liver disease. It primarily results from the formation of microscopic intrapulmonary arteriovenous dilatations in patients with both chronic and the less common acute liver failure. Although the underlying mechanism is yet unknown, the phenomenon could be attributed to the increased liver production or to the decreased liver clearance of vasodilators, potentially involving nitric oxide. The most common symptoms include severe hypoxemia concomitant with the signs of liver diseases, such as hematemesis, black stools, ascites, spider nevus, liver palms, dyspnea, cyanosis, and clubbing. In this study, the majority of the cases presented at least one of these symptoms with/or hypoxemia. Currently, the only prescribed treatment is liver transplantation, which is challenging due to the prolonged wait for the donor liver.
Some studies focused on the advantages of TIPS on treating HPS, which proved that the operation could improve the symptoms by increasing SO2, decreasing A–aPO2, redistributing the blood flow, and reducing the expansion effect of the nerve and humoral factor to pulmonary vasculature. These factors contribute to the short-term effect that is crucial for the patients awaiting liver transplantation. However, there were also other studies stating the adverse events of TIPS and the uncertainty of the treatment. The present study verified the effect of TIPS on HPS and, importantly, optimized the procedure to improve the efficiency and avoid the reverse effects. In the present study, 81 patients diagnosed with HPS underwent TIPS treatment with an 8-mm coated stent and achieved complete shunting of the main portal vein or the left/right portal vein. The portal vein pressure decreased significantly after the operation as compared with the baseline. Reducing the portal vein hypertension may reverse the pathophysiology observed in HPS.[16] The synthesis of nitric oxide and other hormones may be affected, also contributing to decline in the symptoms.[17] The improvement in cardiac output after TIPS might also contribute to improved oxygenation.[18] Thus, TIPS might improve blood oxygenation, providing a theoretical basis and evidence supporting its usage in the treatment of HPS. A recent review of case reports showed that TIPS improved oxygenation in patients with HPS,[19] thereby supporting the present case series. Some case studies also reported improvements in oxygenation after TIPS[17,20–22]; however, these cases only had a small gradient, whereas another study did not report any improvement in the patients with critical (>30 mm Hg) gradients.[23]
Currently, liver transplantation has been universally accepted as an effective method for the treatment of HPS with satisfactory moderate-term outcomes; nevertheless, a large number of studies reporting long-term efficacies are yet lacking. Thus, clear guidelines or consensus on the precise location to establish the portal vein bypass, the main portal vein or the branch, is to be investigated. Clinically, TIPS is conducted based only on the experience and safety of the operation. In the present retrospective study, we counted the cases and conducted group analysis to summarize the experiences. Furthermore, the sample size was relatively large (n = 81), and the impact of 3 different TIPS approaches was investigated on the oxygenation status of patients with HPS. Two previous studies showed that the left-branch TIPS was associated with better efficacy and safety than the right-branch TIPS.[24,25] The results also suggested that the approaches with the left-branch puncture should be superior in improving the oxygenation status; however, the 1-year survival rate is equivalent in the 3 groups, indicating that different procedures only affect the short-term effect. As this study was not designed to address any efficacy issue, a randomized controlled trial might be warranted.
Despite being less morbid and risky than liver transplantation, TIPS also presents some adverse events; for instance, the risk of hepatic encephalopathy and exacerbated bleeding.[26] The most common adverse events include hepatic encephalopathy and hepatic myelopathy in the present study, which are similar to that presented in previous studies. In addition, we observed intraoperative thoracic or abdominal hemorrhage cases at the very beginning of TIPS; however, these could be resolved by adequate training. Moreover, stent dysfunction, also one of the most common events, is now resolved by using a covered stent.
Despite the lack of long-term follow-up study of TIPS for HPS, TIPS has been suggested to be useful for patients awaiting liver transplantation.[11,17] Furthermore, TIPS and liver transplantation lead to satisfactory outcomes.[9,27] In the present study, only group A displayed short-term complications after TIPS; nonetheless, the study was not designed to address the safety issue of the TIPS approach. In addition, the use of TIPS for HPS is yet controversial.[23]
Nevertheless, the present study has some limitations. The design of the study was retrospective, the sample was small, and conducted at a single center. Thus, additional studies are essential for determining the benefits of TIPS for HPS.
In conclusion, TIPS operation significantly decreased the portal vein pressure and improved hypoxemia in HPS cases. The left portal vein for TIPS was recommended, as it could improve the HPS symptoms of hypoxemia and increase the arterial oxygen. For the patients awaiting liver transplantation, TIPS could be an appropriate approach; however, the exact TIPS method has yet to be tested using a controlled trial.
Additional studies are essential to determine the elective TIPS procedures. Currently, we use Fluency stent, which can efficiently decrease the portal vein pressure as well as stent dysfunction. However, a further study utilizing the new stent of Vittor along with a long-term follow-up study is imperative for better evaluation.
Acknowledgments
We thank Hao Zheng for her valuable statistical assistance on this article.
Footnotes
Abbreviations: A–aPO2 = alveolar-to-arterial oxygen partial pressure gradient, CT = computed tomography, HPS = hepatopulmonary syndrome, MRI = magnetic resonance imaging, MRPV = magnetic resonance imaging of portal vein, PaO2 = partial pressure of arterial oxygen, SO2 = oxygen saturation, TIPS = transjugular intrahepatic portosystemic shunt.
This study is supported by Beijing Hospital Authority Clinical Technology Innovation Project in China (XMLX201509).
The authors have no conflicts of interest to disclose.
References
- [1].Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome—a liver-induced lung vascular disorder. N Engl J Med 2008;358:2378–87. [DOI] [PubMed] [Google Scholar]
- [2].Rodriguez-Roisin R, Krowka MJ, Herve P, et al. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004;24:861–80. [DOI] [PubMed] [Google Scholar]
- [3].Mandell MS. Clinical controversies surrounding the diagnosis and treatment of hepatopulmonary syndrome. Minerva Anestesiol 2007;73:347–55. [PubMed] [Google Scholar]
- [4].Palma DT, Fallon MB. The hepatopulmonary syndrome. J Hepatol 2006;45:617–25. [DOI] [PubMed] [Google Scholar]
- [5].Ferreira PP, Camara EJ, Paula RL, et al. Prevalence of hepatopulmonary syndrome in patients with decompensated chronic liver disease and its impact on short-term survival. Arq Gastroenterol 2008;45:34–7. [DOI] [PubMed] [Google Scholar]
- [6].De BK, Sen S, Biswas PK, et al. Occurrence of hepatopulmonary syndrome in Budd-Chiari syndrome and the role of venous decompression. Gastroenterology 2002;122:897–903. [DOI] [PubMed] [Google Scholar]
- [7].Taille C, Cadranel J, Bellocq A, et al. Liver transplantation for hepatopulmonary syndrome: a ten-year experience in Paris, France. Transplantation 2003;75:1482–9. [DOI] [PubMed] [Google Scholar]
- [8].Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: impact of liver transplantation. Hepatology 2005;41:1122–9. [DOI] [PubMed] [Google Scholar]
- [9].Benitez C, Arrese M, Jorquera J, et al. Successful treatment of severe hepatopulmonary syndrome with a sequential use of TIPS placement and liver transplantation. Ann Hepatol 2009;8:71–4. [PubMed] [Google Scholar]
- [10].Martinez-Palli G, Drake BB, Garcia-Pagan JC, et al. Effect of transjugular intrahepatic portosystemic shunt on pulmonary gas exchange in patients with portal hypertension and hepatopulmonary syndrome. World J Gastroenterol 2005;11:6858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Paramesh AS, Husain SZ, Shneider B, et al. Improvement of hepatopulmonary syndrome after transjugular intrahepatic portasystemic shunting: case report and review of literature. Pediatr Transplant 2003;7:157–62. [DOI] [PubMed] [Google Scholar]
- [12].Stewart JK, Kuo WT, Hovsepian DM, et al. Portal venous remodeling after endovascular reduction of pediatric autogenous portosystemic shunts. J Vasc Interv Radiol 2011;22:1199–205. [DOI] [PubMed] [Google Scholar]
- [13].Ho V. Current concepts in the management of hepatopulmonary syndrome. Vasc Health Risk Manag 2008;4:1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fallon MB, Abrams GA. Pulmonary dysfunction in chronic liver disease. Hepatology 2000;32:859–65. [DOI] [PubMed] [Google Scholar]
- [15].Martinez GP, Barbera JA, Visa J, et al. Hepatopulmonary syndrome in candidates for liver transplantation. J Hepatol 2001;34:651–7. [DOI] [PubMed] [Google Scholar]
- [16].Sanyal AJ. The use and misuse of transjugular intrahepatic portasystemic shunts. Curr Gastroenterol Rep 2000;2:61–71. [DOI] [PubMed] [Google Scholar]
- [17].Lasch HM, Fried MW, Zacks SL, et al. Use of transjugular intrahepatic portosystemic shunt as a bridge to liver transplantation in a patient with severe hepatopulmonary syndrome. Liver Transpl 2001;7:147–9. [DOI] [PubMed] [Google Scholar]
- [18].Nakamura T, Nakamura S, Tazawa T, et al. Measurement of blood flow through portopulmonary anastomosis in portal hypertension. J Lab Clin Med 1965;65:114–21. [PubMed] [Google Scholar]
- [19].Tsauo J, Weng N, Ma H, et al. Role of transjugular intrahepatic portosystemic shunts in the management of hepatopulmonary syndrome: a systemic literature review. J Vasc Interv Radiol 2015;26:1266–71. [DOI] [PubMed] [Google Scholar]
- [20].Riegler JL, Lang KA, Johnson SP, et al. Transjugular intrahepatic portosystemic shunt improves oxygenation in hepatopulmonary syndrome. Gastroenterology 1995;109:978–83. [DOI] [PubMed] [Google Scholar]
- [21].Selim KM, Akriviadis EA, Zuckerman E, et al. Transjugular intrahepatic portosystemic shunt: a successful treatment for hepatopulmonary syndrome. Am J Gastroenterol 1998;93:455–8. [DOI] [PubMed] [Google Scholar]
- [22].Wallace MC, James AL, Marshall M, et al. Resolution of severe hepato-pulmonary syndrome following transjugular portosystemic shunt procedure. BMJ Case Rep 2012;2012: pii: bcr0220125811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Corley DA, Scharschmidt B, Bass N, et al. Lack of efficacy of TIPS for hepatopulmonary syndrome. Gastroenterology 1997;113:728–30. [DOI] [PubMed] [Google Scholar]
- [24].Chen L, Xiao T, Chen W, et al. Outcomes of transjugular intrahepatic portosystemic shunt through the left branch vs. the right branch of the portal vein in advanced cirrhosis: a randomized trial. Liver Int 2009;29:1101–9. [DOI] [PubMed] [Google Scholar]
- [25].Bai M, He CY, Qi XS, et al. Shunting branch of portal vein and stent position predict survival after transjugular intrahepatic portosystemic shunt. World J Gastroenterol 2014;20:774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Suhocki PV, Lungren MP, Kapoor B, et al. Transjugular intrahepatic portosystemic shunt complications: prevention and management. Semin Intervent Radiol 2015;32:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cosarderelioglu C, Cosar AM, Gurakar M, et al. Hepatopulmonary syndrome and liver transplantation: a recent review of the literature. J Clin Transl Hepatol 2016;4:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


