Abstract
Rational:
The efficacy of nintedanib, a multitarget receptor tyrosine kinase inhibitor, has been demonstrated in recent randomized controlled trials involving patients with idiopathic pulmonary fibrosis (IPF). However, accelerated disease progression after nintedanib discontinuation has never been reported.
Patient concerns:
We report 2 cases involving patients with a history of IPF who presented with respiratory deterioration at 3 weeks after the discontinuation of nintedanib therapy for IPF. Neither patient fulfilled the definition of “acute exacerbation of IPF” on unilateral computed tomography.
Diagnoses:
Accelerated disease progression after the discontinuation of nintedanib therapy for IPF.
Interventions:
One patient received steroid therapy. The other patient refused to undergo steroid therapy.
Outcomes:
The first patient showed that the affected lobe exhibited volume loss with traction bronchiectasis after receiving steroid therapy, and succumbed to pneumothorax after 3 months. The other patient was transferred to another hospital because of a decline in his general condition.
Lessons:
To our knowledge, this report is the first to document accelerated disease progression after the discontinuation of nintedanib therapy for IPF. Although the accurate mechanism remains unclear, the effects of nintedanib against vascular endothelial growth factor and platelet-derived growth factor receptor may play a role. Our findings suggest that physicians should carefully monitor patients with IPF after nintedanib discontinuation.
Keywords: accelerated disease progression, acute exacerbation, idiopathic pulmonary fibrosis, nintedanib, tyrosine kinase inhibitor
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive pulmonary disorder with an unknown etiology, a poor prognosis, and an increasing prevalence.[1] Nintedanib, a multitarget receptor tyrosine kinase inhibitor (TKI), has been shown to be efficacious in recent randomized controlled trials involving patients with IPF.[2] In addition, nintedanib was found to suppress the acute exacerbation of IPF (AE-IPF),[3] which is associated with high mortality.[4] However, accelerated disease progression after nintedanib discontinuation has never been reported. Here, we report 2 cases of accelerated disease progression 3 weeks after the discontinuation of nintedanib administered for IPF.
2. Case reports
2.1. Case 1
A 71-year-old man with a history of pulmonary tuberculosis presented to our hospital with progressive dyspnea on exertion (DOE). Two years before admission, he had been diagnosed with IPF based on clinical, radiological, and pathological assessments. He was prescribed the antifibrotic agent pirfenidone, which was replaced by nintedanib (300 mg/d) 8 months before admission because of worsening of his DOE and forced vital capacity. Three weeks before admission, he developed diarrhea, forcing discontinuation of nintedanib. Shortly thereafter, his DOE worsened. He did not change his medicine or living environment, and was not exposed to inhaled antigens. On admission, he exhibited tachypnea, respiratory failure, and bilateral fine crackles over the lung. Other vital signs and physical examination findings were unremarkable. Chest computed tomography (CT) showed new ground glass opacities (GGOs) in the left lower lobe (Fig. 1A and B) that were unrelated to the bronchial distribution, and were atypical for pulmonary infection. Empiric antibiotic treatment with piperacillin/tazobactam resulted in no clinical improvement. We administered methylprednisolone pulse therapy (1 g/d for 3 days) followed by prednisolone (1 mg/kg per d). Although the respiratory failure symptoms and GGOs were alleviated, the affected lobe exhibited volume loss with traction bronchiectasis (Fig. 1C). Three months later, he developed secondary spontaneous pneumothorax due to IPF. He refused mechanical ventilation and died soon thereafter.
Figure 1.
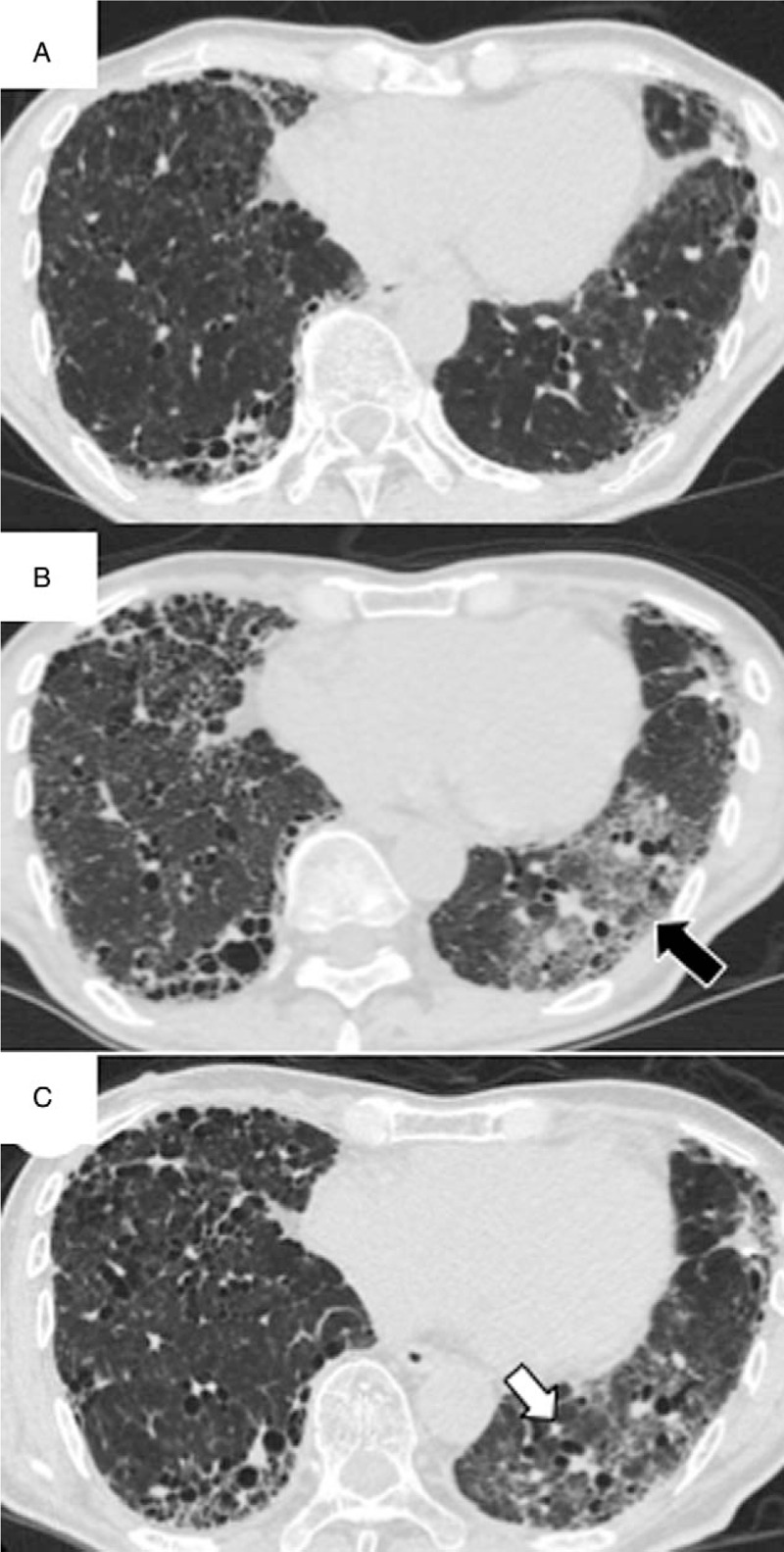
Chest computed tomography (CT) findings for a 71-year-old man with idiopathic pulmonary fibrosis who presented with accelerated disease progression after nintedanib discontinuation. Chest CT findings at 8 months before admission (A), at the time of admission (B), and after treatment with methylprednisolone pulse therapy (1 g/d for 3 days) and prednisolone (C). Note the new ground glass opacities (GGOs) in the left lower lobe on images obtained at the time of admission (B, black arrow). After treatment, the GGOs were alleviated; however, the affected lobe exhibited volume loss with traction bronchiectasis (C, white arrow).
2.2. Case 2
An 86-year-old man presented at our hospital with appetite loss for the past week. Six years before admission, he was diagnosed with IPF based on a definite usual interstitial pneumonia pattern on high-resolution CT. The disease progressed gradually during the follow-up period; nintedanib (300 mg/d) was initiated 3 weeks before admission. He had no diarrhea, nausea, or abdominal pain. He had previously received medications for diabetes mellitus, including insulin. On admission, his oxygen saturation was 98% while breathing 2 L/min of oxygen via a nasal cannula. There were no remarkable findings other than bilateral fine crackles over the lungs. Chest CT showed reticular abnormalities and a honeycomb pattern with traction bronchiectasis (Fig. 2A). After the clinical and radiological confirmation of IPF stability, his continued loss of appetite forced the discontinuation of nintedanib. However, his respiratory condition gradually deteriorated over the next 3 weeks, necessitating administration of 5 L/min of oxygen via a nasal cannula at rest. Chest CT revealed a newly developed GGO with traction bronchiectasis in the left upper lobe (Fig. 2B). Although levofloxacin (500 mg/d) was administered as an empiric antibiotic treatment, his condition showed no improvement. He did not consent to steroid therapy because of the associated risks, such as infection and worsening of diabetes mellitus. He was transferred to a hospital near his home.
Figure 2.
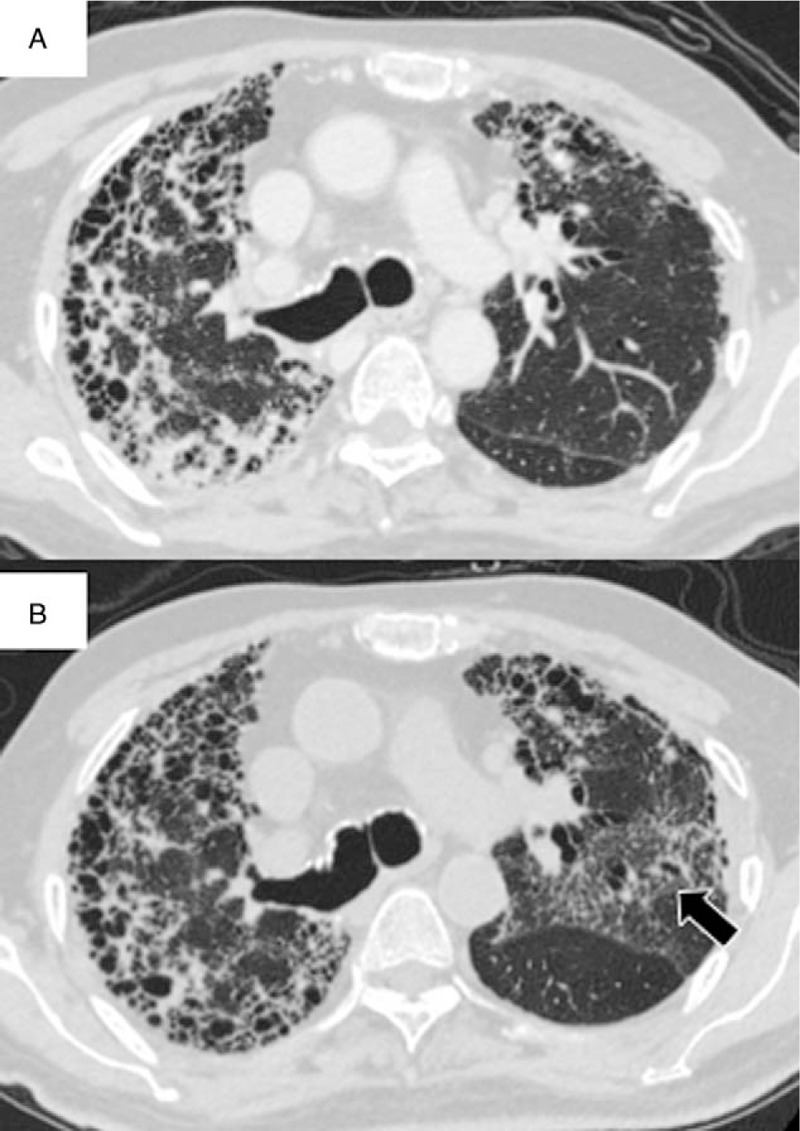
Chest computed tomography (CT) findings for an 86-year-old man with idiopathic pulmonary fibrosis who presented with accelerated disease progression after nintedanib discontinuation. Chest CT findings at the time of nintedanib discontinuation (A) and at 3 weeks after discontinuation (B). Note the new ground glass opacities with traction bronchiectasis in the left upper lobe after discontinuation of nintedanib (B, arrow).
3. Discussion
We described 2 cases of IPF with deteriorated respiratory condition at 3 weeks after nintedanib discontinuation. We believe that these deteriorations were consistent with accelerated disease progression for 2 reasons. First, other possible causes that exacerbate the respiratory condition were unlikely, such as infection, drug toxicity, inhaled antigens, and congestive heart failure. Indeed, we saw no signs of any of the above potential causes in either patient. Second, the new GGOs in high-resolution CT images were accompanied by traction bronchiectasis, which reflects the fibrotic change in the lung.[5] Although their presentations did not fulfill the definition of AE-IPF due to the unilateral CT findings,[4] 1 patient died after 3 months, while the other had deteriorated significantly prior to being transferred to another hospital because of his overall declining health.
Nintedanib is an oral triple TKI targeting vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor receptors, which play critical roles in the pathogenesis of pulmonary fibrosis and several cancers.[2,6,7] In our cases, IPF progressed over 3 weeks after nintedanib discontinuation. Studies on TKI therapy for various cancers such as gastrointestinal stromal tumor, lung cancer, and renal cell carcinoma have reported disease flare after TKI discontinuation.[8–10] However, the exact mechanism underlying this effect has not been elucidated. A study on epidermal growth factor receptor TKIs for epidermal growth factor receptor-driven lung cancers considered the rapid growth of sensitive clones following TKI discontinuation to be 1 possible mechanism.[10] On the other hand, other studies on renal cell carcinoma showed disease flare due to rapid angiogenesis after the discontinuation of TKIs targeting VEGF and PDGF receptors.[11,12] Furthermore, multiple protein tyrosine kinases have been implicated in the development and progression of fibrosis.[13] Notably, VEGF and PDGF have been shown to stimulate lung fibroblast proliferation[14] and may play an important role in human studies regarding AE-IPF.[15] Although the role of angiogenesis in IPF remains unclear,[16] we believe that reactivation of several different receptor tyrosine kinases after TKI discontinuation, including VEGF and PDGF receptors, can result in accelerated disease progression.
To our knowledge, this is the first report documenting accelerated disease progression after nintedanib discontinuation in patients with IPF. In one case, although steroid therapy resulted in respiratory improvement, the affected lung remained impaired. Our findings suggest that physicians should carefully monitor patients with IPF after nintedanib discontinuation to prevent accelerated disease progression, which has an invariably poor prognosis. Further investigation is required to clarify the mechanism underlying accelerated disease progression after nintedanib discontinuation in patients with IPF.
Footnotes
Abbreviations: AE-IPF = acute exacerbation of idiopathic pulmonary fibrosis, CT = computed tomography, DOE = dyspnea on exertion, FVC = forced vital capacity, GGOs = ground glass opacities, IPF = idiopathic pulmonary fibrosis, PDGF = platelet-derived growth factor, TKI = tyrosine kinase inhibitor, VEGF = vascular endothelial growth factor.
Written informed consent was obtained from the patient or the family of the patient.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med 2014;2:566–72. [DOI] [PubMed] [Google Scholar]
- [2].Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071–82. [DOI] [PubMed] [Google Scholar]
- [3].Suissa S, Ernst P. The INPULSIS enigma: exacerbations in idiopathic pulmonary fibrosis. Thorax 2015;70:508–10. [DOI] [PubMed] [Google Scholar]
- [4].Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265–75. [DOI] [PubMed] [Google Scholar]
- [5].Westcott JL, Cole SR. Traction bronchiectasis in end-stage pulmonary fibrosis. Radiology 1986;161:665–9. [DOI] [PubMed] [Google Scholar]
- [6].Roth GJ, Heckel A, Colbatzky F, et al. Design, synthesis, and evaluation of indolinones as triple angiokinase inhibitors and the discovery of a highly specific 6-methoxycarbonyl-substituted indolinone (BIBF 1120). J Med Chem 2009;52:4466–80. [DOI] [PubMed] [Google Scholar]
- [7].Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008;68:4774–82. [DOI] [PubMed] [Google Scholar]
- [8].Abbeele ADVD, Badawi RD, Manola J, et al. Effects of cessation of imatinib mesylate (IM) therapy in patients (pts) with IM-refractory gastrointestinal stromal tumors (GIST) as visualized by FDG-PET scanning. J Clin Oncol 2004;22:3012. [Google Scholar]
- [9].Wolter P, Beuselinck B, Pans S, et al. Flare-up: an often unreported phenomenon nevertheless familiar to oncologists prescribing tyrosine kinase inhibitors. Acta Oncol 2009;48:621–4. [DOI] [PubMed] [Google Scholar]
- [10].Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res 2011;17:6298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Griffioen AW, Mans LA, de Graaf AM, et al. Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res 2012;18:3961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Iacovelli R, Massari F, Albiges L, et al. Evidence and clinical relevance of tumor flare in patients who discontinue tyrosine kinase inhibitors for treatment of metastatic renal cell carcinoma. Eur Urol 2015;68:154–60. [DOI] [PubMed] [Google Scholar]
- [13].Grimminger F, Gunther A, Vancheri C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J 2015;45:1426–33. [DOI] [PubMed] [Google Scholar]
- [14].Beyer C, Distler JH. Tyrosine kinase signaling in fibrotic disorders: translation of basic research to human disease. Biochim Biophys Acta 2013;1832:897–904. [DOI] [PubMed] [Google Scholar]
- [15].Oishi K, Mimura-Kimura Y, Miyasho T, et al. Association between cytokine removal by polymyxin B hemoperfusion and improved pulmonary oxygenation in patients with acute exacerbation of idiopathic pulmonary fibrosis. Cytokine 2013;61:84–9. [DOI] [PubMed] [Google Scholar]
- [16].King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 2011;378:1949–61. [DOI] [PubMed] [Google Scholar]


